2. 山东隆科特酶制剂有限公司, 沂水 276400
2. Shandong Longkete Enzyme Preparation Co., Ltd., Yishui 276400, China
甘露聚糖是存在于植物性饲料中的一类抗营养因子,畜禽采食后会增加消化道内食糜黏度,影响畜禽对饲粮中营养物质的消化和吸收,导致营养物质的消化利用率降低[1]。已知β-甘露聚糖酶由多种细菌、真菌、放线菌、植物和动物产生[2-3],从微生物中提取的β-甘露聚糖酶因其低成本、易获得和高活性等特性[1],受到国内外学者的广泛关注[4],目前已在制浆造纸、制药、食品、石油和纺织工业中得到广泛应用[1, 5-9]。在饲粮中加入β-甘露聚糖酶,可使细胞壁中甘露聚糖分解,并促进被细胞壁结构包裹的营养物质释放,降低消化道内食糜黏度,增加十二指肠和空肠绒毛的高度,从而使表面积增大,提升动物对营养物质的吸收能力[10],达到提高植物性原料的消化利用率和改善动物生产性能的效果。本文主要从β-甘露聚糖酶的酶学特性以及其在畜禽生产中的应用进行综述,为β-甘露聚糖酶在畜牧业中的应用提供科学依据。
1 甘露聚糖与甘露聚糖酶在植物性原料中,甘露聚糖是高等植物细胞壁中半纤维素的主要成分之一,它包括由D-甘露糖、D-半乳糖和D-葡萄糖等单糖合成的线性或支链聚合物[11]。甘露聚糖可分为线性甘露聚糖、葡甘露聚糖、半乳甘露聚糖和半乳葡甘露聚糖(图 1),从结构上看,这些多糖均含有甘露糖或葡萄糖和甘露糖残基组合而成的β-1, 4-主链[12]。此外,甘露聚糖主链可以被侧链的半乳糖α-1, 6-连锁残基取代[13]。由于甘露聚糖具有黏度、表面活性及持水性等物理特性,因此单胃动物采食后会产生抗营养作用。甘露聚糖抗营养作用表现在以下几个方面:一是在畜禽消化道内甘露聚糖溶于水后易形成凝胶状,增加食糜黏度,造成动物饱腹感增加并降低采食量,从而降低生长性能;二是甘露聚糖具有高持水活性,可吸收大量水分,使其物理特性发生改变,抑制肠道的蠕动;三是甘露聚糖的高亲水性使其易与肠黏膜上的脂类微团及多糖蛋白复合物产生相互作用,导致肠黏膜表面水层变厚,降低营养物质吸收;四是未消化的非淀粉多糖(non-starch polysaccharide,NSP)如甘露聚糖等在消化道内沉积,使消化道后段微生物厌氧发酵,产生大量毒素,引起消化道微生物群落的紊乱并抑制动物生长;五是在消化液中甘露聚糖极易与饲粮中带相反电荷的营养物质结合,从而影响营养物质的吸收利用[14-15]。
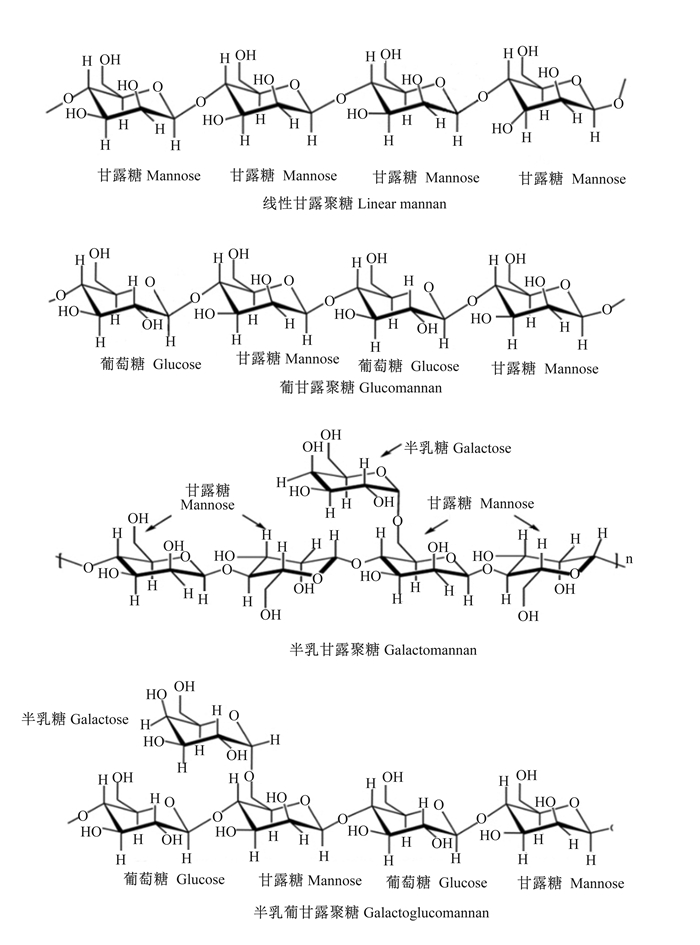
|
图 1 甘露聚糖分类 Fig. 1 Classification of mannan[11] |
各种构型的甘露聚糖是真菌、细菌和病毒等许多病原体的表面成分,可以刺激肠道上皮中先天免疫系统的免疫细胞[1, 16],先天免疫细胞通过病原相关分子模式(pathogen-associated molecular pattern,PAMP)来识别病原体,并与先天免疫细胞中的受体结合激活该类细胞[17]。因此,饲粮中的甘露聚糖会发出肠道中存在病原体的错误信号,引发不合理的免疫激活,导致巨噬细胞和单核细胞增殖增加,并由此产生细胞因子,产生炎症反应以及降低营养物质吸收利用率[18];同时,甘露聚糖使葡萄糖吸收利用率降低,抑制了胰岛素的分泌及胰岛素样生长因子-Ⅰ的表达,从而影响动物机体对能量的利用[18-19]。甘露聚糖的水解可以消除肠道中的这一错误信号,使巨噬细胞、单核细胞和细胞因子的产生减少,血糖浓度升高[18, 20]。
