2. 中国科学院亚热带农业生态过程重点实验室, 动物营养生理与代谢过程湖南省重点实验室, 中国科学院亚热带农业生态研究所, 长沙 410125
2. Key Laboratory of Agro-Ecological Processes in Subtropical Region, Key Laboratory of Animal Nutritional Physiology and Metabolic Process of Hunan, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China
巴马香猪是原产于广西巴马瑶族自治县的脂肪型猪种,有肉质鲜美、性成熟早、抗逆性强等特点,但生长速度缓慢、瘦肉率低,加之传统放养模式,养殖效率低[1]。通过营养调控提高巴马香猪的养殖效率,对生产优质猪肉、提高经济效益有重要意义。β-羟基-β-甲基丁酸(beta-hydroxy-beta-methylbutyrate,HMB)是必需氨基酸亮氨酸的代谢产物,有促进肌肉生长、减少脂肪沉积的作用,但在体内代谢产生的量较少,需通过饲粮补充才能发挥作用[2-4]。研究表明,饲粮添加0.62% HMB可显著降低长大二元杂交生长猪的总脂肪重,改善其脂肪代谢,其作用可能由腺苷酸活化蛋白激酶α(adenosine monophosphate activated protein kinase α,AMPKα)-哺乳动物雷帕霉素靶蛋白(mammaliantargetofrapamycin, mTOR)信号通路所介导[5]。然而,HMB在巴马香猪上的应用效果目前鲜见报道,我们推测饲粮中添加适量HMB可调节巴马香猪生长性能和脂肪代谢。本试验通过在巴马香猪饲粮中添加不同水平的HMB,研究其对巴马香猪生长性能和肝脏脂肪代谢的影响,并筛选出HMB的最适添加量,为HMB在巴马香猪上的应用提供理论依据。
1 材料与方法 1.1 试验设计试验选择32头健康、体重[(8.58±0.21) kg]相近的纯种巴马香猪[阉公猪,(60±2)日龄],随机分为4组(每组8头猪,分别饲喂添加0(对照组)、0.13%、0.64%、1.28% HMB[实际添加物为β-羟基-β-甲基丁酸钙(beta-hydroxy-beta-methylbutyrate calcium, HMB-Ca]的饲粮。所有饲粮均参照我国《猪饲养标准》(NY/T 65—2004)配制而成,饲粮组成及营养水平见表 1。饲养试验于2019年9月8日至2019年11月7日在中国科学院亚热带农业生态研究所动物试验基地开展。试验期间,试验猪在漏粪地板式猪床上单栏饲养,自由采食,自由饮水,每日饲喂次(08:00、12:00、18:00)。消毒及免疫程序按照常规程序进行。试验期60 d。
|
|
表 1 饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of diets (air-dry basis) |
试验结束时,将所有巴马香猪禁食12 h,自由饮水,然后前腔静脉采血后屠宰取样,血液样品静置1 h后于4 ℃、3 000 r/min条件下离心10 min分离血清,将血清收集于1.5 mL EP管后-20 ℃保存以供后续检测。采集肝脏样品,用于肝脏脂肪酸组成和脂肪代谢相关基因表达的测定。
1.3 指标测定及其方法 1.3.1 生长性能试验猪分别于试验开始和结束当天08:00—09:00空腹称重,试验期间记录采食量,用于计算平均日增重(average daily gain,ADG)、平均日采食量(average daily feed intake,ADFI)和料重比(feed/gain,F/G)。
1.3.2 血清脂肪代谢相关生化指标使用全自动血清生化仪(Beckman CX4)检测血清中甘油三酯(triglyceride,TG)、胆固醇(cholesterol,CHOL)、高密度脂蛋白(high density lipoprotein,HDL)和低密度脂蛋白(low density lipoprotein,LDL)的含量。
1.3.3 肝脏脂肪酸组成称取肝脏冻干样品0.3 g左右,经过苯-石油醚(1 ∶ 1,体积比)提取后,经氢氧化钾-甲醇溶液甲酯化30 min,加水分层,取500 mL上层液通过外标-气相色谱-质谱法检测肝脏组织中长链脂肪酸含量,具体操作参照Hu等[6]所述方法进行。各类脂肪酸含量表示为占总脂肪酸的百分比;基于脂肪酸组成计算:多不饱和脂肪酸(polyunsaturated fatty acids,PUFA)/饱和脂肪酸(saturated fatty acids,SFA)比值、n-6/n-3 PUFA比值。
