肠道是动物机体与外界环境直接接触且表面积最大的器官,具有消化、吸收、代谢与免疫等多种生物学功能[1]。肠道上皮屏障具有独特的选择透过性,能够保障机体对饲粮中养分的吸收,并有效地抑制肠腔中病原微生物及有毒有害物质透过屏障进入机体内环境,从而维持动物机体正常的生理机能[2]。
内质网(endoplasmic reticulum,ER)是细胞内由单层膜折叠构成的网状结构细胞器,能够对分泌性蛋白和膜蛋白进行折叠、加工和修饰,同时内质网也参与了机体脂质代谢、能量代谢和细胞内钙离子(Ca2+)稳态的调节[3]。蛋白质在内质网中的折叠、修饰和加工的过程依赖于内质网功能稳态。能量缺乏、氧化应激、Ca2+失衡、代谢异常、蛋白质的糖基化修饰异常和炎症都会引发内质网功能紊乱,未折叠蛋白和错误折叠蛋白在内质网中蓄积,引发内质网应激(endoplasmic reticulum stress,ERS)[4]。有研究表明,断奶应激诱导仔猪肠道中内质网应激相关蛋白表达水平显著升高,提示仔猪肠道功能障碍可能与上皮细胞中内质网应激有关,但具体机制尚不明确[5-6]。本文对内质网应激信号影响猪肠道屏障功能的研究进展进行综述,以期为通过内质网应激信号通路改善猪肠道健康提供理论参考。
1 猪肠道上皮细胞结构与功能肠道是动物机体对营养物质消化吸收的重要器官,也是内外环境之间进行物质交换的媒介。肠道上皮由单层细胞排列而成,是机体内环境与肠腔内容物之间的屏障。肠道上皮通过小肠绒毛及细胞微绒毛的立体结构使肠腔具有更大的单位接触面积,加快了营养物质的消化吸收[7]。仔猪阶段的肠道健康不仅影响仔猪生长性能和成活率,还会间接影响其生长育肥阶段的生长性能。现代规模化猪场为提高母猪年产仔数将仔猪断奶时间提前至21日龄甚至更早。此时受环境变化、饲粮改变、生理以及心理等多方面的影响,早期断奶使仔猪肠绒毛萎缩和消化酶活性降低[8],采食量下降,肠上皮细胞凋亡水平显著升高[9],仔猪肠道和免疫系统出现功能障碍,引发生长发育迟缓等健康问题[10]。
小肠隐窝中的干细胞不断增殖并最终分化为成熟的上皮细胞并向绒毛顶端迁移,补充绒毛顶端因凋亡而脱落的细胞,这种肠道上皮细胞的动态更新机制保障了小肠结构的完整性[11]。成熟的肠道上皮细胞根据功能类型可分为潘氏细胞(Paneth cells)、杯状细胞(goblet cells)、肠内分泌细胞(enteroendocrine cells,EC)和微皱褶细胞(microfold cells,M cells)。潘氏细胞位于隐窝底部,呈椎体形,可分泌大量抗菌活性物质,如溶菌酶和α-防御素,以防止肠腔中有害微生物的侵袭[12]。杯状细胞可分泌黏液、三叶肽因子(trefoil peptides)和抵抗素样分子β(resistin-like molecules β,RELMβ),保护肠上皮屏障。肠内分泌细胞可通过分泌激素肽参与上皮细胞修复和血管生成等生理过程[13]。微皱褶细胞负责将肠腔内细菌和抗原信号向下层免疫细胞传递,是机体识别抗原过程中的重要媒介[14]。
肠道上皮通过跨细胞途径和细胞旁路途径实现营养物质转运并阻止肠腔中有毒有害物质进入机体内环境。跨细胞途径是营养物质消化吸收的主要途径,上皮细胞膜表面的转运载体和离子通道允许肠腔内的水、电解质和小分子营养物质透过屏障进入体内。相邻上皮细胞之间通过紧密连接(tight junctions,TJs)、黏附连接(adherens junctions,AJs)和细胞桥粒等蛋白复合物实现细胞旁路途径的选择透过性,抵御病原、微生物和毒素的入侵[15]。紧密连接蛋白间相互作用形成“吻斑”(kissing points),封闭相邻细胞间的间隙,当紧密连接蛋白表达和分布出现异常时,肠道上皮屏障通透性增加,因此紧密连接蛋白是维持上皮屏障功能的关键限制性因素。
2 未折叠蛋白反应与内质网应激细胞内外环境中多种应激因素(如饥饿、缺氧、感染、代谢紊乱)可导致内质网的蛋白折叠功能紊乱,机体为恢复细胞稳态和内质网的正常功能,启动未折叠蛋白反应(unfolded protein response,UPR),降低蛋白质合成速率,降解异常折叠蛋白,减少错误折叠蛋白累积对细胞生理功能的影响[16-17]。
哺乳动物内质网膜上的肌醇酶1(inositol-requiring kinase 1,IRE1)、蛋白激酶R样内质网激酶(protein kinase RNA-like ER kinase,PERK)和转录活化因子6(activating transcription factor 6,ATF6)是感受内质网应激的重要感应蛋白[18]。在非应激状态下,葡萄糖调节蛋白(GRP78/BiP)作为内质网腔中的分子伴侣能够与3种内质网应激感受蛋白相互结合,阻断其下游信号的通路。当内质网处于应激状态时,GRP78/BiP与异常折叠蛋白结合,从而释放3种内质网应激感受蛋白,进而激活下游信号,恢复内质网功能稳态[19]。
2.1 IRE1信号通路在3种应激感受蛋白中,IRE1是最为保守的内质网应激感受分子。已发现的哺乳动物IRE1有2种,分别为IRE1α和IRE1β,前者在不同组织及细胞中广泛表达,而后者主要位于肠道和呼吸道上皮细胞[20]。细胞受到外来刺激而诱发内质网应激时,IRE1与GRP78/BiP蛋白分离后被磷酸化激活,利用其核酸内切酶活性从X盒结合蛋白1(X-box-binding protein 1,XBP1)的mRNA中特异性剪切26 bp的内含子片段,从而改变XBP1 mRNA的开放阅读框,生成具有功能活性的XBP1剪接异构体(XBP1s)[21]。XBP1s与对应的UPR反应元件结合,在不同细胞或条件下分别调控分泌、脂质代谢、葡萄糖稳态和炎症反应等功能相关基因的转录与翻译[22]。近期有研究表明,IRE1α可诱导XBP1以外的mRNA降解,通过IRE1依赖性降解调控途径(regulated IRE1-dependent decay,RIDD)减少蛋白质的生成,从而缓解内质网应激状态[23-24]。此外,被激活的IRE1α与TNFα受体相关因子2(TNFα receptor-associated factor 2,TRAF2)结合,进而激活核因子-κB(NF-κB)和c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK),促进下游细胞炎症因子和凋亡信号转导[20]。