β-甘露聚糖酶是一种半纤维素水解酶,能破坏甘露聚糖主链的内部糖苷键,释放结构较短的1, 4-甘露低聚糖。根据β-甘露聚糖酶的氨基酸序列,将其主要分为糖苷水解酶家族(glycoside hydrolase family,GH)5、26和113。GH5和GH26都属于最大的糖苷水解酶家族——GH-A。GH-A酶家族具有TIM(α/β)8桶状结构和保留反应机制[21],TIM(α/β)8桶状结构由10股β-链和13个α-螺旋组成,包括3个310-螺旋(残基60~62、64~66和289~294)。β-片状结构由8条位于中部的平行β-链(黄色)组成,形成1个被8个大小为4.5 nm×4.5 nm×4.0 nm的α-螺旋(红色)包围的圆筒(图 2)。β-甘露聚糖酶的活性位点是位于第4个和第7个β折叠的C末端的2个谷氨酸,分别作为酸碱催化位点和亲核催化位点,在所有的β-甘露聚糖酶中绝对保守[21-22]。通常情况下,除了催化模块之外,β-甘露聚糖酶还携带额外的模块,最常见的是碳水化合物结合模块(carbohydrate-binding module,CBM)。CBM根据序列相似性和三维结构至少被分为53个家族。一些β-甘露聚糖酶含有甘露聚糖结合的CBM,对这些模块的结构研究为深入了解甘露聚糖主干识别和侧基调节机制提供了依据。另一些含有纤维素结合的CBM,可显著增加不溶性甘露聚糖纤维素复合物的水解[1, 21]。
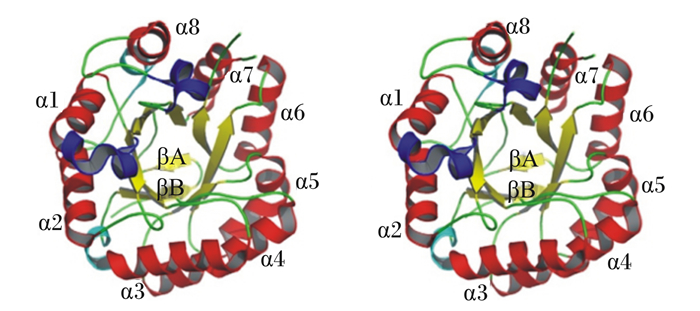
|
图 2 β-甘露聚糖酶的结构 Fig. 2 Structure of β-mannanase[21] |
β-甘露聚糖酶在自然界中普遍存在,已从细菌、真菌、放线菌、植物和动物中分离出来(表 1),饲用β-甘露聚糖酶主要来源于微生物[15],主要原因为胞外酶系安全度高、易获取等;其培养及生产方式主要为深层发酵(submerged fermentation,SMF)和固态发酵(solid-state fermentation,SSF)2种。
|
|
表 1 β-甘露聚糖酶的来源 Table 1 Sources of β-mannanase |
国内外研究学者通常采用有机溶剂沉淀、双水相萃取、层析和离子交换等方法对β-甘露聚糖酶进行分离纯化[34]。β-甘露聚糖酶活性的测定方法有:黏度法、还原糖法以及色原底物法;其中还原糖法中的二硝基水杨酸(dinitrosalicylic acid,DNS)比色法因其方法成熟、操作简单和显色稳定的优点,在β-甘露聚糖酶活性测定中被广泛使用[35-36]。
不同来源的β-甘露聚糖酶构成不同,它们的相对分子质量、酶动力学特性和作用方式也有所不同。β-甘露聚糖酶的最适pH大多数在4.0~7.0,最适温度在40~65 ℃,小于或高于最适温度,大多数酶不稳定。Soni等[37]对土壤曲霉固态培养物中提取的β-甘露聚糖酶进行研究,结果表明纯化的β-甘露聚糖酶的活性为53.75 U/mg,分子质量为49 ku,最佳pH为7.0,最佳温度为70 ℃。You等[38]对从肠杆菌属N18中分离的β-甘露聚糖酶进行研究发现β-甘露聚糖酶分子质量为90 ku;以0.5%瓜尔胶、魔芋粉、豆胶(locust bean gum,LBG)、黄原胶和羧甲基纤维素钠(sodium carboxymethyl cellulose,CMC)作为底物测定β-甘露聚糖酶活性,结果显示LBG作为底物时,β-甘露聚糖酶活性最高,为(8 132±39) U/mg;β-甘露聚糖酶在最佳温度50 ℃(pH=7.0)时超过75%的活性,而在70 ℃时还保留40%以上的活性;一些因素可影响β-甘露聚糖酶的活性,例如1 mmol/L汞离子(Hg2+)显著抑制酶活性,1 mmol/L钙离子(Ca2+)、镍离子(Ni2+)、铜离子(Cu2+)、铁离子(Fe3+)、锌离子(Zn2+)、乙二胺四乙酸(ethylene diamine tetraacetic acid,EDTA)和10%乙醇也可抑制酶活性,而1 mmol/L钴离子(Co2+)、亚铁离子(Fe2+)和十二烷基硫酸钠(sodium dodecyl sulfate,SDS)能显著提高β-甘露聚糖酶的活性。
