2. 岭南现代农业科学与技术广东省实验室茂名分中心, 茂名 525000
2. Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Maoming 525000, China
20世纪40年代初,偶然发现低剂量抗生素可促进动物生长后[1],抗生素便开始作为促生长剂广泛应用于动物生产[2]。然而,随着抗生素应用研究的发展,抗生素通过引起酶结构破坏或失活、药物靶标位点的改变以及阻碍微生物细胞靶标位点的通透性等,导致细菌产生抗性基因,产生抗生素抗性。同时,抗生素随着食物链的生物传递,在人体富集,威胁人类健康[3-4]。
研究发现,益生菌具有促生长、提高饲料利用率[5]、调节动物胃肠道消化吸收及免疫等功能[6-7],与抗生素同样具有促生长作用,可应用于动物生产[8]。罗伊氏乳酸杆菌(Lactobacillus reuteri 1,LR1)是人类、小鼠、猪及鸡等胃肠道主要菌群之一[9-11],用于动物生产具有安全性、稳定性高的特点,其可有效提高动物生长性能、预防腹泻、缓解应激、调节肠道菌群结构,是良好的免疫调节饲料添加剂[9]。研究发现,LR1对仔猪肠上皮细胞(IPEC-1)具有很好的黏附作用,其抑制肠毒性大肠埃希杆菌(enterotoxigenic Escherichia coli,ETEC)对IPEC-1细胞的黏附作用,通过肌球蛋白轻链激酶(myosin light-chain kinase,MLCK)信号通路调节紧密连接蛋白表达,从而保护肠上皮细胞免于大肠杆菌引起的细胞屏障功能的损伤[12-13]。前期研究表明,LR1或抗生素均可有效促进仔猪及生长肥育猪生长[7, 14],LR1与抗生素通过不同的脂肪代谢信号通路调节背最长肌中脂肪酸含量,导致猪肉肌内脂肪含量的差异,从而对猪肉品质产生不同的影响[15]。目前,研究多聚焦在益生菌对动物生长性能、消化吸收功能、肠道微生物菌群结构、肌肉组织代谢及肉品质等研究,然而,长期饲喂LR1对猪皮下脂肪、肝脏组织的脂肪代谢相关基因表达调控的研究鲜有报道。因此,本研究通过在饲粮中添加LR1饲喂21 d断奶仔猪至肥育猪,旨在研究长期饲喂LR1对肥育猪的背脂、腹脂等皮下脂肪以及肝脏脂肪代谢相关基因表达的影响,阐明LR1与抗生素对猪皮下脂肪及肝脏脂肪代谢的影响差异,进一步揭示LR1与抗生素调控猪生长及生理的区别机制,为LR1在生猪养殖应用的可行性及优势提供理论支撑,同时,为其在生猪养殖生产中的应用提供理论依据。
1 材料与方法 1.1 试验动物及设计选取144头体重为(6.49±0.04) kg的21日龄三元杂交(杜×长×大)断奶仔猪,随机分为3组,每组8个重复,每个重复6头。对照1组饲喂基础饲粮[参照NRC(2012)配制],基础饲粮组成及营养水平见表 1,对照2组饲喂在基础饲粮中添加抗生素的试验饲粮,仔猪阶段添加喹乙醇(100 mg/kg)和金霉素(75 mg/kg),在生长肥育猪阶段添加金霉素(75 mg/kg),LR1组饲喂全程在基础饲粮中添加5×1010 CFU/kg LR1的试验饲粮。饲养175 d后进行屠宰、样品采集。试验期间,采取自由采食及饮水方式进行饲养,试验结束后进行称重。
|
|
表 1 基础饲粮组成及营养水平(饲喂基础) Table 1 Composition and nutrient levels of basal diets (as-fed basis) |
试验结束进行动物称重,每个重复选取1头接近平均体重的肥育猪进行电击处死后,立即进行组织分离,将组织样放于冰上。取3 g背脂及腹脂组织,分别分装到3个1.5 mL的EP管中。取肝脏组织约3 g,用预冷磷酸盐缓冲液(PBS)洗去表面血液,并去除表面水分后,分装到3个1.5 mL的EP管中。取左侧胴体第10根肋骨与第11根肋骨间对应部位背部及腹部皮下脂肪组织3 g,分装到3个1.5 mL的EP管中。样品立即放于液氮中,最终转移到-80 ℃保存,以备后续检测。
1.3 脂肪代谢相关基因表达利用实时荧光定量PCR(real-time qPCR)方法检测背脂、腹脂及肝脏组织中胆固醇调节元件结合蛋白(sterol regulatory element binding protein,SREBP)、乙酰辅酶A羧化酶(acetyl CoA carboxylase,ACC)、肉毒碱棕榈酰转移酶-1β(carnitine palmitoyl transferase-1 beta,CPT-1β)、脂肪酸合成酶(Fatty acid synthase,FAS)、过氧化物酶体增殖物激活受体α/γ(peroxisome proliferator-activated receptor alfa/gamma,PPARα/PPARγ)、硬脂酰辅酶A去饱和酶(stearoyl CoA desaturase,SCD)、脂肪酸结合蛋白-1(fatty acid transport protein-1,FATP-1)、甘油三酯脂酶(adipose triglyceride lipase,ATGL)脂肪代谢相关基因的相对表达量。取100 mg皮下脂肪或肝脏组织样品,用Trizol试剂盒提取的总RNA(TaKaRa,日本)溶解在RNase-free水中,利用试剂盒(TaKaRa,Cat. #RR047A)将RNA反转录为cDNA后进行real-time qPCR检测分析。反应体系(20 μL):2 μL cDNA,10 μL SYBR Green mix(Bio-Rad,1725265),上、下游引物各0.8 μL (100 nmol/L),6.4 μL ddH2O。反应条件:预变性95 ℃,3 min;变性95 ℃,15 s;退火30 s;延伸72 ℃,30 s;39个扩增循环。β-肌动蛋白(β-actin)作为内参基因,并采用2-△△Ct方法计算目的基因相对表达量。real-time qPCR引物序列见表 2。
