高环境温度是影响畜禽生长性能的最重要的气候因素之一[1]。随着全球气候变暖的加剧,高温成为了影响畜牧业生产的主要威胁之一[2]。当生物体遭受高温时,热休克蛋白(heat shock proteins, HSPs)会迅速合成[2]。休热克蛋白按分子质量的大小分为热休克蛋白60(heat shock protein 60,HSP60)、热休克蛋白70(heat shock protein 70,HSP70)、热休克蛋白90(heat shock protein 90,HSP90)等。热休克蛋白在应激细胞的存活和内部环境的稳定中起着重要作用[3]。同时,高温可作为细胞凋亡的诱导因子。细胞凋亡是一个复杂的过程。细胞凋亡反应由2个重要的蛋白质家族半胱氨酸天冬氨酸蛋白酶(Caspase)家族和B淋巴细胞瘤-2(B-cell lymphoma-2,Bcl-2)蛋白家族调控[4]。已有研究报道高温可导致DNA损伤[5-6]和蛋白质错误折叠[7],可引起组织损伤和细胞凋亡[8]。然而关于高温是否会引起3T3-L1前体脂肪细胞的凋亡目前鲜有报道。
脂肪含量对畜禽肉质的感官品质具有十分重要的影响[9]。脂肪沉积过程受到多种转录因子和脂肪分泌因子的级联调控,其中调控脂质代谢的相关基因包括脂肪合成基因过氧化物酶体增殖剂激活受体γ(peroxisome proliferators-activated receptor γ,PPARγ)、脂肪酸合成酶(fatty acid synthase,FAS)、乙酰辅酶A羧化酶(acetyl CoA carboxylase,ACC)、脂肪酸结合蛋白2(fatty acid-binding protein 2,AP2)、CCAAT增强子结合蛋α(CCAAT/enhancer-binding protein α,C/EBPα)等,调控脂肪分解的相关基因包括激素敏感脂酶(hormone-sensitive triglayceride lipase,HSL)、脂肪甘油三酯脂酶(adipose triglyceride lipase,ATGL)等。研究发现35 ℃条件下猪皮下脂肪组织中的脂质含量增加[10]。在高温条件下,肉鸡皮下脂肪细胞中脂肪沉积量显著提高,脂肪合成相关基因的mRNA表达量显著增加[11]。然而,目前尚不清楚高温在体外3T3-L1前体脂肪细胞分化模型中的作用以及对于脂肪合成与分解相关基因的影响。因此,本试验旨在研究高温对3T3-L1前体脂肪细胞凋亡和分化过程中脂肪沉积的影响,以期为改善高温对动物福利及畜禽肉品质的影响奠定理论基础,并为预防由高温引起的肥胖提供潜在策略。
1 材料与方法 1.1 试验材料3T3-L1前体脂肪细胞由广西大学遗传育种实验室提供。
1.2 细胞培养和诱导分化将3T3-L1前体脂肪细胞悬浮于DMEM(Gibco,美国)和10%胎牛血清(FBS,Gibco,美国)中,在37 ℃、5%二氧化碳(CO2)培养箱中孵育,每2 d更换1次培养基,完全融合后,分别在37.0(对照)和41.5 ℃(高温处理)条件下用诱导分化培养基[10%FBS、90%DMEM、1%青霉素/链霉素(Sigma-Aldrich,美国)、1 μmol/L地塞米松(Sigma-Aldrich,美国)、10 μg/mL胰岛素(Gibco,美国)和0.5 mmol/L异丁基甲基黄嘌呤(Sigma-Aldrich,美国)]培养细胞,2 d后用10%FBS、90%DMEM和10 μg/mL胰岛素替代分化培养基,继续培养至第6天。
1.3 油红O染色诱导分化培养6 d后,吸弃培养基,用磷酸盐缓冲液(PBS)清洗细胞3次后,用10%的福尔马林溶液固定细胞10 min,吸弃固定液,用PBS漂洗细胞3次,加入稀释好的油红O溶液(北京索莱宝科技有限公司),染色20 min后吸弃染料,最后用PBS将多余染液清洗干净,置于倒置显微镜下观察并拍照。
1.4 总RNA提取及cDNA合成使用Trizol法提取3T3-L1前体脂肪细胞中的总RNA,检测总RNA的浓度和纯度后,按照反转录试剂盒(TaKaRa,大连)说明书反转录成cDNA。反转录体系为:gDNA Eraser 1 μL、5×gDNA Eraser Buffer 2 μL、总RNA 1 μg,RNase Free H2O补足体系至10 μL,得到Mix1溶液,42 ℃保温2 min。在Mix1溶液中加入PrimeScript RT Enzyme Mix Ⅰ 1 μL、5×PrimeScript Buffer Ⅱ 4 μL、RT Primer Mix 1 μL,RNase Free H2O补足体系至20 μL,37 ℃ 15 min,85 ℃ 5 s,获得cDNA,4 ℃保存。