为解决β-甘露聚糖酶在生产应用中存在的问题,大量内源β-甘露聚糖酶基因已被克隆并在外源宿主中表达,主要以原核和真核2种外源表达系统为主[39]。源自细菌的内源β-甘露聚糖酶的基因通常在大肠杆菌表达[40],而真菌来源的β-甘露聚糖酶基因常在帕斯氏酵母和曲霉中表达[41]。原核和真核生物中的内源β-甘露聚糖酶基因大小在978~2 010 kb。完全成熟的重组内源β-甘露聚糖酶蛋白含有326~669个氨基酸残基。从芽孢杆菌属和曲霉真菌属中获得的内源β-甘露聚糖酶,克隆和表达后获得最高酶活性为1 575 U/mL[16]。对枯草杆菌MA139的β-甘露聚糖酶基因进行了密码子优化,得到的重组酶具有较高的酶活性[42]。
3 β-甘露聚糖酶在畜禽生产中的应用 3.1 在家禽生产上的应用研究表明,在家禽饲粮中添加β-甘露聚糖酶可以提高家禽生长性能和免疫力[43-44]。Jackson等[18]研究发现,在饲粮中添加80或110 MU/t的β-甘露聚糖酶时,肉鸡的增重和饲料效率都有所提高,而添加50 MU/t的β-甘露聚糖酶没有显著效果。Zheng等[45]研究发现,饲粮添加β-甘露聚糖酶可提高蛋鸡产蛋前期的采食量、产蛋率以及鸡蛋质量;同时,饲粮添加β-甘露聚糖酶还可增加蛋鸡盲肠中乳酸菌数量,降低氨浓度。β-甘露聚糖酶提高免疫力的一个可能原因是甘露聚糖被降解为甘露寡糖(mannan oligosaccharide,MOS)[43]。研究发现,饲粮添加β-甘露聚糖酶可降低胸腺和法氏囊相对权重以及血清免疫球蛋白G(immunoglobulin G,IgG)和免疫球蛋白M(immunoglobulin M,IgM)含量,β-甘露聚糖酶可抑制由甘露聚糖引起的动物免疫反应,从而减少动物机体免疫反应[46]。也有研究表明,在肉鸡饲粮中添加β-甘露聚糖酶增加了淋巴细胞数量,降低了异核细胞和淋巴细胞比(H ∶ L),从而改善鸡的免疫系统能力[47]。
甘露聚糖具有黏度,可通过减慢胃肠排空、削弱底物与消化酶的结合以及降低营养物质与小肠上皮的接触速率造成营养物质吸收和利用减少[48]。Mehri等[47]研究发现,饲粮添加900 g/t的β-甘露聚糖酶可显著提高小肠绒毛高度、杯状细胞数量、小肠不同部位的隐窝深度和上皮厚度。研究表明,β-甘露聚糖酶提高饲料利用率主要通过3个途径来实现:一是β-甘露聚糖酶分解细胞壁基质,促进被细胞壁结构包裹营养物质的释放;二是降低食糜黏度,提高营养物质的消化率;三是促进十二指肠和空肠绒毛高度的增加,增大吸收表面积,改善肠对营养物质的吸收效率[10]。
3.2 在猪生产上的应用研究发现,在饲粮中添加β-甘露聚糖酶可以提高生长猪和育肥猪的生长性能[49-50],还可以提高哺乳母猪乳脂水平及乳糖含量[51]。Kim等[52]研究发现,在生长猪饲粮中添加400 U/kg的β-甘露聚糖酶能提高其对营养物质消化率以及血糖浓度,营养物质消化率提高可能是由于β-甘露聚糖酶的水解作用增加了细胞壁的通透性,切断了长链多糖,从而加速了营养物质的消化。血糖浓度的提高是由于NSP在后肠被β-甘露聚糖酶分解发酵产生挥发性脂肪酸,而挥发性脂肪酸可代替葡萄糖发挥能源作用。Jeon等[53]研究发现,在饲粮中添加0.5 g/kg的β-甘露聚糖酶可提高生长猪平均日增重(ADG)以及标准回肠氨基酸消化率,因而降低猪粪便氨浓度。Upadhaya等[54]在饲粮中添加0.05%的β-甘露聚糖酶,可减少生长猪肠道中大肠杆菌数量。而消化道中微生物的种类和数量是影响营养物质消化率和肠道健康状况的重要因素,乳酸菌被认为是有益于肠道的细菌,而大肠菌则被认为可以引起腹泻等肠道健康问题[54],因此β-甘露聚糖酶可以改善动物肠道健康。丙二醛(malondialdehyde,MDA)含量是衡量机体氧化应激的重要指标,β-甘露聚糖酶在仔猪生产中的应用有助于提高仔猪机体抗氧化能力,其主要原因是补充β-甘露聚糖酶降低了空肠中MDA的含量[55]。