1.3.4 肝脏脂肪代谢相关基因mRNA表达量采用Trizol试剂盒(Invitrogen公司,美国)从肝脏组织中提取总RNA,经紫外可见光光度计(NanoDrop ND-1000, Thermo Fisher, 美国)检测RNA质量和浓度。取1 000 ng总RNA按照TaKaRa逆转录试剂盒(TaKaRa,RR047A)说明书进行逆转录合成cDNA。采用实时荧光定量PCR试剂盒(TaKaRa,RR820A)检测肝脏中脂肪代谢相关基因的mRNA表达量,以β-肌动蛋白(β-actin)为内参基因,检测乙酰辅酶A羧化酶(acetyl CoA carboxylase,ACC)、激素敏感脂酶(hormone-sensitive triglyceride lipase,HSL)、脂肪酸转运蛋白-1(fatty acid transport protein-1,FATP-1)、脂肪酸移位酶(fatty acid translocase,FAT/CD36)、过氧化物酶增殖体激活受体α(peroxisome proliferator-activated receptor α,PPARα)、肉毒碱棕榈酰基转移酶-1B(carnitine palmitoyl transferase-1B,CPT-1B)、增强子连接蛋白α(CCAAT/enhancer-binding protein α,C/EBPα)、固醇调节元件结合蛋白-1c(sterol regulatory element-binding protein-1c,SREBP-1c)的mRNA表达量,引物序列见表 2。具体测定参照Duan等[3]的方法进行。
|
|
表 2 实时荧光定量PCR引物序列 Table 2 Primer sequences used for real-time quantitative PCR |
试验数据用Excel 2010进行初步处理,用SAS 8.2软件进行单因素方差分析(one-way ANOVA),并用Duncan氏法进行多重比较检验,以P < 0.05作为差异显著性判断标准,以P < 0.01作为差异极显著性判断标准。
2 结果 2.1 饲粮中添加HMB对巴马香猪生长性能的影响由表 3可知,与对照组相比,饲粮中添加0.13%HMB显著增加了巴马香猪的末重和ADG(P < 0.05);但饲粮中添加HMB对巴马香猪的ADFI和F/G无显著影响(P>0.05)。
|
|
表 3 饲粮中添加HMB对巴马香猪生长性能的影响 Table 3 Effects of dietary supplementation of HMB on growth performance of Bama Xiang mini-pigs |
由表 4可知,与对照组相比,饲粮中添加0.13%HMB显著增加了血清中HDL含量(P < 0.05);饲粮中添加1.28%HMB显著增加了血清中CHOL含量(P < 0.05),显著减少了血清中HDL含量(P < 0.05)。此外,血清中LDL含量有随HMB添加量增加而提高的趋势(P=0.08),但饲粮中添加HMB对血清中TG含量无显著影响(P>0.05)。
|
|
表 4 饲粮中添加HMB对巴马香猪血清脂肪代谢相关生化指标的影响 Table 4 Effects of dietary supplementation of HMB on serum lipid metabolism-related biochemical indexes of Bama Xiang mini-pigs |
由表 5可知,与对照组相比,饲粮中添加0.13%HMB显著增加了肝脏中n-3 PUFA(33.33%)、二十二烷酸(C22 ∶ 0,42.31%)、二十二碳六烯酸(C22 ∶ 6n-3,33.33%)含量(P < 0.05),显著降低了n-6/n-3 PUFA比值(28.31%)和二十碳烯酸(C20 ∶ 1,20.00%)、花生四烯酸(C20 ∶ 4n-6,12.99%)含量(P < 0.05);饲粮中添加0.64%HMB显著增加了肝脏中二十碳烯酸(C20 ∶ 1,22.85%)、二十二烷酸(C22 ∶ 0,26.92%)、二十碳三烯酸(C20 ∶ 3n-6,50%)含量(P < 0.05),显著降低了花生四烯酸(C20 ∶ 4n-6,14.76%)含量(P < 0.05);饲粮中添加1.28%HMB显著增加了二十二烷酸(C22 ∶ 0,38.46%)和二十碳三烯酸(C20 ∶ 3n-6,67.64%)含量(P < 0.05),显著降低了十七烷酸(C17 ∶ 0,31.88%)和花生四烯酸(C20 ∶ 4n-6,12.99%)含量(P < 0.05)。
|
|
表 5 饲粮中添加HMB对巴马香猪肝脏脂肪酸组成的影响(占总脂肪酸的百分比) Table 5 Effects of dietary supplementation of HMB on fatty acid composition in liver of Bama Xiang mini-pigs (percentage of total fatty acids) |
如图 1所示,与对照组相比,饲粮中添加0.13%HMB显著提高了肝脏中FAT/CD36和PPARα的mRNA表达量(P < 0.05);饲粮中添加0.64%HMB显著降低了肝脏中HSL的mRNA表达量(P < 0.05);饲粮中添加1.28%HMB显著降低了肝脏中HSL和FATP1的mRNA表达量(P < 0.05)。
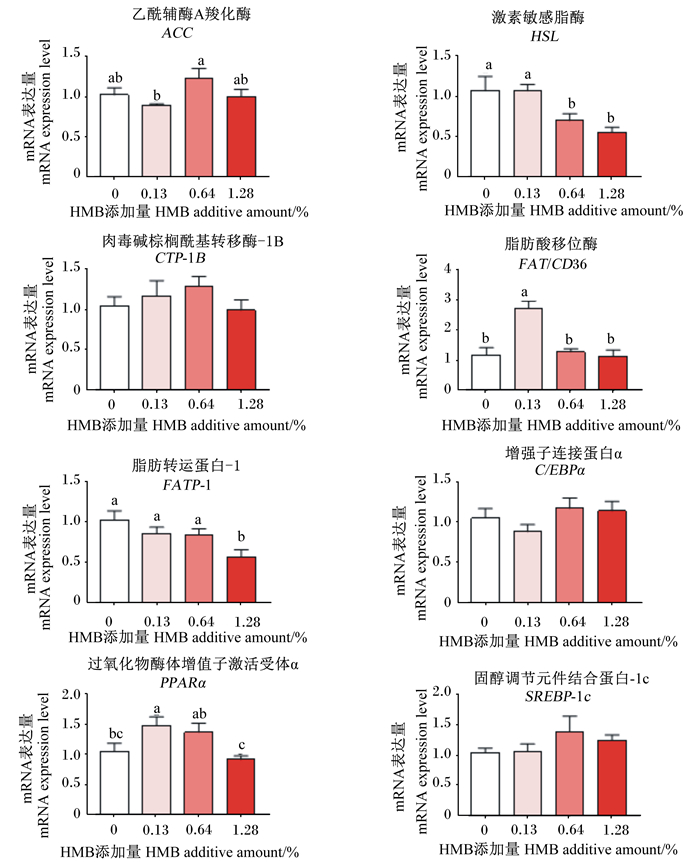
|