2.2 PERK信号通路PERK是一种具有丝氨酸/苏氨酸激酶结构域的Ⅰ型跨膜蛋白,在内质网应激过程中起到重要的信号转导作用。当内质网应激发生时,GRP78/BiP释放出来的PERK形成二聚体,并通过自磷酸化作用而被激活[25]。活化的PERK使真核翻译起始因子2的α亚基(eukaryote initiation factor 2α,eIF2α)磷酸化,抑制蛋白质合成从而降低内质网中错误折叠蛋白的负荷[26]。进一步研究发现,eIF2α磷酸化激活后可选择性促进活化转录因子4(activating transcription factor 4,ATF4)的翻译,进而调控氧化应激和内质网应激介导的细胞凋亡[27]。CCAAT/增强子结合蛋白同源蛋白(CCAAT/enhancer-binding protein homologous protein,CHOP)是内质网应激信号中重要的转录因子,可以被ATF4或其他因子激活,从而介导内质网应激相关的凋亡信号[28]。
2.3 ATF6信号通路ATF6是一种具有羧基端应力感受结构域和氨基端bZip转录因子结构域的跨膜蛋白[29]。目前,在哺乳动物中已发现2种ATF6同源蛋白,即ATF6α和ATF6β。ATF6α具有UPR相关基因激活特性[30]。当内质网腔中未折叠蛋白和错误折叠蛋白蓄积时,ATF6α与GRP78/BiP分离,并从内质网转移至高尔基体,随后其跨膜结构域和高尔基体腔内结构域被高尔基酶位点1蛋白酶(S1P)和高尔基酶位点2蛋白酶(S2P)水解,释放出的ATF6片段(ATF6f)进入细胞核并与内质网应激反应元件(endoplasmic reticulum-stressed response elements,ERSE)结合,上调参与蛋白折叠的靶基因或内质网相关蛋白降解(ER-associated protein degradation,ERAD)信号通路的转录水平,通过促进蛋白折叠或降解错误折叠蛋白的方式应对内质网应激[31-33]。
由上可知,内质网应激是细胞内质网功能稳态被打破的状态,细胞通过IRE1、PERK和ATF6 3条途径激活UPR以减轻错误折叠蛋白和未折叠蛋白在内质网中的负荷,这种适应性修复机制有利于重建细胞稳态,保证细胞存活。但当细胞无法通过UPR进行自我修复而长期处于内质网应激时,细胞凋亡途径被激活,主动清除损伤细胞恢复机体稳态。
3 内质网应激与猪肠道屏障肠道上皮细胞、微生物与营养素通过复杂的互作机制,维持肠屏障功能。肠上皮细胞能够感受环境中的各种信号,并通过适应性机制调节其代谢和生理功能。已有研究表明,内质网应激信号通路与炎症性肠病、肠易激综合症等肠道疾病密切相关[34-36]。
3.1 断奶应激与内质网应激早期断奶的小鼠结肠隐窝深度降低,杯状细胞数量减少,内质网应激相关蛋白,如CHOP、BiP、活化型半胱天冬酶-3(cleaved-caspase-3)等蛋白表达水平升高,这些变化在添加内质网应激相关蛋白的抑制剂后得到明显改善,提示内质网应激与肠道屏障之间可能存在相关性[37-38]。猪由于其消化吸收、物质代谢、肠道微生物组成等与人具有较高的相似性,是多种人类疾病研究的重要动物模型。本团队最近的研究发现,断奶引起仔猪空肠、回肠组织中ATF6α、p-IRE1α和p-eIF2α,以及下游凋亡相关蛋白JNK和CHOP蛋白表达水平显著升高,炎性细胞因子白细胞介素(IL)-1β、肿瘤坏死因子-α(TNF-α)和IL-8蛋白表达水平明显高于同日龄哺乳仔猪。7~21日龄的哺乳仔猪补充谷氨酰胺(每天1.52 g/kg BW)可有效缓解断奶应激引起的空肠内质网应激相关蛋白(BiP、ATF6α、p-IRE1α和p-eIF2α)和凋亡相关蛋白[CHOP、p-JNK、半胱天冬酶-12(caspase-12)、cleaved-caspase-3和B淋巴细胞瘤-2相关X蛋白(Bax)]的表达上调,降低炎性细胞因子(TNF-α、IL-1β、IL-6和IL-8)蛋白的表达[6]。另一项研究发现,7~21日龄的哺乳仔猪额外补充相当于从母乳中摄取的甘氨酸1~2倍的量,能有效缓解断奶应激引发的肠道损伤,降低血清中二胺氧化酶活性,降低断奶应激诱导升高的GRP78/BiP、p-IRE1α、CHOP和p53蛋白的表达水平,提高空肠绒毛高度与隐窝深度比值,增加杯状细胞数量,并上调咬合蛋白(occludin)、闭合蛋白-1(claudin-1)和胞质紧密连接蛋白-1(ZO-1)等紧密连接蛋白表达量[39]。
衣霉素(tunicamycin)是一种由细菌代谢产生的天然抗生素,可通过抑制内质网中新合成蛋白N端糖基化,从而引发内质网应激,因此常用于构建内质网应激的细胞或动物模型[40-42]。Huang等[43]研究发现,枸杞多糖(10 μg/mL)可明显缓解衣霉素诱导p-PERK、p-eIF2α、ATF6、IRE1和CHOP的蛋白表达水平的升高,并抑制细胞凋亡。Li等[44]研究发现,添加β-胡萝卜素(80 mg/kg BW)可缓解早期断奶引起IRE1α和PERK的磷酸化水平,抑制由内质网应激诱导的猪肠上皮细胞凋亡,从而改善猪的肠道健康和生长性能。最近的研究发现,大蒜素可调节猪空肠肌醇酶1/x盒结合蛋白-1s(IRE1/XBP-1s)信号通路,降低eIF2α和ATF4的磷酸化水平,提高紧密连接蛋白的表达,改善早期断奶仔猪肠上皮细胞结构和功能[45]。Jiang等[46]通过体外研究发现,谷氨酰胺(3.0 mmol/L)也可通过IRE-1/XBP-1s信号通路缓解衣霉素诱导的猪小肠上皮细胞(IPEC-J2)内质网应激。这些研究提示,断奶应激通过激活内质网应激信号通路引起上皮细胞凋亡,进而破坏肠道屏障,通过小分子抑制剂或营养物质抑制内质网应激信号可能是潜在的保护肠道屏障的方法。
