白肌肉(PSE肉)最早源于猪肉,具有颜色灰白(pale)、质地松软(soft)及表面渗水严重(exudative)等特征,俗称水猪肉或苍白肉[1]。禽类的部分胸肌中也具有高亮度(L*)值、质地松软及保水性低的特征,因而被称为类PSE肉。我国肉鸡类PSE肉的发生率超过20%(冬季约为21%,夏季约为24%)[2-3],并且类PSE肉一直被公认为是产生低质禽肉的原因之一,给现代养禽业造成了巨大的经济损失[4-5]。研究表明,快速和过度的糖酵解、乳酸的快速积聚和死后早期肌肉中的高温是导致类PSE样综合征的诱因[6]。运输应激被认为是导致肉鸡类PSE肉发生的重要因素之一[7]。
运输应激加速了肉鸡肌肉的能量消耗,使肌肉组织中糖酵解增加,乳酸积累增加,从而引起类PSE肉的发生[8]。有研究表明,宰前长时间运输(>3 h)、长距离运输(≥160 km)及运输小气候等因素能给肉鸡造成严重的应激,导致肉鸡死亡率及类PSE肉发生率增加[9-11]。并且,运输应激能够引起肉鸡血浆中葡萄糖含量显著降低,血浆皮质酮含量急剧升高[12-13]。此外,Xing等[14]研究证实,运输应激会导致宰后早期肌肉中能量水平较低,而随后肌肉中乳酸含量迅速升高,导致肉品质变差。因此,运输应激是与肉品质相关的重要因素之一,应被作为一个关键控制点予以关注[15-16]。基于此,本文从运输应激对肉鸡血液生化指标、肉品质、能量代谢及其机制等方面进行了综述,以期为家禽运输应激研究及能量调控提供参考。
1 运输应激对肉鸡血液生化指标和机体免疫功能的影响随着集约化养殖的不断推行,肉鸡从养殖场运往屠宰场的过程面临着一个多因素的综合压力过程。如宰前禁饲、抓捕、装箱及运输等因素都会给肉鸡带来严重的应激[15, 17],从而激活下丘脑-垂体-肾上腺轴,引起血浆皮质酮和肾上腺素含量增加,造成机体生理代谢紊乱,进而影响肉品质[18];甚至造成肉鸡死亡率的增加,给养禽业和肉禽加工业带来了重大的经济损失[19]。因此,运输应激成为养禽业关注的焦点,也受到国内外越来越多研究者的关注。
运输应激是宰前应激中最主要的应激之一[15, 20]。血浆皮质酮和葡萄糖含量可作为肉鸡运输应激及肌肉损伤的指示物[21-22]。研究表明,与0.5 h运输应激相比,3.0 h运输应激显著降低了肉鸡血液葡萄糖含量[23]。Yue等[24]在慢速生长黄羽肉鸡上的研究同样表明,3.0 h的长途运输应激显著降低了肉鸡血浆葡萄糖含量。这表明运输应激加剧了机体对葡萄糖的消耗。此外,运输应激条件下,家禽血浆皮质酮含量增加,从而参与机体的物质代谢、免疫应答等[25],而肉鸡血浆皮质酮含量增加往往伴随类PSE肉的产生[26]。Zhang等[12]以快速生长的爱拔益加(AA)肉鸡为研究对象,研究发现,与短途运输应激(0.75 h)相比,长途运输应激(3.00 h)可降低肉鸡血浆葡萄糖含量,提高血浆皮质酮含量,表明长途运输应激组肉鸡受到的应激强度大;并且,长途运输应激显著降低了肉鸡体重,这与Nijdam等[27]的研究结果相似。Ondrasovicova等[28]报道,与30 km运输距离相比,120 km运输距离显著提高了肉鸡血浆皮质酮含量。以上结果提示,随着运输时间的延长和运输距离的增加,肉鸡受到的应激强度增大。并且,运输应激还影响血液中相关酶活性,当机体受到外界持续刺激时,肌细胞受损而导致细胞内的酶[如肌酸激酶(creatine kinase,CK)和乳酸脱氢酶(lactate dehydrogenase,LDH)]渗透至外界,从而引起血液中这2种酶活性的增加。邢通[29]研究表明,高温运输应激显著增加了肉鸡血液中CK和LDH活性,这意味着运输应激在一定程度上破坏了肌细胞膜,引起肌细胞损伤和细胞膜破裂。
运输应激除影响血液相关指标外,对机体免疫功能也有一定的影响。运输应激影响机体血液中白细胞数量、中性粒细胞功能和细胞免疫功能[30]。并且,通过将3H-胸腺嘧啶掺入不同有丝分裂原诱导的淋巴细胞中对细胞免疫功能进行研究,结果发现,运输应激抑制了机体细胞免疫功能[30]。Mitchell等[31]研究发现,在3 h的运输应激过程中,随着车内温度、相对湿度及二氧化碳浓度的增加,肉鸡血液中异嗜细胞与淋巴细胞的比率显著增加。张林[25]在肉鸡上的研究同样表明,随着运输时间的增加,肉鸡血液中异嗜细胞与淋巴细胞的比率有增加的趋势。由此可见,运输应激影响了机体的细胞免疫功能,从而降低了机体的免疫功能。