1.5 实时荧光定量PCR(qRT-PCR)以cDNA为模板进行qRT-PCR,反应体系为:TB GreenTM Premix Ex TaqTM Ⅱ(TaKaRa,大连)5 μL、上游引物0.25 μL、下游引物0.25 μL、cDNA 2.5 μL、RNase Free H2O 2 μL。反应程序为:95 ℃预变性30 s,95 ℃变性5 s,60 ℃延伸30 s,45个循环,65 ℃延伸5 s,95 ℃再延伸5 min。以18S rRNA为内参,依据2-△△Ct方法计算目的基因的mRNA表达量。每个样品重复3次。本试验用到的实时荧光定量PCR引物见表 1。
|
|
表 1 实时荧光定量PCR引物 Table 1 qRT-PCR primers |
试验结果以平均值±标准误(mean±SE)表示,并采用SPSS 20.0软件进行单因素方差分析(one-way ANOVA)和LSD法多重比较,P < 0.05表示差异显著,P < 0.01和P < 0.001表示差异极显著。
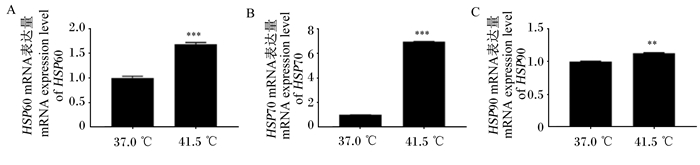
2 结果与分析 2.1 高温对3T3-L1前体脂肪细胞分化过程中热休克蛋白家族基因表达的影响本试验检测了热休克蛋白家族中具有高度保守性的3个基因HSP60、HSP70、HSP90的mRNA表达量。由图 1可知,经高温处理6 d后,HSP60的mRNA表达量是对照组的1.69倍(P < 0.001);HSP70的mRNA表达量是对照组的6.95倍(P < 0.001);HSP90的mRNA表达量是对照组的1.13倍(P < 0.01)。结果表明,3T3-L1前体脂肪细胞经高温处理后,通过高度诱导热休克蛋白家族基因的表达来起到自身保护的作用。
|
“* *”表示差异极显著(P < 0.01),“* * *”表示差异极显著(P < 0.001)。图 3、图 5和图 6同。 "* *" mean extremely significant difference (P < 0.01), "* * *" mean extremely significant difference (P < 0.001). The same as Fig. 3, Fig. 5 and Fig. 6. 图 1 高温处理6 d后热休克蛋白家族基因的mRNA表达量 Fig. 1 mRNA expression levels of heat shock protein family genes after high temperature treatment for 6 days |
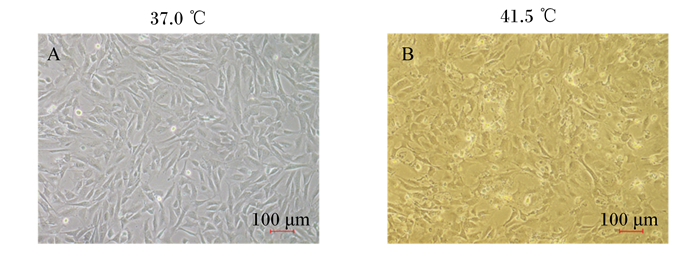
由图 2可知,高温处理4 d后,显微镜下可观察到细胞趋于不规则形态,在没有换液的情况下可见死亡细胞漂浮于培养基中,在表观上说明高温可诱导3T3-L1前体脂肪细胞凋亡。
|
图 2 高温对3T3-L1前体脂肪细胞凋亡的影响 Fig. 2 Effects of high temperature on apoptosis of 3T3-L1 preadipocytes |
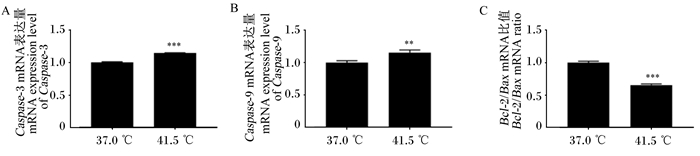
由图 3可知,高温处理4 d后Caspase-3的mRNA表达量是对照组的1.14倍(P < 0.01),Caspase-9的mRNA表达量是对照组的1.13倍(P < 0.01),Bcl-2/Bax mRNA比值较对照组极显著降低(P < 0.001),说明经高温处理后细胞发生凋亡。
|
图 3 高温处理4 d后凋亡相关基因的mRNA表达量 Fig. 3 mRNA expression levels of apoptotic related genes after high temperature treatment for 4 days |
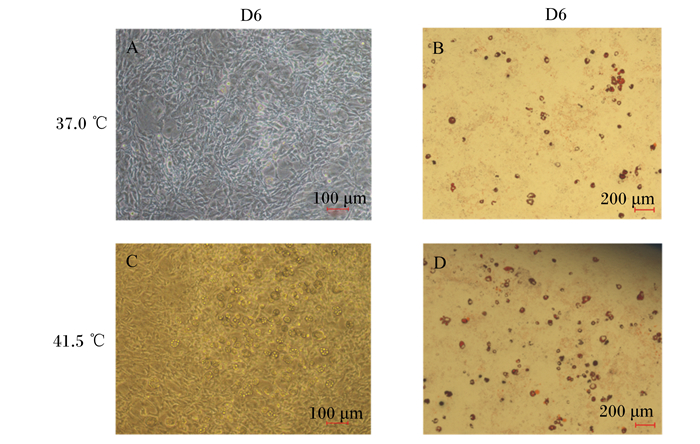
高温处理细胞后,在显微镜下观察高温对细胞诱导分化的情况。由图 4可知,高温诱导分化6 d时,脂滴占据大部分视野,细胞的分化程度高于对照组。油红O染色后可看到细胞经过高温处理后脂滴数量明显增多。
|
D6表示高温处理6 d。图A为在37.0 ℃条件下分化第6天的3T3-L1前体脂肪细胞;图B为在37.0 ℃条件下分化第6天的3T3-L1前体脂肪细胞的油红O染色图;图C为在41.5 ℃条件下分化第6天的3T3-L1前体脂肪细胞;图D为在41.5 ℃条件下分化第6天的3T3-L1前体脂肪细胞的油红O染色图。 D6 denoted high temperature treatment for 6 days. Figure A represented 3T3-L1 preadipocytes on the 6th day of differentiation at 37.0 ℃; figure B represented the oil red O staining image of 3T3-L1 preadipocytes on the 6th day of differentiation at 37℃; figure C represented 3T3-L1 preadipocytes on the 6th day of differentiation at 41.5 ℃; figure D represented the oil red O staining image of 3T3-L1 preadipocytes on the 6th day of differentiation at 41.5 ℃. 图 4 高温对3T3-L1前体脂肪细胞成脂分化的影响 Fig. 4 Effects of high temperature on adipogenic differentiation of 3T3-L1 preadipocytes |