|
|
表 2 实时荧光定量PCR引物序列 Table 2 Primer sequences for real-time qPCR |
试验数据利用Prism 6(Graphpad Software,Inc)软件进行作图,并根据Tukey’s one-way ANOVA方法进行数据统计分析,各组数据以平均值(mean)表示,P<0.05代表具有统计显著性,0.05<P<0.10代表具有差异趋势。
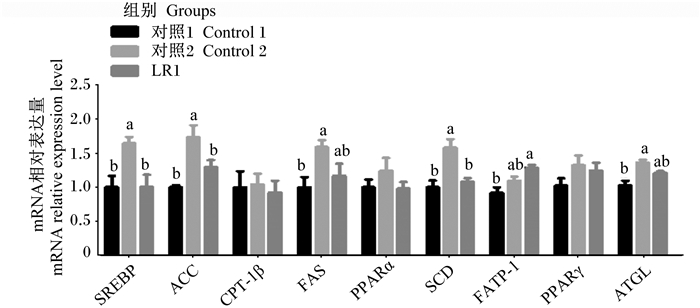
2 结果 2.1 长期饲喂LR1对猪腹脂脂肪代谢相关基因表达的影响由图 1可知,与对照1组相比,对照2组腹脂SREBP、ACC、FAS、SCD及ATGL基因相对表达量显著增加(P<0.05),长期饲喂LR1显著提高腹脂FATP-1基因相对表达量(P<0.05),对照2组及LR1组腹脂CPT-1β、PPARα及PPARγ基因相对表达量均无显著差异(P>0.05)。与对照2组相比,添加LR1显著降低腹脂SREBP、ACC及SCD基因相对表达量(P<0.05),不影响腹脂中CPT-1β、FAS、PPARα、FATP-1、PPARγ及ATGL基因相对表达量(P>0.05)。
|
SREBP:胆固醇调节元件结合蛋白sterol regulatory element binding protein;ACC:乙酰辅酶A羧化酶acetyl CoA carboxylase;CPT-1β:肉毒碱棕榈酰转移酶-1β carnitine palmitoyl transferase-1 beta;FAS:脂肪酸合成酶fatty acid synthase;PPARα/PPARγ:过氧化物酶体增殖物激活受体α/γ peroxisome proliferator-activated receptor alfa/gamma;SCD:硬脂酰辅酶A去饱和酶stearoyl CoA desaturase;FATP-1:脂肪酸结合蛋白-1 fatty acid transport protein-1;ATGL:甘油三酯脂酶adipose triglyceride lipase。 数据柱形标注不同小写字母表示差异显著(P<0.05)。 Data columns with different small letters mean significant differences (P < 0.05). The same as below. 图 1 长期饲喂LR1对猪腹脂脂肪代谢相关基因表达的影响 Fig. 1 Effects of long-term feeding of LR1 on expression of genes related to lipid metabolism in abdominal adipose of pigs (n=8) |
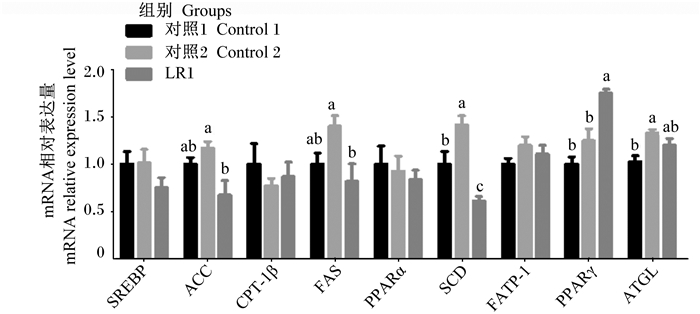
由图 2可知,与对照1、2组相比,添加LR1显著增加背脂PPARγ基因相对表达量(P<0.05)。与对照1组相比,对照2组背脂SCD及ATGL基因相对表达量显著提高(P<0.05),LR1组背脂SCD基因相对表达量显著降低(P<0.05),对照2组及LR1组背脂SREBP、ACC、CPT-1β、FAS、PPARα及FATP-1基因相对表达量无显著差异(P>0.05)。与对照2组相比,LR1组背脂ACC、FAS及SCD基因相对表达量显著降低(P<0.05),SREBP、CPT-1β、PPARα、FATP-1及ATGL基因相对表达量无显著差异(P>0.05)。
|
图 2 长期饲喂LR1对猪背脂脂肪代谢相关基因表达的影响 Fig. 2 Effects of long-term feeding of LR1 on expression of genes related to lipid metabolism in backfat of pigs (n=8) |
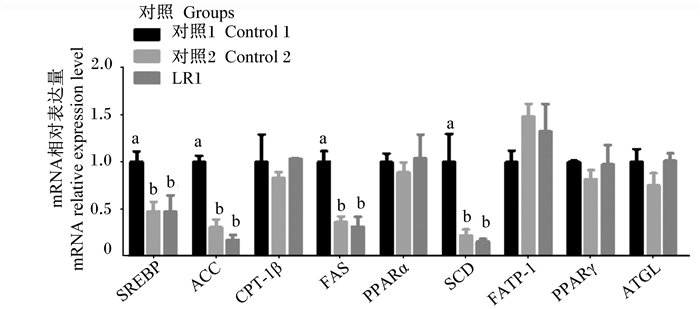
由图 3可知,与对照1组相比,对照2组及LR1组肝脏中SREBP、ACC、FAS及SCD基因相对表达量显著降低(P<0.05),CPT-1β、PPARα、FATP-1、PPARγ及ATGL基因相对表达量无显著差异(P>0.05)。与对照2组相比,LR1组肝脏中SREBP、ACC、CPT-1β、FAS、PPARα、SCD、FATP-1、PPARγ及ATGL基因相对表达量无显著差异(P>0.05)。
|
图 3 长期饲喂LR1对猪肝脏脂肪代谢相关基因表达的影响 Fig. 3 Effects of long-term feeding of LR1 on expression of genes related to lipid metabolism in liver of pigs (n=8) |