3.2 细菌感染与内质网应激肠道中大肠杆菌和沙门氏菌感染是引起猪腹泻及肠炎的常见细菌性疾病。脂多糖(lipopolysaccharide,LPS)作为革兰氏阴性菌外膜表面的重要蛋白,能够被Toll样受体(Toll-like receptors,TLR)识别并诱发肠道炎症反应,引起仔猪腹泻[47]。研究发现,大肠杆菌(109 CFU/kg)感染可导致断奶仔猪空肠和回肠组织中内质网应激信号通路相关蛋白(GRP78/BiP和CHOP)表达水平升高,并上调半胱天冬酶-11(caspase-11)蛋白表达水平,引发细胞凋亡[48]。Jiang等[49]研究发现,LPS可加剧衣霉素诱导的细胞内质网应激及细胞凋亡程度。通过siRNA敲除p53蛋白后,细胞凋亡程度得到明显缓解,这可能是由于p53蛋白可以与GRP78/BiP蛋白互作,调节内质网应激相关信号有关。Yang等[50]研究发现,补充约氏乳杆菌L531(1010 CFU/d,7 d)可明显减轻沙门氏菌诱导的仔猪回肠炎症水平和腹泻的发生,改善肠绒毛形态,这种保护作用与其降低肠上皮中内质网应激相关蛋白GRP78表达有关,这可能是益生菌改善猪肠健康的新机制。
3.3 病毒感染与内质网应激病毒性腹泻是造成猪肠屏障功能障碍的重要因素,给养猪业带来了巨大的经济损失。其中,流行性腹泻、传染性胃肠炎和轮状病毒是引起猪群腹泻的3种常见病毒。猪流行性腹泻是由猪流行性腹泻病毒(PEDV)引发的传染性急性肠病,病理特征表现为严重的肠道炎症、呕吐和腹泻,仔猪感染后致死率极高[51]。Xu等[52]发现,猪流行性腹泻病毒N蛋白可诱导猪肠上皮细胞内质网应激,上调GRP78蛋白表达水平并抑制细胞增殖,提示病毒诱导的肠道损伤与内质网应激信号通路的激活有关。传染性胃肠炎病毒(TGEV)与猪流行性腹泻病毒相似,同属于冠状病毒科[53],仔猪受该病毒感染后空肠和回肠绒毛结构损伤,出现腹泻,严重时可致死[54]。先前的研究发现TGEV病毒N蛋白在病毒转录过程中发挥重要作用,此外Zhang等[55]试验结果表明,TGEV病毒N蛋白也可引起猪肠上皮细胞细胞周期S期阻滞和内质网应激。Xue等[56]研究发现,TGEV可诱导IPEC-J2细胞和仔猪小肠发生内质网应激,激活未折叠蛋白反应的3条经典信号通路,细胞试验结果显示,被激活的PERK-eIF2α轴通过降低内质网内总蛋白质合成效率与促进干扰素IFN-α/β蛋白表达的方式,抑制TGEV在细胞中的复制。Ma等[57]的试验研究发现,TGEV通过磷酸化激活IRE1α抑制宿主细胞miR-30a-5p的表达,从而上调了IFN信号转导的负调控因子(SOCS1和SOCS3),继而下调了IFN-α/β的表达。这2项研究分别从不同角度阐述了TGEV诱导猪肠道内质网应激与细胞抗病毒反应的关系,加深了畜牧科技工作者对内质网应激信号通路在TGEV诱导猪肠道损伤中的作用机制的理解。
3.4 霉菌毒素与内质网应激饲料霉菌毒素污染影响动物生长、发育、繁殖,并影响动物源性食品安全。研究发现,5 μmol/L黄曲霉毒素B1可引发牛乳腺上皮细胞内质网应激并诱导细胞凋亡[58]。Gao等[59]在以Caco-2为模型的研究中发现,黄曲霉毒素M1和赭曲霉毒素A均降低单层细胞跨膜电阻和紧密连接蛋白表达水平,破坏屏障功能。3-乙酰脱氧雪腐镰刀菌烯醇作为呕吐毒素的乙酰化形式,广泛存在于霉菌毒素污染的饲料和食品中。长期以来,3-乙酰脱氧雪腐镰刀菌烯醇对免疫细胞功能的影响并未受到重视。本团队最近试验发现,3-乙酰脱氧雪腐镰刀菌烯醇可诱导巨噬细胞Raw 264.7发生凋亡和DNA损伤,进一步的研究表明,细胞中内质网应激相关蛋白,如ATF6、p-IRE1α和p-eIF2α蛋白表达水平均显著提高,并激活了细胞自噬性细胞死亡,进而影响机体免疫[60]。此外,镰刀菌属细菌产生的玉米赤霉烯酮,也是食品和饲料中常见的污染物[61]。Long等[62]在体外试验中发现,10 μg/mL花青素可显著降低玉米赤霉烯酮诱导的小鼠肠道上皮细胞凋亡比例,并且这种保护效果与CHOP、GRP78和JNK蛋白表达水平下降有关。这项研究表明,花青素可能通过抑制内质网应激信号途径,缓解玉米赤霉烯酮诱导的肠上皮细胞凋亡。因此,通过营养调控手段减少内质网应激对动物肠道带来的影响,有助于提高动物的屏障功能和整体健康。
4 小结肠道健康对于营养物质消化吸收和代谢、动物生长发育和繁殖具有重要影响,并与饲料利用效率和养殖效益密切相关。最近的研究发现,断奶应激、霉菌毒素污染、细菌和病毒等病原微生物直接或间接作用于肠上皮细胞内质网,引起其功能的紊乱,从细胞器水平揭示了其在肠屏障功能中的重要生理学作用(图 1)。开展宿主细胞-微生物-营养素之间互作机制研究,有助于揭示其潜在的分子机制。
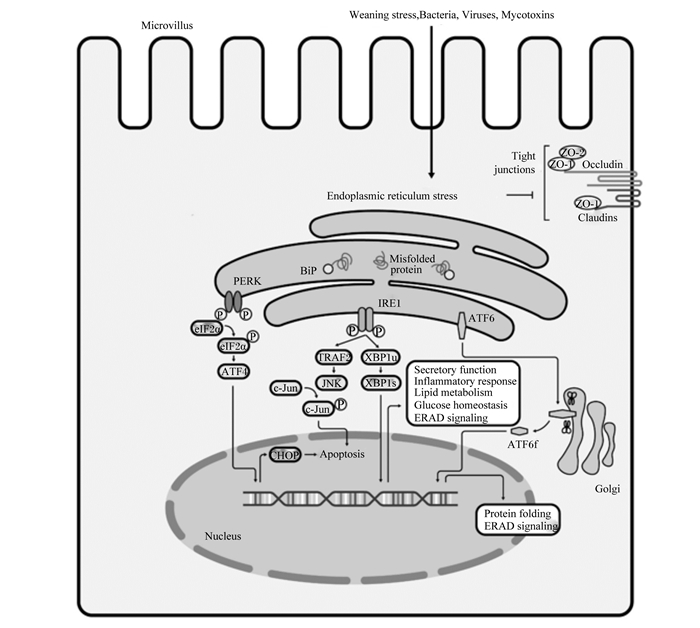
|
Apoptosos:细胞凋亡;ATF4:转录激活因子4 activating transcription factor 4;ATF6:转录激活因子6 activating transcription factor 6;ATF6f:转录激活因子6片段transcriptional activator 6 fragment;BiP:葡萄糖调节蛋白glucose-regulated protein;Bacteria:细菌;Claudins:闭合蛋白;c-JNK:c-Jun N端激酶c-Jun N-terminal kinase;CHOP:CCAAT/增强子结合蛋白同源蛋白CCAAT/ enhancer binding protein homologous protein;Endoplasmic reticulum stress:内质网应激;eIF2α:真核翻译起始因子2的α亚基eukaryote initiation factor 2α;ERAD signaling:内质网相关蛋白降解信号ER-associated protein degradation signaling;Golgi:高尔基体Golgi apparatus;Glucose homeostasis:葡萄糖稳态;IRE1:肌醇酶1 inositol-requiring kinase 1;Inflammatory response:炎症反应;JNK:c-JNK氨基末端激酶c-Jun N-terminal kinase;Microvillus:微绒毛;Mycotoxins:霉菌毒素;Misfolded protein:未折叠蛋白;Nucleus:细胞核;Occludin:咬合蛋白;PERK:蛋白激酶R样内质网激酶protein kinase RNA-like ER kinase;Protein folding:蛋白折叠;Secretory function:分泌功能;Tight junctions:紧密连接;TRAF2:TNFα受体相关因子2 TNFα receptor-associated factor 2;Viruses:病毒;Weaning stress:断奶应激;XBP1:X盒结合蛋白1 X-box-binding protein 1;ZO:胞质紧密连接蛋白cytoplasmic tight junction protein。 