2 运输应激对肉鸡肉品质的影响在运输应激条件下,动物机体能量代谢加强,如葡萄糖、糖原等通过糖酵解作用补充能量,导致宰后肌肉中糖原和乳酸含量变化,从而影响肉品质[25]。随着宰前运输时间的延长,肌肉剧烈收缩,无氧酵解反应增加,使产生的乳酸在肌肉中不断积累,导致肌肉最终(宰后24 h)pH降低[32]。此外,应激会使磷脂酶A2活性增加,激发钙离子(Ca2+)释放,造成线粒体和肌浆中Ca2+浓度增加。Ca2+可激活肌原纤维ATP酶和磷酸化酶使得糖原酵解加剧,提高细胞内乳酸浓度[33],降低肌肉pH。Castellini等[34]研究表明,长途运输应激显著影响了肌肉pH和系水力,使宰后肌肉品质降低。Wang等[4]研究结果显示,与0.5 h运输应激相比,3 h运输应激降低了宰后24 h胸肌pH,增加了胸肌滴水损失;然而,比较有趣的是,与无运输应激相比,3 h运输应激对腿肌的pH和滴水损失没有显著影响。分析其可能原因包括以下几点:1)动物宰杀后,机体氧供应被切断,而肌糖原继续进行无氧酵解,使得肌肉中乳酸大量积累,因而造成pH显著降低;2)由于宰前捕捉、挣扎等应激行为引起体内代谢紊乱,导致肌肉温度较高,死后肌肉冷却时间延长,导致无氧酵解过程增加;3)肌肉肌纤维类型的不同也会影响肌肉的最终pH。研究表明,胸肌(由Ⅱb型肌纤维构成)中乳酸含量高于腿肌(由Ⅰ型、Ⅱa和Ⅱb型肌纤维构成),并且运输应激能够显著降低胸肌最终pH。这可能是由于Ⅱb型肌纤维有着较强的糖酵解代谢能力。因而,糖原含量高的胸肌更易受运输应激的影响而产生类PSE肉[35]。
运输应激不仅影响肌肉的最终pH和系水力,对肌肉的色泽,如L*、黄度(a*)及红度(b*)值等也有不同程度的影响,其中对L*值的影响最大。肌肉pH是衡量肉品质的重要指标,较低的pH会引起肌肉中肌动蛋白和肌球蛋白凝结收缩成颗粒状,空间结构破环,结合水与蛋白质分离形成游离水,游离水的增多导致肌肉系水性下降,而过低的pH和较多的游离水则会对肌肉颜色产生直接影响[23, 36]。此外,在应激条件下,自由基不断产生,活性氧增多,脂质氧化生成大量丙二醛。丙二醛是极活泼的交联剂,会使细胞发生交联从而失去活力,进而使肌肉对水的吸附能力下降。同时,蛋白质变性和脂质氧化会使细胞膜的正常结构和功能受到破坏,细胞内液释出。大量渗出液使肌肉表面潮湿,反射自然光能力增加,导致L*值增加[23]。Zhang等[37]对AA肉鸡进行3.0 h的运输应激,结果发现,与对照组(0.5 h运输应激)相比,3.0 h运输应激显著增加了宰后24 h胸肌L*值,但对a*和b*值没有显著影响,这与Wang等[4]的研究结果一致。但Zhang等[17]及Yue等[24]分别以Ross肉鸡和黄羽肉鸡为研究对象进行研究,结果表明,3.0 h运输应激对宰后24 h胸肌L*、a*和b*值均没有显著影响。造成结果出现差异的原因可能是由于肉鸡品种的不同。除此之外,有研究表明,运输应激使得肉鸡粪便中弯曲杆菌(一种能够引起腹泻的病原菌)的数量增加,从而导致肉鸡胴体感染病原菌的风险加大[38]。以上研究表明,运输应激会引起肉鸡产生强烈的应激反应,导致宰后肉品质下降。
3 运输应激对肉鸡肌肉能量代谢的影响 3.1 运输应激对ATP及磷酸肌酸利用的影响ATP含量及一磷酸腺苷(adenosine monophosphate,AMP)/ATP是典型的评价细胞、组织、器官及整个机体能量状态的指标[4]。在宰后肌肉静息状态下,ATP在肌球蛋白ATP酶和肌浆网Ca2+-ATP酶的作用下持续分解为二磷酸腺苷(adenosine diphosphate,ADP)和无机磷酸盐,为机体代谢提供能量[39]。研究表明,在经历热应激或运输应激的火鸡和肉鸡中,其胸肌肌肉中ATP含量较低,AMP/ATP较高[13, 40]。Xing等[14]选用120羽42日龄AA肉鸡进行研究,结果发现,与无运输组相比,30 min运输应激显著降低了肌肉ATP含量,显著增加了肌肉次黄嘌呤核苷酸(inosine monophosphate,IMP)含量,增加了(AMP+IMP)/ATP。Savenije等[40]研究同样表明,1.5 h运输应激显著降低了宰后1.0 h肌肉ATP含量,这意味着运输应激加速了肌肉能量代谢,增加了肌肉中ATP的分解。
