2. 西南大学动物科学技术学院, 重庆 402460
2. College of Animal Science and Technology, Southwest University, Chongqing 402460, China
动物胃肠道中寄居着数量庞大的微生物菌群,包括细菌、真菌、病毒和原虫,超过机体自身细胞总数,如人体肠道中菌群总数是人体细胞总数的10倍以上,达1014个细菌[1]。肠道菌群通过肠神经、免疫系统、肠内分泌信号等途径,调控机体能量代谢、消化代谢、内分泌、免疫系统及机体稳衡等多方面,被喻为人体新的“功能器官”[2-4]。近年来,随肠道菌群的研究不断深入,肠道菌群与宿主之间存在互作关系,形成肠道菌群参与的脑-肠轴[5]、肠-肺轴[6]、肠-脑-内分泌轴[7-8]、肠-肝轴[9-10]和肝-脑-肠轴[11]等多种等维度间的交互网络[12]。胆汁酸(bile acid,BA)作为机体肠道内重要信号分子之一,由肝脏产生经肠道菌群修饰后在回肠重吸收进入肝脏,作为链接菌群-肠-肝轴的关键介质,参与肠道菌群与宿主的对话过程[2]。胆汁酸及其代谢物与肠道微生物的区系与功能密切相关。研究发现,肠道菌群对胆汁酸进行去共轭化、脱羟基化和差向异构化等生物学修饰,进而影响胆汁酸的合成与代谢[13-14]。同时,肠道菌群也可通过对胆汁酸合成途径中关键酶调节影响胆汁酸合成,如细胞色素P450家族成员甾醇27-羟化酶(recombinant cytochrome P450 27A1,CYP27A1)、胆固醇7α-羟化酶(recombinant cytochrome P450 7A1,CYP7A1)和胆固醇12α-羟化酶(recombinant cytochrome P450 8B1,CYP8B1)等[15]。胆汁酸还在宿主肠免疫中发挥作用,肠内皮细胞、免疫细胞(包括单核细胞、巨噬细胞和树突状细胞等)、肠上皮细胞内均有胆汁酸的相应受体参与肠黏膜免疫调节,对维持肠道先天免疫具有重要作用[16-17]。然而,复杂的体内微环境是制约肠-肝对话机制深入研究的关键瓶颈,肠道菌群与宿主肝脏互作机制研究仍不清楚。本文分别从胆汁酸的菌群生物学修饰、菌群胆汁酸代谢产物功能和次级胆汁酸对肠道黏膜免疫的影响等方面进行探讨,对菌群-胆汁酸-肠道黏膜免疫轴间的复杂关系网络进行最新文献综述,并解析其复杂关系作用网络。
1 菌群介导胆汁酸生物修饰过程初级胆汁酸在多达17种酶催化下由胆固醇合成,包括胆酸(cholic acid,CA)、鹅去氧胆酸(chenodeoxycholic acid,CDCA)和鼠胆酸(muricholic acid,MCA)以及与牛磺酸和甘氨酸形成的共轭胆汁酸[18-19]。初级胆汁酸进入肠腔后先后经去共轭化、脱羟基化、差向异构化、酯化及脱硫反应等生物学过程被肠道菌群修饰为功能性的次级胆汁酸[2]。胆汁酸肠道菌群生物转化过程中,肠道菌群参与到不同的代谢过程并形成复杂的调控网络,具体参考图 1。胆汁酸生物修饰过程包括去共轭作用、脱羟基化作用、差向异构化作用及其他作用。
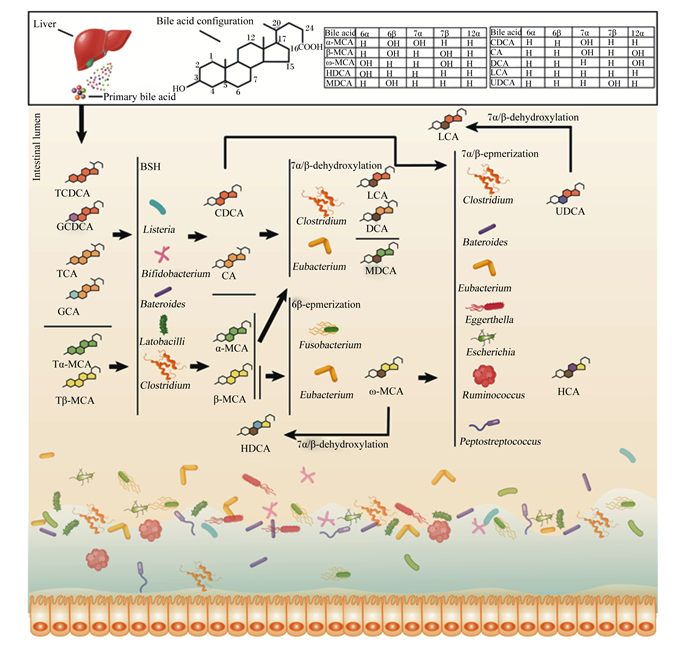
|
TCDCA:牛磺鹅去氧胆酸tauro-chenodeoxycholic acid;GCDCA:甘氨鹅去氧胆酸glyco-chenodeoxycholic acid;TCA:牛磺胆酸tauro-cholic acid;GCA:甘氨胆酸glyco-cholic acid;Tα-MCA:牛磺-α-鼠胆酸tauro-α-muricholic acid;Tβ-MCA:牛磺-β-鼠胆酸tauro-β-muricholic acid;CDCA:鹅去氧胆酸chenodeoxycholic