图 1 肠道上皮细胞内质网应激信号通路 Fig. 1 Endoplasmic reticulum stress signaling pathways in intestinal epithelial cells |
| [1] |
PABST R, RUSSELL M W, BRANDTZAEG P. Tissue distribution of lymphocytes and plasma cells and the role of the gut[J]. Trends in Immunology, 2008, 29(5): 206-208. DOI:10.1016/j.it.2008.02.006 |
| [2] |
JACOBI S K, ODLE J. Nutritional factors influencing intestinal health of the neonate[J]. Advances in Nutrition, 2012, 3(5): 687-696. DOI:10.3945/an.112.002683 |
| [3] |
CAO S S. Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease[J]. Inflammatory Bowel Diseases, 2015, 21(3): 636-644. DOI:10.1097/MIB.0000000000000238 |
| [4] |
GOTOH T, ENDO M, OIKE Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases[J]. International Journal of Inflammation, 2011, 2011: 259462. |
| [5] |
洪盼. β-胡萝卜素对早期断奶仔猪肠道内质网应激及其信号通路的影响[D]. 博士学位论文. 长春: 吉林农业大学, 2018. HONG P. Effects of β-carotene on intestinal endoplasmic reticulum stress and its signaling pathway in early weaned piglets[D]. Ph. D. Thesis. Changchun: Jilin Agricultural University, 2018. (in Chinese). |
| [6] |
HE Y, FAN X X, LIU N, et al. L-glutamine represses the unfolded protein response in the small intestine of weanling piglets[J]. The Journal of Nutrition, 2019, 149(11): 1904-1910. DOI:10.1093/jn/nxz155 |
| [7] |
RODA G, SARTINI A, ZAMBON E, et al. Intestinal epithelial cells in inflammatory bowel diseases[J]. World Journal of Gastroenterology, 2010, 16(34): 4264-4271. DOI:10.3748/wjg.v16.i34.4264 |
| [8] |
HAMPSON D J. Alterations in piglet small intestinal structure at weaning[J]. Research in Veterinary Science, 1986, 40(1): 32-40. DOI:10.1016/S0034-5288(18)30482-X |
| [9] |
YANG H S, XIONG X, WANG X C, et al. Effects of weaning on intestinal upper villus epithelial cells of piglets[J]. PLoS One, 2016, 11(3): e0150216. DOI:10.1371/journal.pone.0150216 |
| [10] |
WIJTTEN P J A, VAN DER MEULEN J, VERSTEGEN M W A. Intestinal barrier function and absorption in pigs after weaning: a review[J]. British Journal of Nutrition, 2011, 105(7): 967-981. DOI:10.1017/S0007114510005660 |
| [11] |
WILLING B P, MALIK G, VAN KESSEL A G. Nutrition and gut health in swine[M]//CHIBA L I. Sustainable swine nutrition. Chichester, UK: John Wiley & Sons, 2012: 197-213.
|
| [12] |
BEVINS C L, SALZMAN N H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis[J]. Nature Reviews Microbiology, 2011, 9(5): 356-368. DOI:10.1038/nrmicro2546 |