3.3 在反刍动物生产上的应用研究表明,β-甘露聚糖酶可以提高反刍动物的生产性能和免疫力。Lee等[56]在山羊饲粮中添加0.1%的β-甘露聚糖酶可以提高其饲料转化率及ADG。Azevedo等[57]研究发现,与对照组相比,饲粮添加0.1%β-甘露聚糖酶可降低奶牛血清结合珠蛋白浓度。结合珠蛋白作为反刍动物主要的急性期蛋白(acute phase protein,APP),可用于检测全身性炎症的发作,包括乳腺炎、子宫内膜炎和肺炎。因此,补充β-甘露聚糖酶可能通过减少急性期反应而发挥抗炎作用。奶牛饲喂β-甘露聚糖酶后,对其血清进行生化分析发现:血清中IgG含量和CD4/CD8值在数值上较高;因此,饲粮添加β-甘露聚糖酶有提高泌乳奶牛免疫力的潜力。Roque等[20]研究发现,饲粮添加0.1%β-甘露聚糖酶不仅能发挥抗炎作用,降低潜在的免疫激活,改善奶牛的能量平衡,还能减少奶牛每次成功受孕所需的授精次数。
4 小结β-甘露聚糖酶作为一种环保的饲料添加剂已经应用于畜禽养殖中。β-甘露聚糖酶通过水解NSP中的甘露聚糖,使植物性饲料中的营养成分充分释放,有利于动物消化吸收。同时,补充β-甘露聚糖酶还能消除饲粮中甘露聚糖的抗营养作用,降低消化道食糜黏性,从而提高饲粮营养物质消化率,并达到保护环境的目的。目前,我国在β-甘露聚糖酶的结构及性质研究中已获得重大突破和进展,但其生产和稳定化技术还未发展成熟,从而限制其在畜牧业中的应用。因此,应进一步加强对β-甘露聚糖酶酶学性质及菌种改良的研究。除通过蛋白质工程改造β-甘露聚糖酶制剂性能,如提高酶活力、耐温和耐酸性等;还应继续从动物、植物及微生物中寻找更多优质β-甘露聚糖酶来源,同时加深对其结构、功能及作用机制的认识,推动其在畜牧业中的应用。
| [1] |
CHAUHAN P S, PURI N, SHARMA P, et al. Mannanases: microbial sources, production, properties and potential biotechnological applications[J]. Applied Microbiology and Biotechnology, 2012, 93(5): 1817-1830. DOI:10.1007/s00253-012-3887-5 |
| [2] |
MEENAKSHI, SINGH G, BHALLA A, et al. Solid state fermentation and characterization of partially purified thermostable mannanase from Bacillus sp. MG-33[J]. BioResources, 2010, 5(3): 1689-1701. |
| [3] |
CHEN C Y, HUANG Y C, YANG T Y, et al. Degradation of konjac glucomannan by Thermobifida fusca thermostable β-mannanase from yeast transformant[J]. International Journal of Biological Macromolecules, 2016, 82: 1-6. DOI:10.1016/j.ijbiomac.2015.10.008 |
| [4] |
赵文鹏, 李浩, 王筱兰. 豆豉中β-甘露聚糖酶高产菌的分离、鉴定及其酶学性质[J]. 江西师范大学学报(自然科学版), 2020, 44(2): 196-201. ZHAO W P, LI H, WANG X L. The isolation, identification of β-mannanase high-yield strains from Douchi and β-mannanase characterization[J]. Journal of Jiangxi Normal University (Natural Science Edition), 2020, 44(2): 196-201 (in Chinese). |