acid;CA:胆酸cholic acid;α-MCA:α-鼠胆酸α-muricholic acid;β-MCA:β-鼠胆酸β-muricholic acid;LCA:石胆酸lithocholic acid;DCA:去氧胆酸deoxycholic acid:MDCA;鼠去氧胆酸murideoxycholic acid;ω-MCA:ω-鼠胆酸ω-muricholic acid;HDCA:猪去氧胆酸hyodeoxycholic acid;HCA:猪胆酸hyocholic acid;UDCA:熊去氧胆酸ursodeoxycholic acid;Bile acid:胆汁酸;Primary bile acid:初级胆汁酸;bile acid configuration:胆汁酸结构;Intestinal lumen:肠腔;Liver:肝脏;BSH:胆盐水解酶bile salt hydrolase;7α/β-dehydroxylation:7α/β-脱羟基化;6β-epmerization:6β-差向异构化;7α/β-epmerization:7α/β-差向异构化;Listeria:李斯特菌属;Bifidobacterium:双歧杆菌属;Bateroides:拟杆菌属;Latobacilli:乳杆菌属;Clostridium:梭菌属;Eubacterium:真杆菌属;Fusobacterium:梭杆菌属;Eggerthella:埃格特菌属;Escherichia:埃希氏菌属;Ruminococcus:瘤胃球菌属;Peptostreptococcus:消化链球菌属。 图 1 肠道菌群介导的胆汁酸生物转化调控网络 Fig. 1 Intestinal microbiota-mediated bile acid bioconversion complex network |
首先是去共轭作用。胆汁酸去共轭化是菌群修饰的起始过程并扮演关键作用,由菌群分泌的胆盐水解酶(bile salt hydrolase,BSH)在C24酰胺键去共轭反应,移除结合型胆汁酸中的牛磺酸和甘氨酸变成游离胆汁酸[20]。具有分泌BSH的肠道菌群属种包括拟杆菌属(Bacteroides)、乳酸杆菌属(Lactobacillus)、双歧杆菌属(Bifidobacterium)、李斯特菌属(Listeria)和布劳特氏菌属(Blautia)等[21-24],详细见表 1。BSH可以将牛磺胆酸(TCA)或甘氨胆酸(GCA)、牛磺鹅去氧胆酸(TCDCA)或甘氨鹅去氧胆酸(GCDCA)、牛磺-α-鼠胆酸(Tα-MCA)或牛磺-β-鼠胆酸(Tβ-MCA)通过去共轭作用形成CA、CDCA和α/β-MCA等游离胆汁酸,调控菌群结构,增加胆汁酸重吸收[25]。最新研究发现,肠道核心菌群分泌的BSH通过水解TCA而抵抗霍乱弧菌(Vibrio cholerae)毒力基因表达,降低腹泻病原体在肠道的定植[26]。
|
|
表 1 具有分泌BSH功能的肠道菌群 Table 1 Gut microbiota with BSH secretion function |
其次是脱羟基化作用。去共轭胆汁酸(如CDCA和CA)在肠道菌群产生的7α-脱羟基酶(7α-dehydroxylase)的作用下发生脱羟基反应。参与7α-脱羟基化的肠道菌群主要是梭菌属,包括平氏梭菌(C. hiranonis)、梭状芽胞杆菌(C. hylemonae)、索氏梭菌(C. sordelli)和闪烁梭菌(C. scindens)等,其可将CA和CDCA分别转换为去氧胆酸(deoxycholic acid,DCA)和石胆酸(lithocholic acid,LCA)[41-42]。而α-MCA、β-MCA则在(3α-,7α-,12α)-脱羟基酶作用下分别转化为鼠脱氧胆酸(murideoxycholic acid,MDCA)和猪去氧胆酸(hyodeoxycholic acid,HDCA)[42]。迟缓埃格特菌属(Eggerthella lenta)、瘤胃球菌属(Ruminococcus gnavus)和毛螺菌属(Lachnospiraceae)2_1_58FAA可将CA、CDCA和DCA分别转换为异胆酸(isoCA)、异鹅去氧胆酸(iso-CDCA)和异去氧胆酸(isoDCA),进而降低对宿主的毒性[13]。