此外,肌酸(creatine,Cr)及其磷酸化形式的磷酸肌酸(phosphocreatine,PCr)是脊椎动物所有活细胞能量传递的关键物质[41]。Cr/PCr系统作为ATP/ADP系统的后备,以便在短时间内存储和调动细胞特别是肌肉细胞中的能量。在细胞中,Cr在肌酸激酶的催化下与ATP结合生成PCr和ADP。并且,当细胞能量水平不足时,PCr可以重新形成Cr,并释放出ATP,为细胞提供所需要的能量[42](图 1)。研究表明,运输应激显著降低了宰后胸肌中Cr含量[13]。Zhang等[37]研究发现,3.0 h运输应激显著降低了胸肌中Cr含量,但对PCr含量及PCr/Cr比值没有显著影响。这表明运输应激激活了肌肉中Cr/PCr系统,PCr在能够通过内源性磷酸肌酸循环来产生部分ATP,为肌肉收缩提供能量。
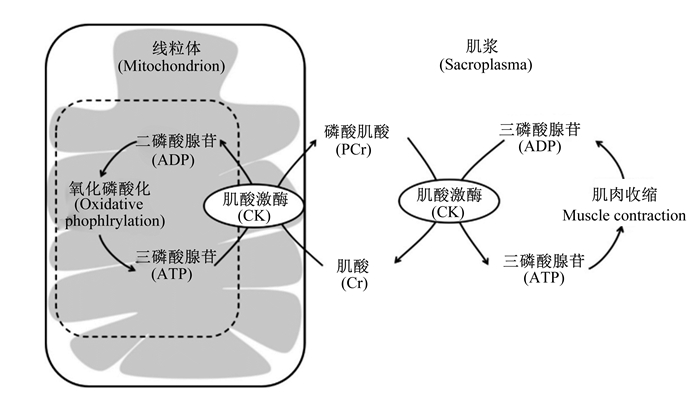
|
图 1 磷酸肌酸"穿梭"系统 Fig. 1 Phosphocreatine "shuttle" system[43] |
肉鸡屠宰后,机体氧供应被切断,细胞的呼吸方式也由有氧呼吸变为无氧呼吸,肌糖原进行无氧酵解。一般认为,肌糖原的降解是糖酵解代谢的第1步。糖原磷酸化酶(glycogen phosphorylase,GP)是参与糖原分解的关键酶,是催化糖原分解的限速步骤。GP通过断裂1,4-糖苷键连接,将葡萄糖分子从糖原链中移除[44]。同时,肌糖酵解代谢途径也受到一些限速酶的高度调控。己糖激酶(hexokinase,HK)是糖酵解途径中的第1个酶,可将葡萄糖转化为葡萄糖-6-磷酸。丙酮酸激酶(pyruvate kinase,PK)和LDH作为糖酵解途径的关键末端酶,在厌氧条件下分别将磷酸烯醇丙酮酸转化为丙酮酸和将丙酮酸转化为乳酸[45]。
宰后糖酵解作用在肌肉变化过程中起着至关重要的作用[46]。糖酵解过程中的糖原含量、糖酵解潜力及糖酵解酶活性均影响着宰后肌肉变化。Zanetti等[20]对肉鸡分别进行90或220 min的运输应激,并利用蛋白组学方法进行研究,结果发现10个蛋白表达的位点出现差异。其中,PK和3-磷酸甘油醛脱氢酶等的表达随运输时间的增加而增加。Xing等[47]在肉鸡上的研究发现,运输应激增加了宰后肌肉中乳酸含量,降低了肌肉pH,增加了糖酵解过程中的2,6-二磷酸果糖酶活性,加速了肌肉的糖酵解。Wang等[4]和Zhang等[13]研究均发现,3.0 h运输应激显著降低了肉鸡胸肌中糖原含量,增加了胸肌乳酸含量及糖酵解潜力{糖酵解潜力(μmol/g)=2[(糖原含量(μmol/g)+葡萄糖含量(μmol/g)+6-磷酸葡萄糖含量(μmol/g)+乳酸含量(μmol/g)]}。同时,3.0 h运输应激也显著增加了胸肌HK、PK及LDH活性。由此可见,运输应激增加了糖酵解酶活性,影响了宰后肌肉糖酵解过程。
4 运输应激对肌肉能量代谢的影响机理 4.1 腺苷酸活化蛋白激酶(AMPK)信号通路介导运输应激条件下肌肉能量代谢AMPK是蛋白激酶信号级联通路的下游组成元件,是由1个催化亚基(AMPK-α)和2个调节亚基(AMPK-β和AMPK-γ)组成的异源三聚体酶,在感应细胞内能量平衡和调控肌肉糖酵解方面发挥着重要的作用[48]。AMPK是体内重要的能量感应器,被称为真核细胞的"能量调节器"。当骨骼肌缺少氧气供应或发生剧烈的肌肉收缩时,ATP被消耗,AMP/ATP升高,AMPK信号通路被激活,从而加速肌肉糖酵解进程[49-50]。然而,当AMPK基因被敲除后,宰后肌肉的糖酵解程度降低[51]。研究表明,α亚基对于AMPK的活化是必需的,α亚基含有激酶结构域,活性高度依赖于α-苏氨酸(Thr)172的可逆磷酸化,而AMPK信号通路的激活主要是通过磷酸化α-Thr172位点来实现的[52]。Xing等[47]利用AA肉鸡为研究对象进行研究,结果发现,与对照组(无运输应激)相比,运输应激增加了宰后0.5~4.0 h肌肉AMPKα的磷酸化水平,而对宰后24 h肌肉AMPKα磷酸化水平没有影响。这表明AMPK信号介导了运输应激条件下肌肉的能量代谢。并且,宰后0.5~24.0 h肌肉AMPKα磷酸化水平逐步降低,表明运输应激可能只是影响了宰后肌肉较早阶段的无氧酵解。
AMPKα由α1和α2 2个亚基构成。其中,AMPKα1广泛分布于肝脏、肾脏、骨骼肌等组织中,参与对肌肉生长的调控;而AMPKα2则主要分布在心肌和骨骼肌等,参与机体代谢[53]。Liang等[54]利用基因小鼠作为模型动物进行研究,结果发现,与AMPKα1基因敲除小鼠相比,AMPKα2基因敲除显著降低了小鼠肌肉的糖酵解潜力,降低了宰后肌肉中乳酸堆积,抑制了AMPK活性。Zhang等[13]研究表明,与0.5 h宰前运输应激相比,3.0 h宰前运输应激显著增加了宰后胸肌AMPKα2 mRNA表达水平,但对AMPKα1 mRNA表达水平没有显著影响,这与Wang等[55]的研究结果一致。以上研究结果提示,AMPKα2而不是AMPKα1介导了宰后肌肉的糖酵解过程,参与了运输应激条件所引起的肌肉能量代谢过程。