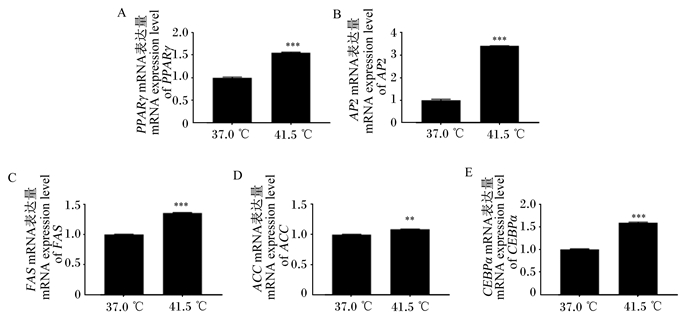
由图 5可知,经高温处理6 d后,PPARγ的mRNA表达量是对照组的1.55倍(P < 0.001);AP2的mRNA表达量是对照组的3.41倍(P < 0.001);FAS的mRNA表达量是对照组的1.36倍(P < 0.001);ACC的mRNA表达量是对照组的1.09倍(P < 0.01);CEBPα的mRNA表达量是对照组的1.60倍(P < 0.001)。上述结果表明,经过高温处理后,3T3-L1前体脂肪细胞中脂肪合成相关基因的表达量极显著上调。
|
图 5 高温处理6 d后脂肪合成相关基因的mRNA表达量 Fig. 5 mRNA expression levels of fat synthesis related genes after 6 days of high temperature treatment |
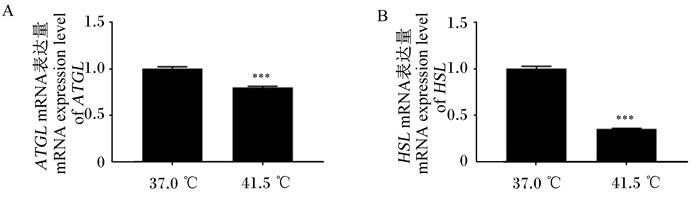
由图 6可知,高温处理6 d后,ATGL和HSL的mRNA表达量均下降,其中ATGL的mRNA表达量较对照组降低了20%(P < 0.001),HSL的mRNA表达量较对照组降低了65%(P < 0.001)。上述结果表明,经过高温处理后,3T3-L1前体脂肪细胞中脂肪分解相关基因的表达量极显著下调。
|
图 6 高温处理6 d后脂肪分解相关基因的mRNA表达量 Fig. 6 mRNA expression levels of fat decomposition related genes after 6 days of high temperature treatment |
高温是畜禽养殖业遇到的最严重的威胁之一。据报道,在热带或亚热带国家,持续高温,特别是夏季高温,是畜禽生产的不利因素。持续高温暴露会降低采食量[20]、生长性能[21]和肉质[22-23]。例如,高温降低了肌内脂肪(IMF)沉积[24],改变了肉的pH[22]。前体脂肪细胞起源于多潜能干细胞,并且尚未积累储存甘油三酯(TG)。脂肪细胞由前体脂肪细胞分化而来,占脂肪组织细胞总数的1/3~2/3,脂肪细胞在脂肪组织的发育中起着决定性的作用,脂肪组织的发育情况决定了机体脂肪的沉积与代谢[25]。因此,在细胞水平上探究高温对细胞凋亡和脂肪沉积与代谢的影响,能够更清楚地了解其发生机理,可为改善高温对畜禽的不利影响以及改善肉品质提供科学理论依据。
细胞以多种方式对高温做出反应,如刺激存活途径或触发受损细胞的死亡,这些应激细胞的初始反应有助于细胞从应激状态中恢复[26]。热休克蛋白的表达是机体对高温的第一反应。热休克蛋白是一种参与应激反应的蛋白质,它保护生物体免受压力的伤害,帮助它们恢复,从而提高细胞存活率[27]。高温会导致猪背最长肌HSP70和HSP90基因的表达量增加[2]。高温条件下肉鸡心脏、肝脏和肾脏中HSP60基因的表达量均上调[28]。本试验对HSP60、HSP70、HSP90 3个基因mRNA表达量的研究结果发现,高温极显著促进3个热休克蛋白的合成。此外,Wang等[29]在HepG2细胞上的研究同样显示出高温对热休克蛋白合成的促进作用。
Caspase家族参与两大凋亡级联即死亡受体途径和线粒体途径,是细胞凋亡的最终执行者。在线粒体途径中,通过二聚作用激活启动子Caspase-9,活化的Caspase-9蛋白水解激活了刽子手Caspases-3,Caspase-3通常以32 ku的酶原形式存在于细胞质中,在细胞凋亡的早期被激活。线粒体的完整性也受Bcl-2蛋白家族的控制。Bcl-2蛋白家族中促凋亡(Bax)和抗凋亡成员(Bcl-2)之间相互作用共同调控细胞凋亡[30-31]。Bax通过结合Bcl-2抑制细胞凋亡。本试验结果表明,高温导致Caspase-9和Caspase-3的mRNA表达量极显著上调,Bcl-2/Bax mRNA比值极显著下降,说明高温会引起3T3-L1前体脂肪细胞的凋亡。