随后是差向异构化作用。CDCA在7α/β-差向异构化作用下生成熊去氧胆酸(ursodeoxycholic acid,UDCA)[43],UDCA通过7β-脱羟基酶转化为LCA。而β-MCA经过6β-差向异构化转化为ω-鼠胆酸(muricholic acid ω,ω-MCA),再通过7β-脱羟基作用产生HDCA,或通过7β-差向异构化形成猪胆酸(hyocholic acid,HCA)[41]。梭菌和真杆菌通过胆汁酸诱导基因(bile acid-induced gene,bai)参与7α/β-脱羟基化作用。目前,胆汁酸诱导操纵子(bile-acid-induced operon,BaiO)已在C. scindens、C. hylemonase、C. hiranonis和C. sordellii中被定位到,其中胆汁酸诱导因子E(bile-acid-induced E,baiE)基因编码胆汁酸7α-脱羟基酶活性,而中胆汁酸诱导子因子I(bile-acid-induced I,baiI)基因编码胆汁酸7β-脱羟基酶活性[44]。拟杆菌属、真杆菌属(Eubacterium)、梭菌属、埃格特菌属(Escherichia)、迟缓埃格特菌属、消化链球菌属(Peptostreptococcus)和瘤胃球菌属在C3、C7和C12的羟基氧化和差向异构化中发挥作用,拟杆菌、真杆菌和乳酸杆菌还参与胆汁酸酯化过程,梭菌、梭杆菌、消化球菌和假单胞菌参与胆汁酸脱硫过程[4, 45-46]。除此之外,迟缓真杆菌、梭杆菌还具有对β-MCA进行6β-差向异构化修饰的能力[47]。
最后是其他作用。如UDCA通过3α/β-差向异构和5α/β-差向异构化分别产生异胆酸(iso-bile acid)和别胆酸(allo-bile acid)[44]。产生异胆酸的细菌包括迟缓真杆菌和产气荚膜梭菌[13],而关于别胆酸的产生机制及过程研究极少。同时,一些细菌属有胆汁酸氧化或酮化的作用,如放线菌门(Actinobacteria)、变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)和拟杆菌属,其能通过产生氧化与酮化的关键酶羟基类固醇脱氢酶(hydroxysteroid dehydrogenase,HSDHs)将环链上3、7或12号位羟基氧化[48]。
2 胆汁酸代谢物调节肠黏膜免疫黏膜免疫系统主要由肠道上皮细胞(intestinal epithelial cells,IEC)、固有层淋巴细胞、肠上皮内淋巴细胞和派伊尔结等组织构成。肠道黏膜组织主要由杯状细胞(goblet cells,GC)、潘氏细胞(Paneth cells,PC)和内分泌细胞(endocrine cells,EC)等IEC单元构成,它们呈单层紧密排列,除能消化吸收肠道营养物质及形成黏膜物理屏障阻碍细菌入侵外,还参与浆细胞分泌的免疫球蛋白A(IgA)抗体转运、抗原呈递和细胞因子分泌等免疫活动。菌群胆汁酸代谢物通过调控肠道黏膜免疫细胞的结构与功能来影响肠道黏膜免疫已被证实[49-50]。