4.2 蛋白质乙酰化影响宰后肌肉能量代谢过程尽管已有研究表明,AMPK参与了宰后肌肉的糖酵解过程,在宰后肌肉糖酵解方面发挥着重要作用[56],但AMPK对宰后肌肉糖酵解的调控机制仍不清楚。Shen等[51]研究表明,经腹腔注入20 mg/kg的AMPK抑制剂显著降低了宰后24 h肌肉中乳酸含量,增加了肌肉pH,降低了AMPKα Thr172的磷酸化表达水平,有效抑制了宰后肌肉糖酵解。AMPK活化后可提高糖酵解过程关键酶HK、磷酸果糖激酶(phosphofructokinase,PFK)和HK等的活性[57-58];然而敲除AMPK基因后,肌肉HK活性明显降低[59]。注入5-氨基-4-甲酰胺咪唑核糖核苷酸(AICAR,一种AMPK激活剂)后,能够显著提高HK活性[60]。以上研究结果表明,AMPK可能通过调节糖酵解过程中关键酶的活性影响肌肉糖酵解过程。
利用蛋白质乙酰化的蛋白质组学分析发现,在细胞质和线粒体中含有大量乙酰化蛋白质,包括参与中间代谢的大多数酶。研究表明,几乎所有参与糖酵解及糖原代谢的酶都被乙酰化[61](图 2),蛋白质乙酰化及脱乙酰化调控着机体的糖酵解进程[62-63]。乙酰化通过多种机制调节代谢酶活性,包括激活或抑制酶活性以及影响蛋白质的稳定性[64]。经腹腔注入AICAR能够显著增加肌肉中AMPK活性,并伴随肌肉总乙酰化水平的升高。而注入组蛋白乙酰转移酶抑制剂Ⅱ(HATⅡ,一种蛋白质乙酰化抑制剂)则能抑制AMPK活性、肌肉总乙酰化蛋白表达水平和肌肉糖酵解速率[65],这意味着AMPK可能通过乙酰化调控肌肉的糖酵解。Li等[6]利用小鼠为模型,宰前强迫游泳2 min模拟宰前应激,结果显示,宰前应激降低了肌肉pH,增加了肌肉乳酸含量及总乙酰蛋白的表达水平。这意味着在运输应激条件下,AMPK可能是通过组蛋白脱乙酰化酶来调节机体的能量代谢。
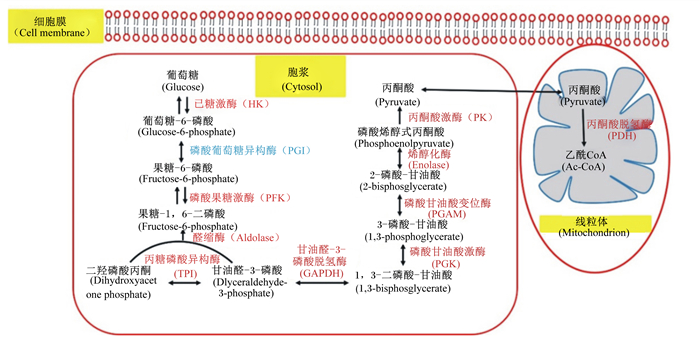
|
底物及产物为黑色字体。若代谢酶的乙酰化已确认,其标记为红色;若还未确定,则标记为蓝色。 Substrates and products are colored in black. Metabolic enzymes are colored in red if their acetylations have been identified or blue if not. 图 2 糖分解过程中代谢酶的乙酰化调控 Fig. 2 Acetylation regulation of metabolic enzymes in glycolysis[64] |
宰前运输引起了肉鸡应激反应,造成肉鸡代谢紊乱,加速了肉鸡能量消耗,使宰后肉鸡肌肉糖酵解活动增强,肌肉pH和系水力降低,从而影响了肉品质。同时,运输应激影响了肌肉无氧酵解过程中关键酶的活性,而参与糖酵解过程的大多数酶是被乙酰化的。因此,今后也可以从糖酵解酶的蛋白乙酰化角度出发,深入研究运输应激对宰后肌肉能量代谢的影响机制。此外,还可以围绕宰前运输管理和宰前营养调控等措施开展研究,降低肉鸡类PSE肉的发生率。
| [1] |
孙皓. 鸡肉类PSE肉与正常肉的功能特性的比较研究[D]. 硕士学位论文. 南京: 南京农业大学, 2013. SUN H. Comparison of functional properties of PSE-like and normal chicken meat[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2013. (in Chinese) |
| [2] |
朱学伸. 家禽"类PSE肉"的品质特性及其改善因素研究[D]. 硕士学位论文. 南京: 南京农业大学, 2011. ZHU X S. Study on characteristics and the improvement factors of pale soft exudative like poultry meat[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2011. (in Chinese) |
| [3] |