| [13] |
GOTO Y, KIYONO H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system[J]. Immunological Reviews, 2012, 245(1): 147-163. DOI:10.1111/j.1600-065X.2011.01078.x |
| [14] |
GILL N, WLODARSKA M, FINLAY B B. Roadblocks in the gut: barriers to enteric infection[J]. Cellular Microbiology, 2011, 13(5): 660-669. DOI:10.1111/j.1462-5822.2011.01578.x |
| [15] |
GROSCHWITZ K R, HOGAN S P. Intestinal barrier function: molecular regulation and disease pathogenesis[J]. Journal of Allergy and Clinical Immunology, 2009, 124(1): 3-20. DOI:10.1016/j.jaci.2009.05.038 |
| [16] |
CORAZZARI M, GAGLIARDI M, FIMIA G M, et al. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate[J]. Frontiers in Oncology, 2017, 7: 78. DOI:10.3389/fonc.2017.00078 |
| [17] |
CAO S S, KAUFMAN R J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease[J]. Antioxidants & Redox Signaling, 2014, 21(3): 396-413. |
| [18] |
SCHRÖDER M, KAUFMAN R J. The mammalian unfolded protein response[J]. Annual Review of Biochemistry, 2005, 74: 739-789. DOI:10.1146/annurev.biochem.73.011303.074134 |
| [19] |
WANG S Y, KAUFMAN R J. The impact of the unfolded protein response on human disease[J]. Journal of Cell Biology, 2012, 197(7): 857-867. DOI:10.1083/jcb.201110131 |
| [20] |
LUO K, CAO S S. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease[J]. Gastroenterology Research and Practice, 2015, 2015: 328791. |
| [21] |
CALFON M, ZENG H Q, URANO F, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA[J]. Nature, 2002, 415(6867): 92-96. DOI:10.1038/415092a |
| [22] |
BYRD A E, BREWER J W. Intricately regulated: a cellular toolbox for fine-tuning XBP1 expression and activity[J]. Cells, 2012, 1(4): 738-753. DOI:10.3390/cells1040738 |
| [23] |
TABAS I, RON D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress[J]. Nature Cell Biology, 2011, 13(3): 184-190. DOI:10.1038/ncb0311-184 |
| [24] |
HOLLIEN J, LIN J H, LI H, et al. Regulated ire1-dependent decay of messenger rnas in mammalian cells[J]. Journal of Cell Biology, 2009, 186(3): 323-331. DOI:10.1083/jcb.200903014 |
| [25] |
CAO S S, ZIMMERMANN E M, CHUANG B M, et al. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice[J]. Gastroenterology, 2013, 144(5): 989-1000. DOI:10.1053/j.gastro.2013.01.023 |
| [26] |
WEK R C, CAVENER D R. Translational control and the unfolded protein response[J]. Antioxidants & Redox Signaling, 2007, 9(12): 2357-2371. |
| [27] |
LU P D, HARDING H P, RON D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response[J]. Journal of Cell Biology, 2004, 167(1): 27-33. DOI:10.1083/jcb.200408003 |