2.1 isoDCA对黏膜免疫系统的影响去共轭脱氧胆酸(deoxycholic acid,DCA)在菌群(如拟杆菌属)3β-羟基化酶的作用下生成具有多种生物学功能的isoDCA。isoDCA通过作用于树突状细胞(dendritic cell,DC)促进初始T细胞(naive T cell,Tn)转化为叉头盒P3调节性T细胞(forkhead box P3 regulatory T cell,FOXP3+ Treg),其作用效果类似于敲除DC细胞中法尼醇X受体(farnesoid X receptor,FXR)而调增Treg细胞[51],提示isoDCA促Treg细胞产生的作用可能是通过拮抗FXR介导的转录调控有关,具有相同功能的次级胆汁酸还包括ω-MCA。前期研究证明isoDCA可与黏膜组织中DC细胞直接作用,通过抑制FXR而调增Treg细胞,其可预防系统性或组织特异性自身免疫或黏膜炎症性病变,也降低Th1细胞和Th17细胞的炎症反应,但对Th2细胞引起的炎症无效[52]。目前文献报道,瘤胃球菌(Ruminococcus gnavus)[13]、迟缓真杆菌(Eubacterium lentum)[53]和产气荚膜梭菌(Clostridium perfringens)[54]可将初级与次级胆汁酸进行3β-羟基差向异构化而转换成3-oxoDCA中间体,并进一步转换成isoDCA。Campbell等[51]研究表明,isoDCA通过增加DC细胞抗炎基因的表达,抑制其免疫刺激活性,从而促进FOXP3+ Treg细胞分化。
通过对敲除FXR的DC细胞的RNA-seq分析发现,isoDCA能够降低多个与抗原加工和呈递、促炎信号识别和转导相关的基因表达。isoDCA也通过诱导负调炎症信号相关的基因表达,说明isoDCA具有广泛抗炎活性。因此,isoDCA能够通过与核受体互作,促进Treg细胞的胸腺外分化。该研究团队还发现将isoDCA的3α-OH基团氧化为-oxo中间体,然后将其还原为3β-OH基团后,这样诱导作用就减弱了,表明胆汁酸的微生物差向异构化修饰可产生具有独特免疫调节特性的代谢产物[51]。isoDCA在生物活性上的研究较少,Devlin等[13]通过探索isoDCA的生物代谢途径发现在生理浓度下,isoDCA较其前体物质DCA具有更小溶解细胞膜脂和引起膜蛋白解离的能力,有利于DCA敏感型肠道共生菌的生长,在维持肠免疫完整性上发挥功能。
2.2 UDCA对肠道黏膜炎症的影响肠道炎症疾病(inflammatory bowel disease,IBD)是一种常见的免疫性疾病,天然产生的次级胆汁酸熊去氧胆酸(ursodeoxycholic acid,UDCA)具有良好的抗炎和细胞保护作用,因此在治疗IBD方面是有效的[55]。IBD早期阶段是由IEC产生的细胞因子对抗病原细菌、毒素或其代谢产物。许多临床研究证明,UDCA及其牛磺酸结合物能够在各种化学物质诱导的肠炎模型中起保护作用,包括结肠三硝基苯磺酸(2, 4, 6-trinitrobenzene sulfonic acid,TNBS)模型[56]、结肠葡聚糖硫酸钠(dextran sulfate sodium,DSS)模型[57]、吲哚美辛(indomethacin,INN)回肠炎模型[58]和化疗诱导肠炎模型[59]。1997年,Kullmann等[58, 60]在雷根斯堡大学进行的2项研究首次证明了UDCA对肠道炎症的保护作用,研究表明,口服UDCA减小了宏观和微观组织损伤评分,并削弱了INN诱导回肠炎模型和TNBS诱导结肠炎模型的体重减轻。