猪的胴体性状直接反映猪的胴体品质,主要包括胴体重、背膘厚、眼肌面积、胴体率及无脂瘦肉指数等,是猪生产性能及经济价值的重要指标。本研究发现,长期饲喂抗生素有提高猪体重的趋势,但不影响肥育猪的胴体重及胴体率。Lowell等[19]指出,长期饲喂抗生素不影响肥育猪体重、胴体重、胴体率及眼肌面积等胴体性状,其研究结果与本研究基本一致,但抗生素使用品种及方式的差异导致其对肥育猪体重影响的差异。目前,LR1多应用于仔猪生产,具有促进仔猪生长、改善肠道微生物菌群结构、缓解应激、提高仔猪免疫等功效[14, 20-21],然而其在生长肥育猪中的研究较少。前期研究发现,长期饲喂LR1具有提高肥育猪体重的趋势,其促生长效果与抗生素无显著差异;而且相比抗生素具有显著增加肥育猪胴体重的优势[7, 14-15]。因此,LR1可作为益生菌饲料添加剂应用于仔猪及生长肥育猪生产。Dowarah等[22]报道,益生菌-乳酸片球菌(Pediococcus acidilactici)不影响生长肥育猪的体重及胴体性状,而Alexopoulos等[23]指出地衣芽孢杆菌(Bacillus licheniformis)及枯草芽孢杆菌(Bacillus subtilis)益生菌的复合使用显著提高猪生长性能及胴体品质的。因此,益生菌的品种及使用剂量影响其对猪生长及生产性能的作用效果,而LR1具有良好的益生作用,促进猪生长,可应用于生猪养殖生产。前期研究也发现,LR1或抗生素均可显著提高仔猪生长性能,长期饲喂LR1或抗生素有提高肥育猪体重的趋势,而不影响肥育猪平均日采食量(ADFI)、平均日增重(ADG)及料重比(F/G)等生长性能,以及胴体重、背膘厚等胴体性状;而与抗生素相比,长期饲喂LR1显著增加肥育猪胴体率,但对终体重、胴体重、背膘厚及眼肌面积的影响不显著[7, 14-15]。因此,与抗生素相比,LR1显著增加肥育猪胴体率,不影响其他胴体性状,LR1具有促生长及提高肥育猪胴体品质的作用,且其总体效果优于抗生素。此外,长期饲喂抗生素降低猪肉风味,而与抗生素相比,LR1通过降低肌肉滴水损失、剪切力以及改变肌纤维特性降低猪肉品质,增加猪肉风味游离氨基酸含量及肌苷酸含量,改善猪肉风味[15]。因此,LR1有效提高生长速度及胴体品质,改善猪肉品质及风味,具有与抗生素同样的促生长作用,其应用于生猪生产是可行的。
3.2 长期饲喂LR1对肥育猪皮下脂肪组织脂肪代谢的影响随着经济的快速发展,人们对猪肉的安全及品质需求日益增加,消费者对猪肉的喜好和要求更明确,对肥肉的需求量低,瘦肉需求量逐渐增加。脂肪沉积是生猪主要的经济性状之一,脂肪组织的沉积部位直接影响胴体性状、肉品质及经济价值。背脂及腹脂等皮下脂肪影响猪的胴体性状,皮下脂肪的脂肪代谢影响猪的脂肪沉积及瘦肉率,从而影响猪的胴体品质的肉用经济价值。脂肪酸通过转运、合成、分解等脂肪代谢过程协同调控机体脂肪沉积[24],因此,皮下脂肪代谢调控的研究对阐明胴体性状、瘦肉率,提高生产性能具有重要的意义。本研究发现,与对照1组相比,对照2组(长期饲喂抗生素)腹脂SREBP、ACC、FAS、SCD及ATGL基因相对表达量提高,而长期饲喂LR1提高腹脂FATP-1基因相对表达量;而与抗生素相比,LR1降低腹脂中SREBP、ACC及SCD基因相对表达量。SREBP作为脂肪合成的候选基因,通过影响胆固醇、脂肪酸、甘油三酯及磷脂等相关酶或基因的表达从而调控脂肪合成代谢[18, 25]。ACC催化乙酰辅酶A羧化生成丙二酸单酰辅酶A,从而促进脂肪酸的从头合成。FAS通过催化乙酰辅酶A及丙二酰辅酶A合成长链脂肪酸,是体内脂肪酸从头合成过程中关键的限速酶,FAS基因相对表达量及活性直接影响动物体脂的沉积[18, 26]。SCD直接调控C16 ∶ 1及C18 ∶ 1单不饱和脂肪酸的从头合成,从而调节脂肪酸代谢[27-28],而ATGL在皮下脂肪组织中高度表达,与脂肪发育及成熟密切相关,水解储存的甘油三酯的第1个酯键,释放非酯化的游离脂肪酸[29-30]。FATP-1一方面特异性结合脂肪酸,尤其是长链不饱和脂肪酸,参与脂肪酸的跨膜转运;另一方面与脂肪酸的酰基化相关,参与动物脂肪代谢及沉积[31-32]。由此可见,长期饲喂抗生素增加肥育猪腹脂组织的合成代谢及分解代谢,而LR1增加肥育猪腹脂组织中脂肪酸的转运。此外,与抗生素相比,长期饲喂LR1显著降低肥育猪腹脂组织中的脂肪合成代谢。因此,抗生素通过SREBP基因上调下游ACC、FAS及SCD基因相对表达量,从而提高肥育猪腹脂的脂肪合成代谢,同时通过ATGL限速酶增加腹脂的脂肪分解代谢;LR1则通过增加脂肪酸转运代谢及降低脂肪酸合成代谢,降低脂肪酸在肥育猪腹脂组织中的沉积。
同时,本研究发现,与对照1相比,长期饲喂抗生素显著提高背脂中SCD及ATGL基因相对表达量,表明抗生素不仅通过诱导SCD基因的表达提高背脂的脂肪合成代谢,同时也通过增加ATGL基因的表达促进背脂的脂肪分解代谢,这说明了抗生素不影响肥育猪背膘厚的原因。本研究结果显示,与抗生素相比,LR1显著降低背脂ACC、FAS及SCD基因相对表达量,同时也增加背脂PPARγ基因相对表达量;而与对照组相比,LR1降低背脂SCD基因相对表达量,提高PPARγ基因相对表达量。PPAR是调节脂肪代谢的重要转录因子之一,而PPARγ作为PPARs超家族成员,主要在脂肪组织中表达,参与调控脂肪分化、转运及细胞中脂质沉积等代谢过程[18, 33-34]。然而,LR1不影响FATP-1基因相对表达量,因此,LR1可能通过PPARγ调控下游其他脂肪酸转运及沉积代谢。由此可见,LR1通过下调肥育猪背脂中ACC、FAS及SCD等相关基因相对表达量,降低背脂的脂肪合成代谢;可能通过上调肥育猪背脂中PPARγ基因相对表达量,提高脂肪酸背脂沉积代谢,从而导致其不影响肥育猪背膘厚的胴体性状。