ZHU X S, XU X L, MIN H H, et al. Occurrence and characterization of pale, soft, exudative-like broiler muscle commercially produced in China[J]. Journal of Integrative Agriculture, 2012, 11(8): 1384-1390. DOI:10.1016/S2095-3119(12)60137-3 |
| [4] |
WANG X, LI J, CONG J, et al. Preslaughter transport effect on broiler meat quality and postmortem glycolysis metabolism of muscles with different fiber types[J]. Journal of Agricultural & Food Chemistry, 2017, 65(47): 10310-10316. |
| [5] |
DESAI M A, JACKSON V, ZHAI W, et al. Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (pectoralis major) meat[J]. Poultry Science, 2016, 95: 2696-2706. DOI:10.3382/ps/pew213 |
| [6] |
LI Z, LI X, WANG Z, et al. Antemortem stress regulates protein acetylation and glycolysis in postmortem muscle[J]. Food Chemistry, 2016, 20: 294-298. |
| [7] |
HUANG J, YANG J, HUANG M, et al. Effect of pre-slaughter shackling and wing flapping on plasma parameters, postmortem metabolism, AMPK, and meat quality of broilers[J]. Poultry Science, 2018, 97: 1841-1847. DOI:10.3382/ps/pey019 |
| [8] |
HAMBRECHT E, EISSEN J J, NEWMAN D J, et al. Preslaughter handling effects on pork quality and glycolytic potential in two muscles differing in fiber type composition[J]. Journal of Animal Science, 2005, 83(4): 900-907. DOI:10.2527/2005.834900x |
| [9] |
CAFFREY N P, DOHO O. Factors affecting mortality risk during transportation of broiler chickens for slaughter in Atlantic Canada[J]. Preventive Veterinary Medicine, 2017, 147: 199-208. DOI:10.1016/j.prevetmed.2017.09.011 |
| [10] |
BIANCHI M, PETRACCI M, CAVANI C. Effects of transport and lairage on mortality, liveweight loss and carcass quality in broiler chickens[J]. Italian Journal of Animal Science, 2005, 4(Suppl.2): 516-518. |
| [11] |
MITCHELL M A, KETTLEWELL P J. Welfare of poultry during transport-a review[C]//In proceedings of the 8th European symposium on poultry welfare. Italy: [s. n. ], 2009.