| [28] |
PALAM L R, BAIRD T D, WEK R C. Phosphorylation of EIF2 facilitates ribosomal bypass of an inhibitory upstream orf to enhance CHOP translation[J]. Journal of Biological Chemistry, 2011, 286(13): 10939-10949. DOI:10.1074/jbc.M110.216093 |
| [29] |
ADACHI Y, YAMAMOTO K, OKADA T, et al. Atf6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum[J]. Cell Structure and Function, 2008, 33(1): 75-89. DOI:10.1247/csf.07044 |
| [30] |
WU J, RUTKOWSKI D T, DUBOIS M, et al. Atf6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress[J]. Developmental Cell, 2007, 13(3): 351-364. DOI:10.1016/j.devcel.2007.07.005 |
| [31] |
CHEN X Q, KARNOVSKY A, SANS M D, et al. Molecular characterization of the endoplasmic reticulum: insights from proteomic studies[J]. Proteomics, 2010, 10(22): 4040-4052. DOI:10.1002/pmic.201000234 |
| [32] |
HAN J, BACK S H, HUR J, et al. Er-stress-induced transcriptional regulation increases protein synthesis leading to cell death[J]. Nature Cell Biology, 2013, 15(5): 481-490. DOI:10.1038/ncb2738 |
| [33] |
NAMBA T, ISHIHARA T, TANAKA K I, et al. Transcriptional activation of atf6 by endoplasmic reticulum stressors[J]. Biochemical and Biophysical Research Communications, 2007, 355(2): 543-548. DOI:10.1016/j.bbrc.2007.02.004 |
| [34] |
BISCHOFF S C, BARBARA G, BUURMAN W, et al. Intestinal permeability-a new target for disease prevention and therapy[J]. BMC Gastroenterology, 2014, 14: 189. DOI:10.1186/s12876-014-0189-7 |
| [35] |
MICHIELAN A, D'INCÀ R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut[J]. Mediators of Inflammation, 2015, 2015: 628157. |
| [36] |
SZABO G. Gut-liver axis in alcoholic liver disease[J]. Gastroenterology, 2015, 148(1): 30-36. DOI:10.1053/j.gastro.2014.10.042 |
| [37] |
LI B, LEE C, ZANI A, et al. Early maternal separation induces alterations of colonic epithelial permeability and morphology[J]. Pediatric Surgery International, 2014, 30(12): 1217-1222. DOI:10.1007/s00383-014-3611-x |
| [38] |
LI B, ZANI A, LEE C, et al. Endoplasmic reticulum stress is involved in the colonic epithelium damage induced by maternal separation[J]. Journal of Pediatric Surgery, 2016, 51(6): 1001-1004. DOI:10.1016/j.jpedsurg.2016.02.073 |
| [39] |
FAN X X, LI S, WU Z L, et al. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: a functional role of chop signaling[J]. Amino Acids, 2019, 51(3): 463-473. DOI:10.1007/s00726-018-2681-9 |
| [40] |
YUAN L, CAO Y, KNÖCHEL W. Endoplasmic reticulum stress induced by tunicamycin disables germ layer formation in Xenopus laevis embryos[J]. Developmental Dynamics, 2007, 236(10): 2844-2851. DOI:10.1002/dvdy.21299 |
| [41] |