脂滴是参与脂质储存和动员的关键细胞器[32-33]。几乎所有的细菌和真核细胞都能积累中性脂质,并将其储存在脂滴中。脂滴通常呈球形,含有多种中性脂质,并被单层磷脂和外周蛋白所包围[34]。脂滴参与TG的合成,并且在降解TG方面也有重要作用[35]。同时脂滴可作为前体脂肪细胞分化成熟的标志。Qu等[36]提出,猪皮下脂肪细胞在41.5 ℃温度下会刺激脂滴的形成,并且增加血清中TG含量。本试验通过油红O染色发现,高温能够明显促进脂滴的生成,从表观上证明了高温对3T3-L1前体脂肪细胞成脂分化的促进作用。研究表明,PPARγ在3T3-L1前体脂肪细胞中以低水平表达,在正常细胞分化条件下被显著诱导[13]。C/EBPα作为关键转录因子,可促进FAS和AP2基因的表达,从而促进脂肪细胞分化[37]。在分化末期,参与TG代谢的基因AP2、ACC、FAS的表达量显著增加,导致细胞内的形态发生改变和脂质积累[38]。高温可显著提高肉鸡肝脏中ACC、FAS和腹脂中FAS mRNA的表达量[39]。另外,41.5 ℃可促进猪皮下脂肪细胞PPARγ和C/EBPα的mRNA表达,并且在第6天达到最大值[36]。细胞内的脂解酶包括ATGL和HSL[40-42]。HSL是第1个被发现在哺乳动物脂肪组织中水解TG的酶。第2个被发现的是ATGL,它是催化TG水解的初始步骤,在小鼠和人类的脂肪组织中高表达,定位于脂滴上[43-45]。研究发现,高温降低了猪皮下脂肪组织中ATGL和HSL的mRNA表达量[46]。本试验检测了高温处理6 d后脂肪合成与分解相关基因的mRNA表达量,结果显示,高温处理使得PPARγ、AP2、FAS、C/EBPα和ACC的mRNA表达量被极显著上调,ATGL和HSL的mRNA表达量被极显著下调,说明高温会促进脂肪合成,抑制脂肪分解,最终导致脂质积累。油红O染色的果与此结论相一致。
本试验结果表明,高温促进了热休克蛋白的表达,引起了3T3-L1前体脂肪细胞的凋亡。高温刺激了脂滴的生成,促进了分化过程中参与脂肪生成的多个基因的表达,降低了脂肪分解相关基因的表达。这些结果可为高温期间的3T3-L1前体脂肪细胞反应提供依据,并为改善高温对畜禽肉品质的不良影响奠定理论基础。
4 结论高温会引起3T3-L1前体脂肪细胞的热休克反应,导致细胞凋亡,促进脂肪酸的合成,抑制脂肪酸的分解,增加脂质累积。
| [1] |
RENAUDEAU D, GOURDINE J L, ST-PIERRE N R. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs[J]. Journal of Animal Science, 2011, 89(7): 2220-2230. DOI:10.2527/jas.2010-3329 |
| [2] |
MA X Y, WANG L, SHI Z B, et al. Mechanism of continuous high temperature affecting growth performance, meat quality, and muscle biochemical properties of finishing pigs[J]. Genes & Nutrition, 2019, 14: 23. |
| [3] |
GABAI V L, MERⅡN A B, MOSSER D D, et al. Hsp70 prevents activation of stress kinases.A novel pathway of cellular thermotolerance[J]. Journal of Biological Chemistry, 1997, 272(29): 18033-18037. DOI:10.1074/jbc.272.29.18033 |
| [4] |
MARKSTROM E, SVENSSON E, SHAO R, et al. Survival factors regulating ovarian apoptosis-dependence on follicle differentiation[J]. Reproduction, 2002, 123(1): 23-30. DOI:10.1530/rep.0.1230023 |
| [5] |
PAUL C, MURRAY A A, SPEARS N, et al. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice[J]. Reproduction, 2008, 136(1): 73-84. DOI:10.1530/REP-08-0036 |
| [6] |
BRUSKOV V I, MALAKHOVA L V, MASALIMOV Z K, et al. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA[J]. Nucleic Acids Res, 2002, 30(6): 1354-1363. DOI:10.1093/nar/30.6.1354 |
| [7] |