数据柱无字母或标注相同字母表示差异不显著(P>0.05),标注不同字母表示差异显著(P < 0.05)。 Values columns with no letter or the same letter indicated no significant difference (P>0.05), while with different letters indicated significant difference (P < 0.05). 图 1 饲粮中添加HMB对巴马香猪肝脏脂肪代谢相关基因mRNA表达量的影响 Fig. 1 Effects of dietary supplementation of HMB on mRNA expression levels of lipid metabolism-related genes in liver of Bama Xiang mini-pigs |
添加剂应用的前提条件是确保其安全性,大量研究表明HMB的使用是安全的,并且具有改善机体健康状况的潜力。据报道,动物连续16周摄入8~5 000 mg/(kg·d) HMB未见不良影响[7-8]。本试验结果显示,给巴马香猪分别饲喂含有0.13%、0.64%和1.28% HMB的饲粮60 d,对生长性能无任何负面影响,且适宜添加量的HMB(0.13%)还可改善生长性能,与上述试验结果一致。
3.2 饲粮中添加HMB对巴马香猪血清脂肪代谢相关生化指标的影响血清TG、CHOL、HDL和LDL含量是反映机体血脂肪代谢的常用指标。HDL和LDL是CHOL的运输载体,HDL可将CHOL运输至肝脏使其代谢生成其他物质,具有清除血浆多余CHOL的作用,LDL则负责将肝脏中CHOL运输至肝外组织,被认为是心血管疾病的制动剂,两者共同维持机体CHOL的稳态[9-10]。血清中高含量的TG和CHOL是心血管疾病的重要风险因素[11-12]。前期研究表明,HMB(1%,质量体积分数)可改善高脂诱导肥胖小鼠的血脂代谢,降低血清中TG和CHOL含量[13]。在长大二元杂猪上的研究显示,饲粮中添加0.62%HMB显著降低了二元杂猪血清LDL含量[14]。本试验结果显示,在饲粮中添加0.13%HMB显著提高了巴马香猪血清中HDL含量,表明饲粮中添加适量HMB后改善了巴马香猪的血脂代谢。此外,本试验结果还显示饲粮中添加1.28%HMB降低了巴马香猪血清中HDL含量,升高了CHOL含量,推测饲粮中添加高水平的HMB可通过在体内代谢生成羟甲基戊二酸单酰辅酶A(合成CHOL的前体物质)而增加血清中CHOL含量[15-16]。这与我们前期的试验结果[13]一致,前期试验结果显示高水平HMB处理对高脂诱导肥胖小鼠脂肪代谢并无改善效果。综上可知,饲粮中添加HMB可改善巴马香猪的血脂代谢,其适宜添加量为0.13%,添加高水平HMB并未取得叠加的改善效果;此外,可能存在低于0.13%HMB的更适宜添加量,还需进一步的试验研究。
3.3 饲粮中添加HMB对巴马香猪肝脏脂肪酸组成的影响脂肪酸可分为SFA、单不饱和脂肪酸(monounsaturated fatty acids,MUFA)和PUFA,根据首个双键距离分子末端甲基位置的不同又可将PUFA分为n-3和n-6 PUFA[17]。n-3 PUFA具有调节机体血脂、抗炎等生理功能,而n-6 PUFA的生理功能则与之相反,具有促炎作用。n-6/n-3 PUFA比值低的膳食被认为可预防心血管疾病、癌症等疾病[18]。因此,通过饲粮途径增加猪肉产品中n-3 PUFA含量并降低n-6 PUFA含量被认为是改善猪肉品质的一种有效有段[19]。值得注意的是,机体n-3 PUFA含量并非越高越好,过量的n-3 PUFA会导致猪肉过氧化、生物膜受到损伤等问题。因此,提高猪肉产品中n-3 PUFA含量的同时平衡其n-6/n-3 PUFA比值,对生产出符合人类健康需求的肉制品就显得非常重要[18]。本试验结果表明,饲粮中添加0.13%HMB显著提高了肝脏中C22 ∶ 6n-3含量,并降低了花生四烯酸含量和n-6/n-3 PUFA比值,这可能是由于饲粮中添加0.13% HMB促进了巴马香猪对营养物质吸收并促进了肝脏从血液中摄取n-3 PUFA。C22 ∶ 6n-3是一种n-3 PUFA,可提高血清中HDL含量,降低LDL、TG和CHOL含量,从而降低血液黏度,改善血液循环,发挥降脂、抗炎和预防心血管疾病等作用[19-20]。我们推测,饲粮中添加HMB可通过提高巴马香猪肝脏中C22 ∶ 3n-3的含量来增加血清中HDL的含量,从而减少机体CHOL的含量,进而改善机体脂肪代谢。此外,在禁食情况下,机体会动员肌肉和脂肪组织中的TG,运输至肝脏分解为脂肪酸为机体供能[21],因此,肝脏中脂肪酸的组成情况可在一定程度上反映猪肉中脂肪酸的组成情况。综上可知,饲粮中添加HMB可通过提高n-3 PUFA含量和降低n-6/n-3 PUFA比值来改善猪肉品质,且其添加量为0.13%时效果最佳。此外,可能存在低于0.13%HMB的更适宜添加量,还需进一步的试验研究。