|
| [12] |
ZHANG L, LI J L, GAO T, et al. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress[J]. Animal, 2014, 8(12): 1955-1962. DOI:10.1017/S1751731114001906 |
| [13] |
ZHANG L, WANG X, LI J, et al. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers[J]. Journal of Agricultural & Food Chemistry, 2017, 65(32): 6991-6999. |
| [14] |
XING T, XU X, JIANG N, et al. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer[J]. Animal Science Journal, 2016, 87: 299-307. DOI:10.1111/asj.12426 |
| [15] |
SCHWARTZKOPF-GENSWEIN K S, FAUCITANO L, DADGAR S, et al. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: a review[J]. Meat Science, 2012, 92(3): 227-243. DOI:10.1016/j.meatsci.2012.04.010 |
| [16] |
SPEER N C, SLACK G, TROYER E. Economic factors associated with livestock transportation[J]. Journal of Animal Science, 2001, 79: E166-E170. DOI:10.2527/jas2001.79E-SupplE166x |
| [17] |
ZHANG C, GENG Z, CHEN K, et al. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status[J]. Poultry Science, 2019, 98: 4648-4655. DOI:10.3382/ps/pez164 |
| [18] |
NIJDAM E, DELEZIE E, LAMBOOIJ E, et al. Comparison of bruises and mortality, stress parameters, and meat quality in manually and mechanically caught broilers[J]. Poultry Science, 2005, 84(3): 467-474. DOI:10.1093/ps/84.3.467 |
| [19] |
VECEREK V, VOSLAROVA E, CONTE F, et al. Negative trends in transport-related mortality rates in broiler chickens[J]. Asian Australasian Journal of Animal Sciences, 2016, 29(12): 1796-1804. DOI:10.5713/ajas.15.0996 |
| [20] |
ZANETTI E, MASI A, PIVATO M, et al. A note on protein expression changes in chicken breast muscle in response to time in transit before slaughtering[J]. Proteome Science, 2013, 34(11): 1-10. |
| [21] |
ZHANG L, YUE H Y, ZHANG H J, et al. Transport stress in broilers: Ⅰ.Blood metabolism, glycolytic potential, and meat quality[J]. Poultry Science, 2009, 88(10): 2033-2041. DOI:10.3382/ps.2009-00128 |
| [22] |
VOSLAROVA E, CHLOUPEK P, VOSMEROVA P, et al. Time course changes in selected biochemical indices of broilers in response to pretransport handling[J]. Poultry Science, 2011, 90(10): 2144-2152. DOI:10.3382/ps.2011-01473 |
| [23] |
张岩. 肉鸡宰前管理初步调查及宰前应激对鸡肉品质影响[D]. 硕士学位论文. 南京: 南京农业大学, 2012. ZHANG Y. Preliminary investigation of broiler pre-slaughter management and effect of pre-slaughter stress on broiler meat quality[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2012. (in Chinese) |
| [24] |
YUE H Y, ZHANG L, WU S G, et al. Effects of transport stress on blood metabolism, glycolytic potential, and meat quality in meat-type yellow-feathered chickens[J]. Poultry Science, 2010, 89(3): 413-419. DOI:10.3382/ps.2009-00550 |
| [25] |
张林. 运输应激对肉仔鸡肌肉品质的影响及其机理[D]. 硕士学位论文, 杨陵: 西北农林科技大学, 2009. ZHANG L. Effects of transport stress on meat quality and mechanism in broiler chickens[D]. Master's Thesis. Yangling: Northwest Agriculture & Forestry University, 2009. (in Chinese) |
| [26] |
KANNAN G, HEATH J L, WABECK C J, et al. Elevated plasma corticosterone concentrations influence the onset of rigor mortis and meat color in broilers[J]. Poultry Science, 1998, 77: 322-328. DOI:10.1093/ps/77.2.322 |
| [27] |
NIJDAM E, DELEZIE E, LAMBOOIJ E, et al. Comparison of bruises and mortality, stress parameters, and meat quality in manually and mechanically caught broilers[J]. Poultry Science, 2005, 84: 467-474. DOI:10.1093/ps/84.3.467 |
| [28] |
ONDRASOVICOVA O, SABA L, SMIRJAKOVA S, et al. Effects of vehicle-road transport on blood profile in broiler chickens[J]. Medycyna Weterynaryjna, 2008, 64(3): 292-293. |
| [29] |
邢通. 高温运输应激诱导类PSE鸡肉的形成机理研究[D]. 博士学位论文. 南京: 南京农业大学, 2018. XING T. Study on the mechanism of transport under high temperature-induced PSE-like meat[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2018. (in Chinese) |