ROTI J L R. Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events[J]. International Journal of Hyperthermia, 2008, 24(1): 3-15. DOI:10.1080/02656730701769841 |
| [8] |
LI X S, WANG C Y, LI N, et al. Combined effects of elevated temperature and crude oil pollution on oxidative stress and apoptosis in sea cucumber (Apostichopus japonicus, Selenka)[J]. International Journal of Environmental Research and Public Health, 2021, 18(2): 801. DOI:10.3390/ijerph18020801 |
| [9] |
GAO Z M, LIU F H, YIN P, et al. Inhibition of heat-induced apoptosis in rat small intestine and IEC-6 cells through the AKT signaling pathway[J]. BMC Veterinary Research, 2013, 9: 241. DOI:10.1186/1746-6148-9-241 |
| [10] |
LARA L J, ROSTAGNO M H. Impact of heat stress on poultry production[J]. Animals, 2013, 3(2): 356-369. DOI:10.3390/ani3020356 |
| [11] |
罗静娴. EGCG对热应激肉鸡脂肪沉积的影响及作用机制[D]. 硕士学位论文. 荆州: 长江大学, 2019. LUO J X. Effect of EGCG on fat deposition in broilers under heat stress and its mechanis[D]. Master's Thesis. Jingzhou: Yangtze University, 2019. (in Chinese) |
| [12] |
LIU Z, YAO X Y, DU J, et al. Selenium deficiency augments the levels of inflammatory factors and heat shock proteins via the redox regulatory pathway in the skeletal muscles of mice[J]. Biological Trace Element Research, 2018, 182(2): 309-316. DOI:10.1007/s12011-017-1100-5 |
| [13] |
LUMBERA W M L, CRUZ J D, YANG S H, et al. Heat shock protein augmentation of Angelica gigas Nakai root hot water extract on adipogenic differentiation in murine 3T3-L1 preadipocytes[J]. Asian-Australasian Journal of Animal Sciences, 2016, 29(3): 419-427. DOI:10.5713/ajas.15.0677 |
| [14] |
GUPTA J, TIKOO K. Involvement of insulin-induced reversible chromatin remodeling in altering the expression of oxidative stress-responsive genes under hyperglycemia in 3T3-L1 preadipocytes[J]. Gene, 2012, 504(2): 181-191. DOI:10.1016/j.gene.2012.05.027 |
| [15] |
SCHAEFER L, MACAKOVA K, RASLIK I, et al. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction[J]. The American Journal of Pathology, 2002, 160(3): 1181-1191. DOI:10.1016/S0002-9440(10)64937-1 |
| [16] |