3.4 饲粮中添加HMB对巴马香猪肝脏脂肪代谢相关基因mRNA表达量的影响肝脏是猪脂肪代谢的主要场所之一,ACC、HSL、FAT/CD36、FATP1、PPARα等是参与脂肪代谢的关键酶。其中,ACC和HSL分别是脂肪酸合成与分解的关键限速酶[22-23];FAT/CD36是将脂肪酸转运至胞内的转运载体[24],其表达量升高暗示肝脏从血液中摄取的脂肪酸增多;FATP1是一种对胰岛素敏感的长链脂肪酸转运载体[25],其表达量降低说明转运入肝脏中的脂肪酸减少;转录因子PPARα是肝脏脂质代谢的主要调节因子,其激活可促进肝脏脂肪酸的摄取、利用和分解代谢[26],亦可促进HDL和C22 ∶ 6n-3的生成[27-28]。本试验结果显示,饲粮中添加0.13%HMB可增加巴马香猪肝脏中FAT/CD36和PPARα的mRNA表达量,而添加1.28%HMB则能降低肝脏中HSL和FATP1的mRNA表达量。因此,本试验结果表明,饲粮中添加0.13%HMB可促进肝脏从血液中摄取脂肪酸,通过PPARα促进C22 ∶ 6n-3的生成,进而提高血液中HDL含量,改善机体脂肪代谢,而添加1.28%HMB则促进了肝脏脂肪酸合成并抑制了脂肪酸分解。
4 结论① 在巴马香猪饲粮中添加HMB可改善其生长性能,并可提高肝脏中C22 ∶ 6n-3含量,改善肝脏脂肪代谢,其适宜添加量为0.13%。
② HMB改善巴马香猪肝脏脂肪代谢的机制可能与PPARα信号通路相关。
| [1] |
宋明彤, 祝倩, 马文强, 等. 饲粮添加甜菜碱对巴马香猪血常规指标和器官生长的影响[J]. 动物营养学报, 2020, 32(4): 1908-1915. SONG M T, ZHU Q, MA W Q, et al. Effects of dietary betaine addition on blood routine indexes and organ growth of Bama mini-pigs[J]. Chinese Journal of Animal Nutrition, 2020, 32(4): 1908-1915 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.04.051 |
| [2] |
MOLFINO A, GIOIA G, ROSSI FANELLI F, et al. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials[J]. Amino Acids, 2013, 45(6): 1273-1292. DOI:10.1007/s00726-013-1592-z |
| [3] |
DUAN Y H, DUAN Y M, LI F N, et al. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs[J]. Amino Acids, 2016, 48(9): 2131-2144. DOI:10.1007/s00726-016-2223-2 |
| [4] |
KORNASIO R, RIEDERER I, BUTLER-BROWNE G, et al. β-hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways[J]. Biochimica et Biophysica Acta: Molecular Cell Research, 2009, 1793(5): 755-763. DOI:10.1016/j.bbamcr.2008.12.017 |
| [5] |
DUAN Y H, ZHANG L Y, LI F N, et al. Beta-hydroxy-beta-methylbutyrate modulates lipid metabolism in adipose tissues of growing pigs[J]. Food & Function, 2018, 9(9): 4836-4846. |
| [6] |
HU C J, JIANG Q Y, ZHANG T, et al. Dietary supplementation with arginine and glutamic acid enhances key lipogenic gene expression in growing pigs[J]. Journal of Animal Science, 2017, 95(12): 5507-5515. DOI:10.2527/jas2017.1703 |
| [7] |
HOFFMAN R, COOPER J, WENDELL M, et al. Effects of beta-hydroxy beta-methylbutyrate on power performance and indices of muscle damage and stress during high-intensity training[J]. Journal of Strength and Conditioning Research, 2004, 18(4): 747-752. |
| [8] |
FIOROTTO M L, SCHWARTZ R J, DELAUGHTER M C, et al. Persistent IGF-Ⅰ overexpression in skeletal muscle transiently enhances DNA accretion and growth[J]. FABSE Journal, 2003, 17(1): 59-60. |