最近,Ward等[61]研究了UDCA对结肠IBD的调节作用,并确定了胆汁酸菌群代谢物对治疗反应的影响,他们利用小鼠DSS黏膜损伤模型对UDCA和其结肠代谢物LCA进行了评估,并监测了这些胆汁酸对体外培养的结肠上皮细胞和小鼠结肠组织释放细胞因子肿瘤坏死因子-α(tumor necrosis factor alpha,TNF-α)、白细胞介素-6(interleukin 6,IL-6)、白细胞介素-1β(interleukin-1 beta,IL-1β)和干扰素-γ(interferon-γ,IFN-γ)的影响,结果显示,UDCA在体外能抑制结肠上皮细胞释放促炎性细胞因子,在体内对结肠炎症有保护作用,而UDCA的主要代谢物LCA的治疗比UDCA更能抑制上皮细胞因子的释放和保护DSS诱导的黏膜炎症[61]。推测UDCA可能是一种用于减轻或预防慢性肠道炎症的新疗法,但菌群参与胆汁酸代谢是发挥其治疗作用的必要条件。同时,相似研究表明[62],UDCA对大鼠坏死性结肠炎模型中肠道组织结构和免疫表型具有改善作用。从以上结果推测,UDCA代谢物LCA很可能是UDCA作用的重要介质。
Martinez-Moya等[50]研究了用于治疗试验性结肠炎的UDCA剂量标准,采用3种UDCA剂量[10、25和50 mg/(kg·d)]作用于TNBS诱导的大鼠结肠炎模型。根据动物一些宏观和生化参数表征发现,当UDCA剂量为50 mg/(kg·d)时能改善试验性结肠炎症表型,而UDCA剂量为25或10 mg/(kg·d)时并没有显著影响。相比低剂量组,高剂量UDCA组动物体重恢复更快,黏膜受损总面积更小,碱性磷酸酶活性更低,证明了UDCA具有IBD治疗效果。这些结果表明,在适当的剂量下,UDCA是一种有效的减轻肠道炎症、增强肠黏膜免疫的药物,具有潜在改善炎症性肠病的作用。而在400 mg/(kg·d)的UDCA过高剂量下,小鼠肠道炎症会持续恶化[63]。综合分析,UDCA具有剂量依赖性,适宜剂量UDCA对肠道及黏膜免疫系统起保护作用,而过高剂量会导致过度刺激,引发更强烈的炎症反应。而UDCA在啮齿类结肠炎模型中的治疗作用是否可转化为人类IBD患者,以及这种作用对肝胆汁酸生成、肠道菌群结构和黏膜免疫系统影响程度仍有待阐明。
UDCA作为抗氧化剂维持细胞膜结构稳定和抑制细胞凋亡中发挥保护作用,改善因化疗引起的肠道黏膜炎症反应。Seung等[59]通过构建5-氟尿嘧啶诱导肠黏膜炎小鼠模型,研究不同剂量UDCA对该模型的改善作用,发现当UDCA剂量为10和100 mg/(kg·d)时,可有效减缓体重减轻症状,降低炎症因子(TNF-α、IL-6)水平并抑制肠绒毛损伤,表明了UDCA可被用作化疗相关的肠道黏膜炎症治疗的可能性。
2.3 LCA及其衍生物对黏膜免疫的影响LCA是一种脂溶性较强的次级胆汁酸,对肝脏有一定的毒性。在胆汁淤积中,通常认为高水平LCA诱导细胞凋亡,从而引起肝脏损伤。研究表明,这种超生理水平的LCA会引起氧化应激和DNA损伤,并诱导肝细胞和结肠上皮细胞进行程序性凋亡[64]。作为UDCA的菌群代谢物,有人认为LCA的这种毒性作用会限制UDCA对肠黏膜免疫机制的增强作用。然而结果显示,通过限制UDCA代谢为LCA反而还会削弱UDCA对DSS肠炎模型的治疗效果[61]。由此可见,LCA在肠黏膜免疫中可能也会发挥关键作用。Kozoni等[65]研究表明,以灌肠的方法给小鼠补充LCA可以有效防止结肠上皮细胞的凋亡,可能促进黏膜免疫屏障功能。同时,也有体外试验证明结肠生理水平(10 μmol/L)的LCA可以有效阻碍结肠上皮细胞释放TNF-α[61]。该研究团队也发现了LCA在预防DSS诱导的肠炎方面效果也很好,LCA治疗组小鼠肠黏膜中细胞因子(TNF-α、IL-6、IL-1β和IFN-γ)的释放被显著限制,效果甚至高于UDCA。