KAWADA K, IEKUMO T, SAITO R, et al. Aberrant neuronal differentiation and inhibition of dendrite outgrowth resulting from endoplasmic reticulum stress[J]. Journal of Neuroscience Research, 2014, 92(9): 1122-1133. DOI:10.1002/jnr.23389 |
| [42] |
SUGANYA N, BHAKKIYALAKSHMI E, SURIYANARAYANAN S, et al. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells[J]. Cell Proliferation, 2014, 47(3): 231-240. DOI:10.1111/cpr.12102 |
| [43] |
HUANG C, YAO R Y, ZHU Z K, et al. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress[J]. International Journal of Biological Macromolecules, 2019, 130: 508-514. DOI:10.1016/j.ijbiomac.2019.02.157 |
| [44] |
LI R N, YANG Y, HONG Z P, et al. β-carotene attenuates weaning-induced apoptosis via inhibition of perk-chop and ire1-jnk/p38 mapk signalling pathways in piglet jejunum[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(1): 280-290. DOI:10.1111/jpn.13216 |
| [45] |
JIANG Q, TIAN J Q, LIU G, et al. Endoplasmic reticulum stress and unfolded protein response pathways involved in the health-promoting effects of allicin on the jejunum[J]. Journal of Agricultural and Food Chemistry, 2019, 67(21): 6019-6031. DOI:10.1021/acs.jafc.9b02180 |
| [46] |
JIANG Q, CHEN J S, LIU S J, et al. L-glutamine attenuates apoptosis induced by endoplasmic reticulum stress by activating the ire1α-XBP1 axis in ipec-j2:a novel mechanism of l-glutamine in promoting intestinal health[J]. International Journal of Molecular Sciences, 2017, 18(12): 2617. DOI:10.3390/ijms18122617 |
| [47] |
ARCE C, RAMIREZ-BOO M, LUCENA C, et al. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 And IPI-2I) in response to lps from Salmonella typhimurium[J]. Comparative Immunology, Microbiology and Infectious Diseases, 2010, 33(2): 161-174. DOI:10.1016/j.cimid.2008.08.003 |
| [48] |
JIANG Q, CHEN S, REN W K, et al. Escherichia coli aggravates endoplasmic reticulum stress and triggers chop-dependent apoptosis in weaned pigs[J]. Amino Acids, 2017, 49(12): 2073-2082. DOI:10.1007/s00726-017-2492-4 |
| [49] |
JIANG Q, LIU G, CHEN J S, et al. Crosstalk between nuclear glucose-regulated protein 78 and tumor protein 53 contributes to the lipopolysaccharide aggravated apoptosis of endoplasmic reticulum stress-responsive porcine intestinal epithelial cells[J]. Cellular Physiology and Biochemistry, 2018, 48(6): 2441-2455. DOI:10.1159/000492682 |
| [50] |
YANG G Y, XIA B, SU J H, et al. Anti-inflammatory effects of Lactobacillus johnsonii L531 in a pig model of salmonella infantis infection involves modulation of CCR6+ T cell responses and ER stress[J]. Veterinary Research, 2020, 51(1): 26. DOI:10.1186/s13567-020-00754-4 |