| [9] |
丁浩, 黄攀, 章文明, 等. 饲粮添加枯草芽孢杆菌对保育猪生长性能和血浆生化参数的影响[J]. 动物营养学报, 2020, 32(2): 605-612. DING H, HUANG P, ZHANG W M, et al. Effects of dietary Bacillus subtilis on growth performance and plasma biochemical parameters of nursery piglets[J]. Chinese Journal of Animal Nutrition, 2020, 32(2): 605-612 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.02.016 |
| [10] |
SHARMAN M J, FERNANDEZ M L, ZERN T L, et al. Replacing dietary carbohydrate with protein and fat decreases the concentrations of small LDL and the inflammatory response induced by atherogenic diets in the guinea pig[J]. The Journal of Nutritional Biochemistry, 2008, 19(11): 732-738. DOI:10.1016/j.jnutbio.2007.09.008 |
| [11] |
ZERN T L, WEST K L, FERNANDEZ M L. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs[J]. The Journal of Nutrition, 2003, 133(7): 2268. DOI:10.1093/jn/133.7.2268 |
| [12] |
RAMJIGANESH T, ROY S, FREAKE H C, et al. Corn fiber oil lowers plasma cholesterol by altering hepatic cholesterol metabolism and up-regulating LDL receptors in guinea pigs[J]. Journal of Nutrition, 2002, 132(3): 335-340. DOI:10.1093/jn/132.3.335 |
| [13] |
DUAN Y H, ZHONG Y Z, XIAO H, et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets[J]. FASEB Journal, 2019, 33(9): 10019-10033. DOI:10.1096/fj.201900665RR |
| [14] |
ZHONG Y Z, SONG B, ZHENG C B, et al. α-ketoisocaproate and β-hydroxy-β-methyl butyrate regulate fatty acid composition and lipid metabolism in skeletal muscle of growing pigs[J]. Journal of Animal Physiology and Animal Nutrition, 2019, 103(3): 846-857. DOI:10.1111/jpn.13077 |
| [15] |
HOLECEK M, MUTHNY T, KOVARIK M, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues[J]. Food and Chemical Toxicology, 2009, 47(1): 255-259. DOI:10.1016/j.fct.2008.11.021 |
| [16] |
DUAN Y H, LI F N, LI Y H, et al. The role of leucine and its metabolites in protein and energy metabolism[J]. Amino Acids, 2016, 48(1): 41-51. DOI:10.1007/s00726-015-2067-1 |
| [17] |
ROSSI R, PASTORELLI G, CANNATA S, et al. Recent advances in the use of fatty acids as supplements in pig diets: a review[J]. Animal Feed Science and Technology, 2010, 162(1/2): 1-11. |
| [18] |