LCA菌群代谢衍生物3-氧代石胆酸(3-oxolithocholic acid,3-oxoLCA)和异别石胆酸(isoallolithocholic acid,isoalloLCA)通过调控鼠Th17细胞和Treg细胞分化功能而影响宿主肠黏膜免疫,但不会影响肠道共生菌群落结构[66]。该研究表明,3-oxoLCA可以通过与Th17细胞转录因子视黄酸相关孤儿受体γt配体(retinoic acid receptor-related orphan receptor-γt,RORγt)的结合域结合,抑制RORγt转录活性,进而抑制Th17细胞分化[66]。而isoalloLCA通过产生线粒体活性氧(mitochondrial reactive oxygen,mitoROS)和增加FOXP3启动子区域H3K27乙酰化水平,增加FOXP3的表达,促进Treg细胞分化。在正常机体条件下,促炎性Th17细胞会引发炎症来抑制肠道感染,一旦威胁消除后,Treg细胞就会抑制炎症。Th17细胞过度活化会引发肠道异常炎症,促进自身免疫性疾病并损害肠道健康。根据Hang等[66]研究,3-oxoLCA和isoalloLCA在回肠部位作用显著,具有良好的抗炎效应。FOXP3+ Treg细胞和RORγ+调节性T细胞(RORγ+ Treg细胞)位于肠固有层,对调节肠道炎症至关重要。多形拟杆菌(Bacteroides thetaiotaomicron)和脆弱拟杆菌(Bacteroides fragilis)被证实可直接诱导RORγ+ Treg细胞增殖,同时也可通过调控初级和次级胆汁酸去共轭化和羟基化反应来调控RORγ+ Treg细胞平衡[67]。Wang等[68]研究认为,肠道菌群胆汁酸代谢产物可诱导结肠RORγ+ Treg细胞增殖并对维持其功能至关重要,测试来自菌群氧化以及脱羟基产生的8种主要次级胆汁酸,均可显著增多小鼠结肠RORγ+ Treg细胞和FOXP3+ Treg细胞的数量。关于胆汁酸在肠炎上的作用,还有研究称DCA通过抑制炎症性环氧合酶信号转导控制产气荚膜梭菌诱导的鸡坏死性肠炎[68]。
3 菌群-胆汁酸-肠黏膜免疫轴的复杂网络胆汁酸介导下的菌群代谢与肠黏膜免疫间的串扰关系十分复杂,对“菌群-胆汁酸-肠黏膜免疫系统”轴的复杂网络机制解析尚不清晰。肠黏膜表面的免疫细胞能够快速识别和清除致病微生物,同时也具有对于肠道共生菌群和无害抗原的耐受性[69-70]。肠道同时也面临着大量外来抗原以及食物、菌群、宿主等代谢产物的威胁。为应对此类威胁,人类及啮齿类动物的肠道上分布着庞大的免疫细胞库,包括功能及表型迥异的CD4+FOXP3+ Treg细胞、CD4+RORγt+细胞、CD8+ Treg细胞或抗原呈递细胞(antigen presenting cells,APCs)等免疫细胞[71-74]。胆汁酸经过肠道菌群的生物修饰后可与肠黏膜免疫发生互作,并加强其屏障功能,预防或治疗黏膜炎症和其他肠道疾病[75-77]。如图 2所示,经主要肠道菌群修饰后的胆汁酸通过与T细胞、DCs和肠上皮细胞中核受体维生素D受体(VDR)和FXR以及膜受体G蛋白偶联胆汁酸受体(TGR5)等作用,调控FOXP3+ Treg细胞与RORγ+ Treg细胞增殖与分化、炎症因子TNF-α等产生与分泌等来增强肠黏膜免疫。
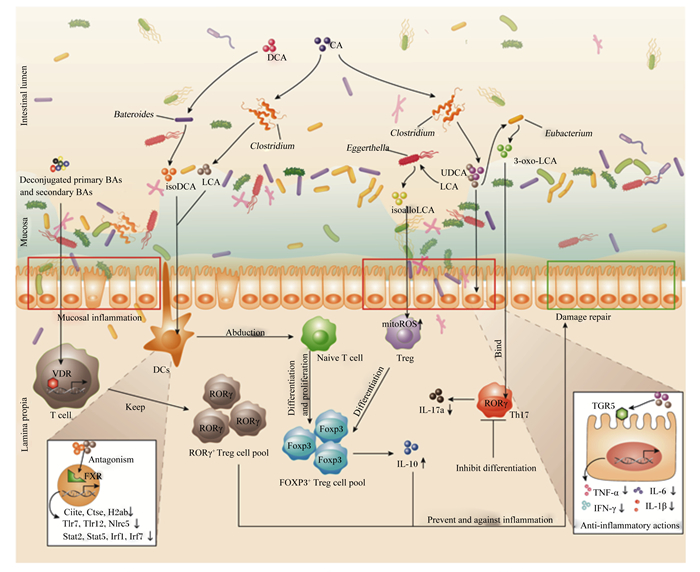
|