| [30] |
MACKENZIE A, DRENNAN M, ROWAN T, et al. Effect of transportation and weaning on humoral immune responses of calves[J]. Research in Veterinary Science, 1997, 63: 227-230. DOI:10.1016/S0034-5288(97)90025-4 |
| [31] |
MITCHELL M A, KETTLEWELL P J. Physiological stress and welfare of broiler chickens in transit: solutions not problems[J]. Poultry Science, 1998, 77: 1803-1814. DOI:10.1093/ps/77.12.1803 |
| [32] |
YOUNG O A, WEST J, HART A L, et al. A method for early determination of meat ultimate pH[J]. Meat Science, 2004, 66(2): 493-498. DOI:10.1016/S0309-1740(03)00140-2 |
| [33] |
姚军虎. 应激引起PSE肉的机理及其营养控制[J]. 国外畜牧科技, 1994, 21: 28-30. YAO J H. Mechanism of stress induced PSE meat and its nutritional control[J]. Foreign Animal Husbandry Science and Technology, 1994, 21: 28-30 (in Chinese). |
| [34] |
CASTELLINI C, MATTIOLI S, PIOTTOLI L, et al. Effect of transport length on oxidative status and breast meat characteristics in outdoor-reared chicken genotypes[J]. Italian Journal of Animal Science, 2016, 15(2): 191-199. DOI:10.1080/1828051X.2016.1174082 |
| [35] |
LEFAUCHEUR L. A second look into fibre typing-relation to meat quality[J]. Meat Science, 2010, 84(2): 257-270. DOI:10.1016/j.meatsci.2009.05.004 |
| [36] |
BEAUCLERCQ S, NADAL-DESBARATS L, HENNEQUET-ANTIER C, et al. Serum and muscle metabolomics for the prediction of ultimate pH, a key factor for chicken-meat quality[J]. Journal of Proteome Research, 2016, 15(4): 1168-1178. DOI:10.1021/acs.jproteome.5b01050 |
| [37] |
ZHANG L, LI J L, WANG X F, et al. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers[J]. Poultry Science, 2019, 98: 3223-3232. DOI:10.3382/ps/pez052 |
| [38] |
WHYTE P, COLLINS J, MCGILL K, et al. The effect of transportation stress on excretion rates of campylobacters in market-age broilers[J]. Poultry Science, 2001, 80(6): 817-820. DOI:10.1093/ps/80.6.817 |
| [39] |
OBANOR F O. Biochemical basis of the effect of pre-slaughter stress and post-slaughter processing conditions on meat tenderness[D]. Ph. D. Thesis. California: Lincoln University, 2002.
|
| [40] |
SAVENIJE B, LAMBOOIJ E, GERRITZEN M, et al. Effects of feed deprivation and transport on preslaughter blood metabolites, early postmortem muscle metabolites, and meat quality[J]. Poultry Science, 2002, 81: 699-708. DOI:10.1093/ps/81.5.699 |
| [41] |
MARKUS W, RIMA K D. Creatine and creatinine metabolism[J]. Physiological Reviews, 2000, 80: 1107-1213. DOI:10.1152/physrev.2000.80.3.1107 |
| [42] |
赵敏孟. 胚蛋注射丙酮酸肌酸调控肉鸡能量代谢和肌肉发育的作用机理研究[D]. 博士学位论文. 南京: 南京农业大学, 2017. ZHAO M M. Regulatory mechanism of in ovo feeding of creatine pyruvate on energy metabolism and muscle development of broiler chickens[D]. Ph. D. Thesis. Nanjing: Nanjing Agriculture University, 2017. (in Chinese) |
| [43] |
LUCAS G F. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles[J]. Einstein(Sao Paulo), 2014, 12: 126-131. DOI:10.1590/S1679-45082014RB2741 |
| [44] |
YADGARY L, UNI Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development[J]. Poultry Science, 2012, 91: 444-453. DOI:10.3382/ps.2011-01669 |
| [45] |
LIU Y, LI J L, LI Y J, et al. Effects of dietary supplementation of guanidinoacetic acid and combination of guanidinoacetic acid and betaine on postmortem glycolysis and meat quality of finishing pigs[J]. Animal Feed Science and Technology, 2015, 205: 82-89. DOI:10.1016/j.anifeedsci.2015.03.010 |
| [46] |
郭谦, 沈清武, 罗洁. 畜禽宰后肌肉能量代谢与肉品质研究进展[J]. 食品工业科技, 2020, 41: 357-361. GUO Q, SHEN Q W, LUO J. Progress on muscle energy metabolism and meat quality after the slaughter of livestock and poultry[J]. Science and Technology of Food Industry, 2020, 41: 357-361 (in Chinese). |
| [47] |
XING T, XU X, JIANG N, et al. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer[J]. Animal Science Journal, 2015, 87(2): 299-307. |