段叶辉, 李凤娜, 李丽立, 等. n-6/n-3多不饱和脂肪酸比例对机体生理功能的调节[J]. 天然产物研究与开发, 2014, 26(4): 626-631. DUAN Y H, LI F N, LI L L, et al. The regulation of n-6/n-3 polyunsaturated fatty acid ratio in physiological function of the body[J]. Natural Product Research and Development, 2014, 26(4): 626-631 (in Chinese). |
| [19] |
段叶辉, 印遇龙, 李丽立, 等. n-3 PUFAs对脂质代谢和炎症-免疫的调节机制[J]. 生物技术通报, 2012(3): 28-34. DUAN Y H, YIN Y L, LI L L, et al. Review on the regulatory mechanisms of n-3 PUFAs on lipid metabolism and inflammation-immunity[J]. Biotechnology Bulletin, 2012(3): 28-34 (in Chinese). |
| [20] |
PARK Y, HARRIS W S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance[J]. Journal of Lipid Research, 2003, 44(3): 455-463. DOI:10.1194/jlr.M200282-JLR200 |
| [21] |
ALVES-BEZERRA M, COHEN D E. Triglyceride metabolism in the liver[J]. Comprehensive Physiology, 2017, 8(1): 1-8. |
| [22] |
ZIMMERMANN R, STRAUSS J G, HAEMMERLE G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase[J]. Science, 2004, 306(5700): 1383-1386. DOI:10.1126/science.1100747 |
| [23] |
KOONEN D P, GLATZ J F C, BONEN A, et al. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle[J]. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 2005, 1736(3): 163-180. DOI:10.1016/j.bbalip.2005.08.018 |
| [24] |
ZHOU J, ZHAI Y G, MU Y, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway[J]. Journal of Biological Chemistry, 2006, 281(21): 15013-15020. DOI:10.1074/jbc.M511116200 |
| [25] |
WU Q, ORTEGON A M, TSANG B, et al. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity[J]. Molecular and Cellular Biology, 2006, 26(9): 3455-3467. DOI:10.1128/MCB.26.9.3455-3467.2006 |
| [26] |
KERSTEN S. Integrated physiology and systems biology of PPARα[J]. Molecular Metabolism, 2014, 3(4): 354-371. DOI:10.1016/j.molmet.2014.02.002 |
| [27] |
HEO Y R, CLAYCOMBE K, JONES B H, et al. Effects of fatty (FA) allele and high-fat diet on adipose tissue leptin and lipid metabolism[J]. Hormone and Metabolic Research, 2002, 34(11/12): 686-690. DOI:10.1055/s-2002-38264 |
| [28] |
张云, 何秋霞, 侯海荣, 等. 过氧化物酶体增殖物激活受体α的研究进展[J]. 现代生物医学进展, 2013, 13(29): 5798-5800. ZHANG Y, HE Q X, HOU H R, et al. Advances in peroxisome proliferator activated receptor alpha research[J]. Progress in Modern Biomedicine, 2013, 13(29): 5798-5800. |