WANG F, WEI Z L, SUN X R, et al. Apoptosis inducing factor is involved in stretch-induced apoptosis of myoblast via a caspase-9 independent pathway[J]. Journal of Cellular Biochemistry, 2017, 118(4): 829-838. DOI:10.1002/jcb.25759 |
| [17] |
MEISTER S, SCHUBERT U, NEUBERT K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition[J]. Cancer Research, 2007, 67(4): 1783-1792. DOI:10.1158/0008-5472.CAN-06-2258 |
| [18] |
BALI B, NAGY Z, KOVÁCS K J. Oxygen-glucose deprivation-induced changes in organotypic cultures of the rat hippocampus[J]. Ideggyogyaszati Szemle, 2007, 60(3/4): 140-143. |
| [19] |
崔悦悦. 白藜芦醇通过脂联素信号通路调节猪脂肪沉积的机制研究[D]. 硕士学位论文. 南宁: 广西大学, 2019. CUI Y Y. Study on the mechanism of resveratrol regulating fat deposition through adiponectin signaling pathway in pig[D]. Master's Thesis. Nanning. Guangxi University, 2019. (in Chinese) |
| [20] |
LE BELLEGO L, VAN MILGEN J, NOBLET J. Effect of high temperature and low-protein diets on the performance of growing-finishing pigs[J]. Journal of Animal Science, 80(3): 691-701. DOI:10.2527/2002.803691x |
| [21] |
COLLIN A, VAN MILGEN J, DUBOIS S, et al. Effect of high temperature and feeding level on energy utilization in piglets[J]. Journal of Animal Science, 2001, 79(7): 1849-1857. DOI:10.2527/2001.7971849x |
| [22] |
LEFAUCHEUR L, LE DIVIDICH J, MOUROT J, et al. Influence of environmental temperature on growth, muscle and adipose tissue metabolism, and meat quality in swine[J]. Journal of Animal Science, 1991, 69(7): 2844-2854. DOI:10.2527/1991.6972844x |
| [23] |
WITTE D P, ELLIS M, MCKEITH F K, et al. Effect of dietary lysine level and environmental temperature during the finishing phase on the intramuscular fat content of pork[J]. Journal of Animal Science, 2000, 78(5): 1272-1276. DOI:10.2527/2000.7851272x |
| [24] |
ČANDEK-POTOKAR M, ŽLENDER B, LEFAUCHEUR L, et al. Effects of age and/or weight at slaughter on longissimus dorsi muscle: biochemical traits and sensory quality in pigs[J]. Meat Science, 1998, 48(3/4): 287-300. |
| [25] |
AILHAUD G, GRIMALDI P, NÉGREL R. Cellular and molecular aspects of adipose tissue development[J]. Annual Review of Nutrition, 1992, 12: 207-233. DOI:10.1146/annurev.nu.12.070192.001231 |