| [48] |
HARDIE D G. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism[J]. Proceedings of the Nutrition Society, 2011, 70(1): 92-99. DOI:10.1017/S0029665110003915 |
| [49] |
GOWANS G J, HARDIE D G. AMPK: a cellular energy sensor primarily regulated by AMP[J]. Biochemical Society Transactions, 2014, 42(1): 71-75. DOI:10.1042/BST20130244 |
| [50] |
HALSE R, FRYER L G, MCCORMACK J G, et al. Regulation of glycogen synthase by glucose and glycogen a possible role for AMP-activated protein kinase[J]. Diabetes, 2003, 52(1): 9-15. DOI:10.2337/diabetes.52.1.9 |
| [51] |
SHEN Q W, GERRARD D E, DU M. Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem[J]. Meat Science, 2008, 78(3): 323-330. DOI:10.1016/j.meatsci.2007.06.023 |
| [52] |
HORMAN S, HUSSAIN N, DILWORTH S M, et al. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation[J]. Comparative Biochemistry & Physiology Part B: Biochemistry & Molecular Biology, 2005, 142(4): 374-382. |
| [53] |
李泽. AMPK活性对宰后羊肉能量代谢和肉质的影响及其机理研究[D]. 博士学位论文. 呼和浩特: 内蒙古农业大学, 2010. LI Z. Effect of AMPK activity on energy metabolism and meat quality of postmortem lamb and its mechanism research[D]. Ph. D. Thesis. Hohhot: Inner Mongolia Agricultural University, 2010. (in Chinese) |
| [54] |
LIANG J, YANG Q, ZHU M J, et al. AMP-activated protein kinase (AMPK) α2 subunit mediates glycolysis in postmortem skeletal muscle[J]. Meat Science, 2013, 95(3): 536-541. DOI:10.1016/j.meatsci.2013.05.025 |
| [55] |
WANG P, ZHANG R Y, SONG J, et al. Loss of AMP-activated protein kinase-α2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle[J]. Diabetes, 2012, 61(5): 1051-1061. DOI:10.2337/db11-1180 |
| [56] |
LIANG J, YANG Q, ZHU M J, et al. AMP-activated protein kinase (AMPK) alpha2 subunit mediates glycolysis in postmortem skeletal muscle[J]. Meat Science, 2013, 95(3): 536-541. DOI:10.1016/j.meatsci.2013.05.025 |
| [57] |
SHEN Q W, MEANS W J, UNDERWOOD K R, et al. Early post-mortem AMP-activated protein kinase (AMPK) activation leads to phosphofructokinase-2 and -1(PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle[J]. Journal of Agricultural & Food Chemistry, 2006, 54(15): 5583-5589. |
| [58] |
MARSIN A S, BOUZIN C, BERTRAND L, et al. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase[J]. Journal of Biological Chemistry, 2002, 277(34): 30778-30783. DOI:10.1074/jbc.M205213200 |
| [59] |
DU M, SHEN Q W, ZHU M J. Role of beta-adrenoceptor signaling and AMP-activated protein kinase in glycolysis of postmortem skeletal muscle[J]. Journal of Agricultural & Food Chemistry, 2005, 53(8): 3235-3239. |
| [60] |
WINDER W W, HOLMES B F, RUBINK D S, et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle[J]. Journal of Applied Physiology, 2000, 88(6): 2219-2226. DOI:10.1152/jappl.2000.88.6.2219 |
| [61] |
ZHAO S, XU W, JIANG W, et al. Regulation of cellular metabolism by protein lysine acetylation[J]. Science, 2010, 327(5968): 1000-1004. DOI:10.1126/science.1179689 |
| [62] |
HALLOWS W C, YU W, DENU J M. Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation[J]. Journal of Biological Chemistry, 2012, 287(6): 3850-3858. DOI:10.1074/jbc.M111.317404 |
| [63] |
LI T, LIU M, FENG X, et al. Glyceraldehyde-3-phosphate dehydrogenase is activated by lysine 254 acetylation in response to glucose signal[J]. Journal of Biological Chemistry, 2014, 289(6): 3775-3785. DOI:10.1074/jbc.M113.531640 |
| [64] |
GUAN K L, XIONG Y. Regulation of intermediary metabolism by protein acetylation[J]. Trends in Biochemical Sciences, 2011, 36(2): 108-116. DOI:10.1016/j.tibs.2010.09.003 |
| [65] |
LI Q, LI Z, LOU A, et al. HAT inhibitors antagonize AMPK in postmortem glycolysis[J]. Asian-Australas Journal of Animal Science, 2017, 30: 857-864. |