| [5] |
RAHMANI N, KASHIWAGI N, LEE J M, et al. Mannan endo-1, 4-β-mannosidase from Kitasatospora sp. isolated in Indonesia and its potential for production of mannooligosaccharides from mannan polymers[J]. AMB Express, 2017, 7: 100. DOI:10.1186/s13568-017-0401-6 |
| [6] |
ZANG H Y, XIE S S, WU H J, et al. A novel thermostable GH5_7β-mannanase from Bacillus pumilus GBSW19 and its application in manno-oligosaccharides (MOS) production[J]. Enzyme and Microbial Technology, 2015, 78: 1-9. DOI:10.1016/j.enzmictec.2015.06.007 |
| [7] |
WANG C H, ZHANG J K, WANG Y, et al. Biochemical characterization of an acidophilic β-mannanase from Gloeophyllum trabeum CBS900.73 with significant transglycosylation activity and feed digesting ability[J]. Food Chemistry, 2016, 197: 474-481. DOI:10.1016/j.foodchem.2015.10.115 |
| [8] |
FAVARO C P, BARALDI I J, CASCIATORI F P, et al. β-mannanase production using coffee industry waste for application in soluble coffee processing[J]. Biomolecules, 2020, 10(2): 227. DOI:10.3390/biom10020227 |
| [9] |
SINGH S, SINGH G, KHATRI M, et al. Thermo and alkali stable β-mannanase: characterization and application for removal of food (mannans based) stain[J]. International Journal of Biological Macromolecules, 2019, 134: 536-546. DOI:10.1016/j.ijbiomac.2019.05.067 |
| [10] |
ADIBMORADI M, MEHRI M. Effects of β-mannanase on broiler performance and gut morphology[C]//Proceedings of the 16th European Symposium on Poultry Nutrition. Strasbourg: World Poultry Science Association, 2007: 471-474.