VDR:维生素D受体vitamin D receptor;FXR:法尼醇X受体farnesoid X receptor;TGR5:G蛋白偶联胆汁酸受体G protein-coupled bile acid receptor Gpbar1;DCs.:树突状细胞dendritic cells;Treg:调节性T细胞regulatory T cells;mitROS:线粒体活性氧mitochondria reactive oxygen species;DCA:去氧胆酸deoxycholic acid;LCA:石胆酸lithocholic acid;CA:胆酸cholic acid;UDCA:熊去氧胆酸ursodeoxycholic acid;isoDCA:异去氧胆酸3β-hydroxydeoxycholic acid;isoalloLCA:异别石胆酸isoallolithocholic acid;3-oxo-LCA:3-氧代石胆酸3-oxolithocholic acid;Deconjugated primary BAs:解共轭初级胆汁酸;Secondary BAs:次级胆汁酸;mitoROS:线粒体活性氧mitochondrial reactive oxygen;TNF-α:肿瘤坏死因子-α tumor necrosis factor alpha;IL-6:白细胞介素-6 interleukin-6;IL-1β:白细胞介素-1β interleukin-1 beta;IFN-γ:干扰素-γ interferon-γ;IL-17a:白细胞介素-17a interleukin-17a;Intestinal lumen:肠腔;Lamina propia:固有层;Mucosa:黏膜层;Abduction:诱导;Inhibit differentiation:抑制分化;Bind:结合;Proliferation:增殖;Inflammation:炎症;Mucosal inflammation:黏膜炎症;Anti-inflammatory actions:抗炎反应;Damage repair:损伤修复;Antagonism:拮抗;DCs:树突状细胞dendritic cell;T cell:T细胞;Treg:调节性T细胞regulatory cells;Naive T cell:初始T细胞;RORγ+ Treg cell pool:视黄酸相关孤儿受体γt调节性T细胞池;FOXP3+ Treg cell pool:叉头盒P3调节性T细胞池;Th17:辅助性T细胞17 T helper cell 17;Bateroids:拟杆菌属;Clostridium:梭菌属;Eggerthella:埃格特菌属;Eubacterium:真杆菌属;Ciita:MHC Ⅱ类反式激活蛋白MHC class Ⅱ transactivator;Ctse:组织蛋白酶E Cathepsin E;H2ab:组蛋白1簇recombinant histone cluster 1;Tlr7:Toll样受体7 Toll like receptor 7;Nlrc5:NOD样受体5 nod-like receptors 5;Stat2:信号传导及转录激活蛋白2 signal transducer and activator of transcription 2;Irf1:干扰素调节因子1 interferon regulatory factor 1;Irf17:干扰素调节因子7 interferon regulatory factor 7。 图 2 胆汁酸菌群代谢产物参与肠道黏膜免疫的复杂网络 Fig. 2 Complex network of intestinal microbiota mediated bile acids on intestinal mucosal immunity |