Rejinders等[35]指出万古霉素及阿莫西林抗生素上调脂肪组织的氧化信号通路,而Renu等[36]报道阿霉素通过PPARγ降低脂肪组织的脂肪合成,通过ATGL及PPARα降低脂肪组织的氧化代谢。Torres等[37]研究指出益生菌通过调节肠道微生物菌群结构,以及脂肪组织的脂肪因子、炎症因子等调控脂肪组织的代谢,调节体重及健康。Kim等[38]报道高加索酸奶乳杆菌(Lactobacillus kefiri DH5)通过诱导PPAR-α、FABP4及CPT1基因的表达,增加脂肪组织的脂肪酸氧化分解,从而抵抗肥胖。而Park等[39]指出淀粉乳杆菌KU4(Lactobacillus amylovorus KU4)通过调控PPARγ-PGC-1α转录复合体,促进白色脂肪向褐色脂肪的转化,从而减少皮下脂肪的脂肪沉积。因此,益生菌菌种或抗生素种类的差异可能通过不同的信号通路及靶标基因调节脂肪组织的脂肪代谢。
3.3 长期饲喂LR1对肥育猪肝脏脂肪代谢的影响除了脂肪组织,肝脏也是脂肪合成的重要场所,其在机体脂肪代谢中起到关键性作用。食物脂肪经胃肠道消化吸收生成甘油三酯,其与载脂蛋白结合形成乳糜颗粒进入血液,一方面,经由肝脏合成脂肪酸后,与载脂蛋白或胆固醇结合形成极低密度脂蛋白微粒后,通过血液运输到身体各部位,进行脂肪储存或利用;另一方面,机体动员贮存脂肪水解为甘油及脂肪酸,释放到血液后被肝细胞摄取,通过线粒体β氧化作用产生能量,供机体生理活动需要。因此,肝脏是机体脂类合成及分解的代谢中心,其通过脂肪酸氧化、合成及脂蛋白的摄取、分泌动态平衡,调控机体脂肪代谢,该平衡被破坏则导致脂肪代谢疾病。本研究结果表明,抗生素及LR1均显著降低肝脏中SREBP、ACC、FAS及SCD基因相对表达量,而不影响PPARα、CPT-1β、FATP、PPARγ及ATGL基因相对表达量。PPARα调控肝脏脂肪分化及β氧化作用[40],而CPT-1β是脂肪酸β氧化过程中的关键限速酶[41],ATGL是脂肪酸水解作用的关键限速酶[29],PPARγ通过控制下游FATP等脂肪酸转运蛋白调控脂肪酸转运,影响脂肪转运及沉积[18, 31-32]。由此可见,抗生素及LR1均通过SREBP调控下游基因ACC、FAS及SCD的表达,从而降低肝脏的脂肪合成代谢,而不影响肝脏的分解、转运及沉积。Cho等[42]也指出抗生素或益生菌可调控肝脏脂肪代谢,而Renu等[36]报道抗生素的使用损伤肝脏脂肪代谢。Yi等[43]研究发现LR1调控断奶仔猪肝脏脂肪代谢,Wang等[44]报道谷物乳杆菌(Lactobacillus frumenti)可提高仔猪肝脏脂肪酸的β氧化作用,而与本研究结果存在差异,可能由于菌种或猪的生长阶段导致研究结果的差异。
此外,前期研究结果表明,抗生素通过上调SREBP基因相对表达量诱导下游基因ACC的表达,提高背最长肌的脂肪合成代谢,而通过上调PPARα及ATGL基因相对表达量,增加背最长肌中脂肪酸的氧化及分解代谢,显著降低背最长肌中肌内脂肪的含量。然而,LR1通过诱导背最长肌中SCD基因相对表达量,促进背最长肌的合成代谢,而通过上调PPARα基因相对表达量提高背最长肌的分解代谢,而不影响肌内脂肪含量。此外,LR1通过诱导PPARα、MyOD及PGC1α基因促进肌纤维的增殖及分化[15, 40]。综上所述,LR1与抗生素对不同组织的脂肪代谢调控存在差异,二者通过不同的基因调控皮下脂肪及肌肉组织的脂肪代谢。
4 结论① 长期饲喂LR1促进皮下脂肪的脂肪转运代谢,而长期饲喂抗生素增加皮下脂肪的脂肪合成及分解代谢;与抗生素相比,LR1降低皮下脂肪的脂肪合成代谢。
② 长期饲喂LR1或抗生素均降低肥育猪肝脏的脂肪合成代谢,而不影响肝脏的转运及分解代谢。
③ 抗生素及LR1对肥育猪脂肪代谢调控存在组织差异性,两者通过不同的基因调控皮下脂肪的脂肪代谢,但二者对肝脏脂肪代谢的调控无显著差异。
| [1] |
GUSTAFSON R H, BOWEN R E. Antibiotic use in animal agriculture[J]. Journal of Applied Microbiology, 1997, 83(5): 531-541. DOI:10.1046/j.1365-2672.1997.00280.x |
| [2] |
CHATTOPADHYAY M K. Use of antibiotics as feed additives: a burning question[J]. Frontiers in Microbiology, 2014, 5: 334. |
| [3] |
LI J Y. Current status and prospects for in-feed antibiotics in the different stages of pork production-a review[J]. Asian-Australasian Journal of Animal Sciences, 2017, 30(12): 1667-1673. DOI:10.5713/ajas.17.0418 |
| [4] |
LEE J H. Perspectives towards antibiotic resistance: from molecules to population[J]. Journal of Microbiology, 2019, 57(3): 181-184. DOI:10.1007/s12275-019-0718-8 |