| [51] |
LEE C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus[J]. Virology Journal, 2015, 12: 193. DOI:10.1186/s12985-015-0421-2 |
| [52] |
XU X G, ZHANG H L, ZHANG Q, et al. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression[J]. Veterinary Microbiology, 2013, 164(3/4): 212-221. |
| [53] |
MASTERS P S. The molecular biology of coronaviruses[J]. Advances in Virus Research, 2006, 66: 193-292. |
| [54] |
CRUZ J L G, SOLA I, BECARES M, et al. Coronavirus gene 7 counteracts host defenses and modulates virus virulence[J]. PLoS Pathogens, 2011, 7(6): e1002090. DOI:10.1371/journal.ppat.1002090 |
| [55] |
ZHANG Q, XU Y, CHANG R, et al. Transmissible gastroenteritis virus n protein causes endoplasmic reticulum stress, up-regulates interleukin-8 expression and its subcellular localization in the porcine intestinal epithelial cell[J]. Research in Veterinary Science, 2018, 119: 109-115. DOI:10.1016/j.rvsc.2018.06.008 |
| [56] |
XUE M, FU F, MA Y L, et al. The perk arm of the unfolded protein response negatively regulates transmissible gastroenteritis virus replication by suppressing protein translation and promoting type Ⅰ interferon production[J]. Journal of Virology, 2018, 92(15): e00431-18. |
| [57] |
MA Y L, WANG C L, XUE M, et al. The coronavirus transmissible gastroenteritis virus evades the type Ⅰ interferon response through ire1α-mediated manipulation of the microrna mir-30a-5p/socs1/3 axis[J]. Journal of Virology, 2018, 92(22): e00728-18. |
| [58] |
PARK W, PARK M Y, SONG G, et al. Exposure to aflatoxin B1 attenuates cell viability and induces endoplasmic reticulum-mediated cell death in a bovine mammary epithelial cell line (MAC-T)[J]. Toxicology in Vitro, 2019, 61: 104591. DOI:10.1016/j.tiv.2019.104591 |
| [59] |
GAO Y N, LI S L, WANG J Q, et al. Modulation of intestinal epithelial permeability in differentiated Caco-2 cells exposed to aflatoxin M1 and ochratoxin a individually or collectively[J]. Toxins (Basel), 2017, 10(1): 13. DOI:10.3390/toxins10010013 |
| [60] |
LIU N, YANG Y, CHEN J Q, et al. 3-acetyldeoxynivalenol induces lysosomal membrane permeabilization-mediated apoptosis and inhibits autophagic flux in macrophages[J]. Environmental Pollution, 2020, 265: 114697. DOI:10.1016/j.envpol.2020.114697 |
| [61] |
MARIN S, RAMOS A J, CANO-SANCHO G, et al. Mycotoxins: occurrence, toxicology, and exposure assessment[J]. Food and Chemical Toxicology, 2013, 60: 218-237. DOI:10.1016/j.fct.2013.07.047 |
| [62] |
LONG M, CHEN X L, WANG N, et al. Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines[J]. Molecules, 2018, 23(7): 1508. DOI:10.3390/molecules23071508 |