|
| [11] |
MOREIRA L R S, FILHO E X F. An overview of mannan structure and mannan-degrading enzyme systems[J]. Applied Microbiology and Biotechnology, 2008, 79(2): 165-178. DOI:10.1007/s00253-008-1423-4 |
| [12] |
LIEPMAN A H, NAIRN C J, WILLATS W G T, et al. Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants[J]. Plant Physiology, 2007, 143(4): 1881-1893. DOI:10.1104/pp.106.093989 |
| [13] |
PETKOWICZ C L O, SCHAEFER S, REICHER F. The mannan from Schizolobium parahybae endosperm is not a reserve polysaccharide[J]. Carbohydrate Polymers, 2007, 69(4): 659-664. DOI:10.1016/j.carbpol.2007.02.002 |
| [14] |
杨鸿昆, 陈权军, 罗永发, 等. β-甘露聚糖特性、抗营养作用及应用[J]. 饲料研究, 2007(12): 18-20. YANG H K, CHEN Q J, LUO Y F, et al. Characteristics, antinutritional effects and application of β-mannan[J]. Feed Research, 2007(12): 18-20 (in Chinese). DOI:10.3969/j.issn.1002-2813.2007.12.005 |
| [15] |
谢志恒, 崔细鹏, 周平发, 等. 饲用β-甘露聚糖酶的研究进展[J]. 饲料博览, 2012(12): 29-32. XIE Z H, CUI X P, ZHOU P F, et al. Research progress of feed β-mannanase[J]. Feed Review, 2012(12): 29-32 (in Chinese). DOI:10.3969/j.issn.1001-0084.2012.12.011 |
| [16] |
DUNCAN C J G, PUGH N, PASCO D S, et al. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta[J]. Journal of Agricultural and Food Chemistry, 2002, 50(20): 5683-5685. DOI:10.1021/jf020267c |
| [17] |
FORSBERG N E, WANG Y Q. Nutrition and immunity in dairy cattle: implications to hemorrhagic bowel syndrome[C]//Proceedings of 2006 Mid-South Ruminant Nutrition Conference. Dallas: Texas Animal Nutrition Council, 2006.
|
| [18] |
JACKSON M E, GERONIAN K, KNOX A, et al. A dose-response study with the feed enzyme beta-mannanase in broilers provided with corn-soybean meal based diets in the absence of antibiotic growth promoters[J]. Poultry Science, 2004, 83(12): 1992-1996. DOI:10.1093/ps/83.12.1992 |
| [19] |
HSIAO H Y, ANDERSON D M, DALE N M. Levels of β-mannan in soybean meal[J]. Poultry Science, 2006, 85(8): 1430-1432. DOI:10.1093/ps/85.8.1430 |
| [20] |
ROQUE B M, REYES G C, TEWOLDEBRHAN T A, et al. Exogenous β-mannanase supplementation improved immunological and metabolic responses in lactating dairy cows[J]. Journal of Dairy Science, 2019, 102(5): 4198-4204. DOI:10.3168/jds.2018-15568 |
| [21] |
赵月菊, 薛燕芬, 马延和. β-甘露聚糖酶的结构生物学研究现状和展望[J]. 微生物学报, 2009, 49(9): 1131-1137. ZHAO Y J, XUE Y F, MA Y H. Recent advances and prospect on structural biology of β-mannanase-a review[J]. Acta Microbiologica Sinica, 2009, 49(9): 1131-1137 (in Chinese). DOI:10.3321/j.issn:0001-6209.2009.09.001 |
| [22] |
DAVID A, CHAUHAN P S, KUMAR A, et al. Coproduction of protease and mannanase from Bacillus nealsonii PN-11 in solid state fermentation and their combined application as detergent additives[J]. International Journal of Biological Macromolecules, 2018, 108: 1176-1184. DOI:10.1016/j.ijbiomac.2017.09.037 |
| [23] |
PRADEEP G C, CHO S S, CHOI Y H, et al. An extremely alkaline mannanase from Streptomyces sp. CS428 hydrolyzes galactomannan producing series of mannooligosaccharides[J]. World Journal of Microbiology and Biotechnology, 2016, 32(5): 84. DOI:10.1007/s11274-016-2040-5 |
| [24] |
BLIBECH M, FARHAT-KHEMAKHEM A, KRIAA M, et al. Optimization of β-mannanase production by Bacillus subtilis US191 using economical agricultural substrates[J]. Biotechnology Progress, 2020, 36(4): e2989. |
| [25] |
JANA U K, SURYAWANSHI R K, PRAJAPATI B P, et al. Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae[J]. Bioresource Technology, 2018, 268: 308-314. DOI:10.1016/j.biortech.2018.07.143 |
| [26] |
SONI H, RAWAT H K, AHIRWAR S, et al. Screening, statistical optimized production, and application of β-mannanase from some newly isolated fungi[J]. Engineering in Life Sciences, 2017, 17(4): 392-401. DOI:10.1002/elsc.201600136 |
| [27] |