| [5] |
LIU W C, DEVI S, PARK J, et al. Effects of complex probiotic supplementation in growing pig diets with and without palm kernel expellers on growth performance, nutrient digestibility, blood parameters, fecal microbial shedding and noxious gas emission[J]. Animal Science Journal, 2018, 89(3): 552-560. DOI:10.1111/asj.12965 |
| [6] |
ZHANG D Y, JI H F, LIU H, et al. Changes in the diversity and composition of gut microbiota of weaned piglets after oral administration of Lactobacillus or an antibiotic[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10081-10093. DOI:10.1007/s00253-016-7845-5 |
| [7] |
TIAN Z M, CUI Y Y, LU H J, et al. Effects of long-term feeding diets supplemented with Lactobacillus reuteri 1 on growth performance, digestive and absorptive function of the small intestine in pigs[J]. Journal of Functional Foods, 2020, 71: 104010. DOI:10.1016/j.jff.2020.104010 |
| [8] |
WANG X Q, YANG F, LIU C, et al. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets[J]. The Journal of Nutrition, 2012, 142(1): 7-13. DOI:10.3945/jn.111.147074 |
| [9] |
HOU C L, ZENG X F, YANG F J, et al. Study and use of the probiotic Lactobacillus reuteri in pigs: a review[J]. Journal of Animal Science and Biotechnology, 2015, 6(1): 14. DOI:10.1186/s40104-015-0014-3 |
| [10] |
OH P L, BENSON A K, PETERSON D A, et al. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution[J]. The ISME Journal, 2010, 4(3): 377-387. DOI:10.1038/ismej.2009.123 |
| [11] |
WANG T W, TENG K L, LIU G, et al. Lactobacillus reuteri HCM2 protects mice against enterotoxigenic Escherichia coli through modulation of gut microbiota[J]. Scientific Reports, 2018, 8(1): 17485. DOI:10.1038/s41598-018-35702-y |
| [12] |
WANG Z L, WANG L, CHEN Z, et al. In vitro evaluation of swine-derived Lactobacillus reuteri: probiotic properties and effects on intestinal porcine epithelial cells challenged with enterotoxigenic Escherichia coli K88[J]. Journal of Microbiology and Biotechnology, 2016, 26(6): 1018-1025. DOI:10.4014/jmb.1510.10089 |
| [13] |
YI H B, WANG L, XIONG Y X, et al. Lactobacillus reuteri LR1 improved expression of genes of tight junction proteins via the MLCK pathway in IPEC-1 cells during infection with enterotoxigenic Escherichia coli K88[J]. Mediators of Inflammation, 2018, 2018: 6434910. |
| [14] |