| [26] |
FULDA S, GORMAN A M, HORI O, et al. Cellular stress responses: cell survival and cell death[J]. International Journal of Cell Biology, 2010, 2010: 214074. |
| [27] |
CHEN D, TOONE W M, MATA J, et al. Global transcriptional responses of fission yeast to environmental stress[J]. Molecular Biology of the Cell, 2003, 14(1): 214-229. DOI:10.1091/mbc.e02-08-0499 |
| [28] |
YAN J Y, BAO E D, YU J M. Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature[J]. Research in Veterinary Science, 2009, 86(3): 533-538. DOI:10.1016/j.rvsc.2008.09.002 |
| [29] |
WANG T, YU Q J, CHEN J, et al. PP2A mediated AMPK inhibition promotes HSP70 expression in heat shock response[J]. PLoS One, 2010, 5(10): e13096. DOI:10.1371/journal.pone.0013096 |
| [30] |
MAES M E, SCHLAMP C L, NICKELLS R W. BAX to basics: how the BCL2 gene family controls the death of retinal ganglion cells[J]. Progress in Retinal and Eye Research, 2017, 57: 1-25. DOI:10.1016/j.preteyeres.2017.01.002 |
| [31] |
ADAMS J M, CORY S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets[J]. Cell Death & Differentiation, 2018, 25(1): 27-36. |
| [32] |
MEYERS A, WEISKITTEL T M, DALHAIMER P. Lipid droplets: formation to breakdown[J]. Lipids, 2017, 52(6): 465-475. DOI:10.1007/s11745-017-4263-0 |
| [33] |
JARC E, PETAN T. Lipid droplets and the management of cellular stress[J]. The Yale Journal of Biology and Medicine, 2019, 92(3): 435-452. |
| [34] |
ZEHMER J K, HUANG Y G, PENG G, et al. A role for lipid droplets in inter-membrane lipid traffic[J]. Proteomics, 2009, 9(4): 914-921. DOI:10.1002/pmic.200800584 |
| [35] |
BARTZ R, ZEHMER J K, ZHU M F, et al. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation[J]. Journal of Proteome Research, 2007, 6(8): 3256-3265. DOI:10.1021/pr070158j |
| [36] |
QU H, DONKIN S S, AJUWON K M. Heat stress enhances adipogenic differentiation of subcutaneous fat depot-derived porcine stromovascular cells[J]. Journal of Animal Science, 2015, 93(8): 3832-3842. DOI:10.2527/jas.2015-9074 |
| [37] |