CHAI S Y, BAKAR F D A, MAHADI N M, et al. A thermotolerant endo-1, 4-β-mannanase from Trichoderma virens UKM1:cloning, recombinant expression and characterization[J]. Journal of Molecular Catalysis B: Enzymatic, 2016, 125: 49-57. DOI:10.1016/j.molcatb.2015.12.011 |
| [28] |
HUANG J W, CHEN C C, HUANG C H, et al. Improving the specific activity of β-mannanase from Aspergillus niger BK01 by structure-based rational design[J]. Biochimica et Biophysica Acta: Proteins and Proteomics, 2014, 1844(3): 663-669. DOI:10.1016/j.bbapap.2014.01.011 |
| [29] |
ERKAN S B, BASMAK S, OZCAN A, et al. Mannooligosaccharide production by β-mannanase enzyme application from coffee extract[J]. Journal of Food Processing and Preservation, 2020, e14668. |
| [30] |
CARRILLO-BARRAL N, MATILLA A J, DEL CARMEN RODRÍGUEZ-GACIO M, et al. Mannans and endo-β-mannanase transcripts are located in different seed compartments during Brassicaceae germination[J]. Planta, 2018, 247(3): 649-661. DOI:10.1007/s00425-017-2815-4 |
| [31] |
KIM M K, AN Y J, JEONG C S, et al. Expression at 279 K, purification, crystallization and preliminary X-ray crystallographic analysis of a novel cold-active β-1, 4-d-mannanase from the Antarctic springtail Cryptopygus antarcticus[J]. Acta Crystallographica Section F: Structural Biology Communications, 2013, 69(9): 1007-1010. DOI:10.1107/S1744309113020538 |
| [32] |
LARSSON A M, ANDERSON L, XU B Z, et al. Three-dimensional crystal structure and enzymic characterization of β-mannanase Man5A from blue mussel Mytilus edulis[J]. Journal of Molecular Biology, 2006, 357(5): 1500-1510. DOI:10.1016/j.jmb.2006.01.044 |
| [33] |
ZAHURA U A, RAHMAN M M, INOUE A, et al. An endo-β-1, 4-mannanase, AkMan, from the common sea hare Aplysia kurodai[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2010, 157(1): 137-143. DOI:10.1016/j.cbpb.2010.05.012 |
| [34] |
李宝坤. CXJZ菌株分泌β-甘露聚糖酶的分离纯化及其酶学性质研究[D]. 硕士学位论文. 乌鲁木齐: 新疆农业大学, 2005: 8-9. LI B K. Study on purification and characterization of β-mannanase from CXJZ strain[D]. Master's Thesis. Urumqi: Xinjiang Agricultural University, 2005: 8-9. (in Chinese) |
| [35] |
吴琪, 谢红云, 段垒. β-甘露聚糖酶的酶学性质研究[J]. 饲料研究, 2011(1): 46-48. WU Q, XIE H Y, DUAN L. Study on the enzymatic properties of β-mananase[J]. Feed Research, 2011(1): 46-48 (in Chinese). |
| [36] |
张玮, 强莉, 宫玲玲. 饲用β-甘露聚糖酶酶活力的测定方法及影响因素[J]. 养殖与饲料, 2017(12): 44-46. ZHANG W, QIANG L, GONG L L. Determination of β-mannanase activity in feed and its influencing factors[J]. Animals Breeding and Feed, 2017(12): 44-46 (in Chinese). DOI:10.3969/j.issn.1671-427X.2017.12.024 |
| [37] |
SONI H, RAWAT H K, PLETSCHKE B I, et al. Purification and characterization of β-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass[J]. 3 Biotech, 2016, 6(2): 136. DOI:10.1007/s13205-016-0454-2 |
| [38] |
YOU J, LIU J F, YANG S Z, et al. Low-temperature-active and salt-tolerant β-mannanase from a newly isolated Enterobacter sp. strain N18[J]. Journal of Bioscience and Bioengineering, 2016, 121(2): 140-146. DOI:10.1016/j.jbiosc.2015.06.001 |
| [39] |
秦玲丽. β-甘露聚糖酶产生菌株的筛选、基因克隆与表达及酶学性质研究[D]. 硕士学位论文. 南京: 华南理工大学, 2017: 12-13. QIN L L. The isolation, gene cloing and expression of β-mannanase and characteration of the recombinant enzyme[D]. Master's Thesis. Nanjing: South China University of Technology, 2017: 12-13. (in Chinese) |
| [40] |
SRIVASTAVA P K, RAO A R G A, KAPOOR M. Metal-dependent thermal stability of recombinant endo-mannanase (ManB-1601) belonging to family GH 26 from Bacillus sp. CFR1601[J]. Enzyme and Microbial Technology, 2016, 84: 41-49. DOI:10.1016/j.enzmictec.2015.12.010 |
| [41] |
VAN DYK J S, SAKKA M, SAKKA K, et al. Identification of endoglucanases, xylanases, pectinases and mannanases in the multi-enzyme complex of Bacillus licheniformis SVD1[J]. Enzyme and Microbial Technology, 2010, 47(3): 112-118. DOI:10.1016/j.enzmictec.2010.05.004 |