YI H B, WANG L, XIONG Y X, et al. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs[J]. Journal of Animal Science, 2018, 96(6): 2342-2351. DOI:10.1093/jas/sky129 |
| [15] |
TIAN Z M, CUI Y Y, LU H J, et al. Effect of long-term dietary probiotic Lactobacillus reuteri 1 or antibiotics on meat quality, muscular amino acids and fatty acids in pigs[J]. Meat Science, 2021, 171: 108234. DOI:10.1016/j.meatsci.2020.108234 |
| [16] |
中华人民共和国农业部. GB/T 8467-1987瘦肉型种猪性能测定技术规程[S]. 北京: 中国标准出版社, 1988. Ministry of Agriculture of the People's Republic of China. GB/T 8467-1987 Regulation for performance testing techniques of lean-type breeding pigs[S]. Beijing: China Standard Press, 1988. (in Chinese) |
| [17] |
NPPC. National pork producers council[S]. Des Moines, I.A. : National Pork Producers Council, 1991.
|
| [18] |
田志梅, 马现永, 王丽, 等. 长期饲喂不同蛋白质水平饲粮对猪脂肪代谢相关基因表达的影响[J]. 动物营养学报, 2017, 29(10): 3761-3772. TIAN Z M, MA X Y, WANG L, et al. Effects of long-term feeding diets with different protein levels on gen expressions related to lipid metabolism of pigs[J]. Chinese Journal of Animal Nutrition, 2017, 29(10): 3761-3772 (in Chinese). DOI:10.3969/j.issn.1006-267X.2017.10.039 |
| [19] |
LOWELL J E, BOHRER B M, WILSON K B, et al. Growth performance, carcass quality, fresh belly characteristics, and commercial bacon slicing yields of growing-finishing pigs fed a subtherapeutic dose of an antibiotic, a natural antimicrobial, or not fed an antibiotic or antimicrobial[J]. Meat Science, 2018, 136: 93-103. DOI:10.1016/j.meatsci.2017.10.011 |
| [20] |
LE M H A, GALLE S, YANG Y, et al. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs[J]. Journal of Animal Science, 2016, 94(11): 4677-4687. DOI:10.2527/jas.2016-0693 |
| [21] |
YANG J J, WANG C L, HUANG K H, et al. Compound Lactobacillus sp.administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets[J]. Applied Microbiology and Biotechnology, 2020, 104(15): 6749-6765. DOI:10.1007/s00253-020-10727-4 |
| [22] |
DOWARAH R, VERMA A K, AGARWAL N, et al. Efficacy of species-specific probiotic Pediococcus acidilactici FT28 on blood biochemical profile, carcass traits and physicochemical properties of meat in fattening pigs[J]. Research in Veterinary Science, 2018, 117: 60-64. DOI:10.1016/j.rvsc.2017.11.011 |
| [23] |