LIU Q Q, YUAN B B, LO K A, et al. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(36): 14568-14573. DOI:10.1073/pnas.1211611109 |
| [38] |
PAULAUSKIS J D, SUL H S. Cloning and expression of mouse fatty acid synthase and other specific mRNAs.Developmental and hormonal regulation in 3T3-L1 cells[J]. Journal of Biological Chemistry, 1988, 263(15): 7049-7054. DOI:10.1016/S0021-9258(18)68602-8 |
| [39] |
刘梅. 急性热应激对肉仔鸡生长性能及脂肪代谢的影响[J]. 动物营养学报, 2011, 23(5): 862-868. LIU M. Effects of acute heat stress on growth performance and lipid metabolism of broilers[J]. Chinese Journal of Animal Nutrition, 2011, 23(5): 862-868 (in Chinese). DOI:10.3969/j.issn.1006-267x.2011.05.022 |
| [40] |
TOWNSEND K L, TSENG Y H. Brown fat fuel utilization and thermogenesis[J]. Trends in Endocrinology & Metabolism, 2014, 25(4): 168-177. |
| [41] |
LAN Y L, LOU J C, LYU W, et al. Update on the synergistic effect of HSL and insulin in the treatment of metabolic disorders[J]. Therapeutic Advances in Endocrinology and Metabolism, 2019, 10. DOI:10.1177/2042018819877300 |
| [42] |
LI Z, YANG P, LIANG Y, et al. Effects of liraglutide on lipolysis and the AC3/PKA/HSL pathway[J]. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 2019, 12: 1697-1703. DOI:10.2147/DMSO.S216455 |
| [43] |
SCHREIBER R, XIE H, SCHWEIGER M. Of mice and men: the physiological role of adipose triglyceride lipase (ATGL)[J]. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 2019, 1864(6): 880-899. DOI:10.1016/j.bbalip.2018.10.008 |
| [44] |
SATHYANARAYAN A, MASHEK M T, MASHEK D G. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism[J]. Cell Reports, 2017, 19(1): 1-9. DOI:10.1016/j.celrep.2017.03.026 |
| [45] |
LEE F T, MOUNTAIN A J, KELLY M P, et al. Enhanced efficacy of radioimmunotherapy with 90Y-CHX-A"-DTPA-hu3S193 by inhibition of epidermal growth factor receptor (EGFR) signaling with EGFR tyrosine kinase inhibitor AG1478[J]. Clinical Cancer Research, 2005, 11(19 Pt 2): 7080S-7086S. |
| [46] |
FERNANDEZ M V S, JOHNSON J S, ABUAJAMIEH M, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs[J]. Physiological Reports, 2015, 3(2): e12315. DOI:10.14814/phy2.12315 |