| [42] |
QIAO J Y, RAO Z H, DONG B, et al. Expression of Bacillus subtilis MA139β-mannanase in Pichia pastoris and the enzyme characterization[J]. Applied Biochemistry and Biotechnology, 2010, 160(5): 1362-1370. DOI:10.1007/s12010-009-8688-7 |
| [43] |
ZOU X T, QIAO X J, XU Z R. Effect of β-mannanase (Hemicell) on growth performance and immunity of broilers[J]. Poultry Science, 2006, 85(12): 2176-2179. DOI:10.1093/ps/85.12.2176 |
| [44] |
LATHAM R E, WILLIAMS M P, WALTERS H G, et al. Efficacy of β-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan[J]. Poultry Science, 2018, 97(2): 549-556. DOI:10.3382/ps/pex309 |
| [45] |
ZHENG L, CHO S H, KANG C W, et al. Effects of β-mannanase on egg production performance, egg quality, intestinal microbiota, viscosity, and ammonia concentration in laying hens[J]. Brazilian Journal of Poultry Science, 2020, 22(1): eRBCA-2019-1180. |
| [46] |
LI Y H, CHEN X, CHEN Y Q, et al. Effects of β-mannanase expressed by Pichia pastoris in corn-soybean meal diets on broiler performance, nutrient digestibility, energy utilization and immunoglobulin levels[J]. Animal Feed Science and Technology, 2010, 159(1/2): 59-67. |
| [47] |
MEHRI M, ADIBMORADI M, SAMIE A, et al. Effects of β-mannanase on broiler performance, gut morphology and immune system[J]. African Journal of Biotechnology, 2010, 9(37): 6221-6228. |
| [48] |
KACZMAREK S A, ROGIEWICZ A, MOGIELNICKA M, et al. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets[J]. Poultry Science, 2014, 93(7): 1745-1753. DOI:10.3382/ps.2013-03739 |
| [49] |
YOON S Y, YANG Y X, SHINDE P L, et al. Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs[J]. Journal of Animal Science, 2010, 88(1): 181-191. DOI:10.2527/jas.2008-1741 |
| [50] |
KIM J S, INGALE S L, LEE S H, et al. Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs[J]. Animal Feed Science and Technology, 2013, 186(1/2): 64-70. |
| [51] |
KIM J S, HOSSEINDOUST A, JU I K, et al. Effects of dietary energy levels and β-mannanase supplementation in a high mannan-based diet during lactation on reproductive performance, apparent total tract digestibility and milk composition in multiparous sows[J]. Italian Journal of Animal Science, 2018, 17(1): 128-134. DOI:10.1080/1828051X.2017.1345663 |
| [52] |
KIM J S, INGALE S L, HOSSEINDOUST A R, et al. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs[J]. Animal, 2017, 11(2): 202-208. DOI:10.1017/S1751731116001385 |
| [53] |
JEON S M, HOSSEINDOUST A, CHOI Y H, et al. Comparative standardized ileal amino acid digestibility and metabolizable energy contents of main feed ingredients for growing pigs when adding dietary β-mannanase[J]. Animal Nutrition, 2019, 5(4): 359-365. DOI:10.1016/j.aninu.2019.07.001 |
| [54] |
UPADHAYA S D, PARK J W, LEE J H, et al. Efficacy of β-mannanase supplementation to corn-soya bean meal-based diets on growth performance, nutrient digestibility, blood urea nitrogen, faecal coliform and lactic acid bacteria and faecal noxious gas emission in growing pigs[J]. Archives of Animal Nutrition, 2016, 70(1): 33-43. DOI:10.1080/1745039X.2015.1117697 |
| [55] |
TIWARI U P, CHEN H Y, KIM S W, et al. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models[J]. Animal Feed Science and Technology, 2018, 245: 77-90. DOI:10.1016/j.anifeedsci.2018.07.002 |
| [56] |
LEE J J, SEO J, JUNG J K, et al. Effects of β-mannanase supplementation on growth performance, nutrient digestibility, and nitrogen utilization of Korean native goat (Capra hircus coreanae)[J]. Livestock Science, 2014, 169: 83-87. DOI:10.1016/j.livsci.2014.08.018 |
| [57] |
AZEVEDO M, TEWOLDEBRHAN T, APPUHAMY R, et al. 1398 Supplementation of β-mannanese (CTCZYME) tends to improve immune traits in early lactating dairy cows[J]. Journal of Animal Science, 2016, 94(Suppl.5): 677. |