ALEXOPOULOS C, GEORGOULAKIS I E, TZIVARA A, et al. Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs[J]. Journal of Veterinary Medicine Series A, 2004, 51(6): 306-312. DOI:10.1111/j.1439-0442.2004.00637.x |
| [24] |
ZHAO S M, REN L J, CHEN L, et al. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition[J]. Lipids, 2009, 44(11): 1029. DOI:10.1007/s11745-009-3356-9 |
| [25] |
XU H F, LUO J, MA G Z, et al. Acyl-CoA synthetase short-chain family member 2(ACSS2) is regulated by SREBP-1 and plays a role in fatty acid synthesis in caprine mammary epithelial cells[J]. Journal of Cellular Physiology, 2018, 233(2): 1005-1016. DOI:10.1002/jcp.25954 |
| [26] |
RITTNER A, PAITHANKAR K S, HIMMLER A, et al. Type Ⅰ fatty acid synthase trapped in the octanoyl-bound state[J]. Protein Science, 2020, 29(2): 589-605. DOI:10.1002/pro.3797 |
| [27] |
GREEN C D, OZGUDEN-AKKOC C G, WANG Y, et al. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species[J]. Journal of Lipid Research, 2010, 51(7): 1871-1877. DOI:10.1194/jlr.M004747 |
| [28] |
ZHANG J, CUI L, MA J, et al. Transcriptome analyses reveal genes and pathways associated with fatty acid composition traits in pigs[J]. Animal Genetics, 2017, 48(6): 645-652. DOI:10.1111/age.12597 |
| [29] |
DEIULⅡS J A, SHIN J, BAE D, et al. Developmental, hormonal, and nutritional regulation of porcine adipose triglyceride lipase (ATGL)[J]. Lipids, 2008, 43(3): 215-225. DOI:10.1007/s11745-007-3146-1 |
| [30] |
ZIMMERMANN R, STRAUSS J G, HAEMMERLE G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase[J]. Science, 2004, 306(5700): 1383-1386. DOI:10.1126/science.1100747 |
| [31] |
CHEN X L, LUO Y L, WANG R S, et al. Effects of fatty acid transport protein 1 on proliferation and differentiation of porcine intramuscular preadipocytes[J]. Animal Science Journal, 2017, 88(5): 731-738. DOI:10.1111/asj.12701 |
| [32] |
SHU G, ZHU X T, WANG X Q, et al. Identification and gene expression of porcine fatty acid transport protein 1 isoforms[J]. Journal of Animal Physiology and Animal Nutrition, 2009, 93(4): 439-446. DOI:10.1111/j.1439-0396.2008.00825.x |
| [33] |
MADEIRA M S, PIRES V M R, ALFAIA C M, et al. Combined effects of dietary arginine, leucine and protein levels on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of crossbred pigs[J]. British Journal of Nutrition, 2014, 111(9): 1521-1535. DOI:10.1017/S0007114513004029 |
| [34] |
FEIGE J N, GELMAN L, MICHALIK L, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions[J]. Progress in Lipid Research, 2006, 45(2): 120-159. DOI:10.1016/j.plipres.2005.12.002 |
| [35] |
REIJNDERS D, GOOSSENS G H, HERMES G D A, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial[J]. Cell Metabolism, 2016, 24(1): 63-74. DOI:10.1016/j.cmet.2016.06.016 |
| [36] |
RENU K, SRUTHY K B, PARTHIBAN S, et al. Elevated lipolysis in adipose tissue by doxorubicin via PPARα activation associated with hepatic steatosis and insulin resistance[J]. European Journal of Pharmacology, 2019, 843: 162-176. DOI:10.1016/j.ejphar.2018.11.018 |
| [37] |
TORRES S, FABERSANI E, MARQUEZ A, et al. Adipose tissue inflammation and metabolic syndrome.The proactive role of probiotics[J]. European Journal of Nutrition, 2019, 58(1): 27-43. DOI:10.1007/s00394-018-1790-2 |
| [38] |
KIM D H, JEONG D, KANG I B, et al. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue[J]. Molecular Nutrition & Food Research, 2017, 61(11): 1700252. |
| [39] |
PARK S S, LEE Y J, KANG H, et al. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling[J]. Scientific Reports, 2019, 9(1): 20152. DOI:10.1038/s41598-019-56817-w |
| [40] |
崔悦悦. 白藜芦醇通过脂联素信号通路调节猪脂肪沉积的机制研究[D]. 硕士学位论文. 南宁: 广西大学, 2019. CUI Y Y. Study on the mechanism of resveratrol regulating fat deposition through adiponectin signaling pathway in pig[D]. Master's Thesis. Nanning: Guangxi University, 2019. (in Chinese) |
| [41] |
DAI J Y, LIANG K, ZHAO S, et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis[J]. Proceedings of the National Academy Sciences of the United States of America, 2018, 115(26): E5896-E5905. DOI:10.1073/pnas.1801745115 |
| [42] |
CHO M S, KIM S Y, SUK K T, et al. Modulation of gut microbiome in nonalcoholic fatty liver disease: pro-, pre-, syn-, and antibiotics[J]. Journal of Microbiology, 2018, 56(12): 855-867. DOI:10.1007/s12275-018-8346-2 |
| [43] |
YI H B, YANG G D, XIONG Y X, et al. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut-liver axis of weaned pigs[J]. Food & Function, 2019, 10(11): 7387-7396. |
| [44] |
WANG Z C, HU J, ZHENG W Y, et al. Lactobacillus frumenti mediates energy production via fatty acid β-oxidation in the liver of early-weaned piglets[J]. Journal of Animal Science and Biotechnology, 2019, 10: 95. DOI:10.1186/s40104-019-0399-5 |