一方面,一些去共轭胆汁酸与次级胆汁酸的混合物通过调控T细胞中的VDR来维持RORγ+ Treg细胞池的平衡。isoDCA与LCA通过拮抗DCs核内受体FXR下调与抗原加工和呈递、促炎信号识别和转导相关的基因表达,进一步诱导初始T细胞分化增殖为FOXP3+ Treg细胞,由此上调白细胞介素-10(IL-10)的分泌[51]。另一方面,UDCA与LCA可直接作用于肠上皮细胞TGR5受体下调炎症因子TNF-α、IL-6和IL-1β等的表达[61]。LCA代谢物3-oxoLCA与isoalloLCA同样在黏膜免疫上发挥重要作用,3-oxoLCA通过与Th17细胞RORγ结合域结合抑制Th17细胞的分化,减少促炎因子白细胞介素-17a(interleukin-17a,IL-17a)的分泌,从而降低炎症反应[66]。isoalloLCA通过上调Treg细胞线粒体活性氧水平和增加FOXP3启动子区域的H3K27乙酰化促进Treg细胞分化,进而调控FOXP3+ Treg细胞池的大小[66]。这些代谢通路共同构成了“菌群-胆汁酸-肠黏膜免疫系统”轴的复杂作用网络。
4 小结与展望胆汁酸通过核受体和肠黏膜免疫细胞等重要途径调控肠黏膜免疫功能和维持肠道键康稳态,本文系统地阐述了胆汁酸在肠道菌群与肠黏膜免疫系统对话机制中的生物学作用,有关肠道菌群胆汁酸的生物修饰和次级代谢产物的功能研究还需进一步深入。目前,应更多关注胆汁酸生物修饰的关键菌种和胆汁酸菌群代谢物在肠道黏膜免疫方面的功能,更多聚焦于菌群-胆汁酸-肠黏膜免疫轴中关键作用靶点,实现通过调控菌群生长或胆汁酸代谢过程来预防、控制或治疗相关疾病,为后续研究胆汁酸代谢规律以及在各种生物轴功能提供有力基础。
| [1] |
CLEMENTE J C, URSELL L K, PARFREY L W, et al. The impact of the gut microbiota on human health: an integrative view[J]. Cell, 2012, 148(6): 1258-1270. DOI:10.1016/j.cell.2012.01.035 |
| [2] |
JIA W, XIE G X, JIA W P. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nature Reviews.Gastroenterology & Hepatology, 2018, 15(2): 111-128. |
| [3] |
QUINN R A, MELNIK A V, VRBANAC A, et al. Global chemical effects of the microbiome include new bile-acid conjugations[J]. Nature, 2020, 579(7797): 123-129. DOI:10.1038/s41586-020-2047-9 |
| [4] |
RIDLON J M, KANG D J, HYLEMON P B, et al. Bile acids and the gut microbiome[J]. Current Opinion in Gastroenterology, 2014, 30(3): 332-338. DOI:10.1097/MOG.0000000000000057 |
| [5] |
TAN H E, SISTI A C, JIN H, et al. The gut-brain axis mediates sugar preference[J]. Nature, 2020, 580(7804): 1-6. DOI:10.1038/d41586-020-01035-y |
| [6] |
DANG A T, MARSLAND B J. Microbes, metabolites, and the gut-lung axis[J]. Mucosal Immunology, 2019, 12(4): 843-850. DOI:10.1038/s41385-019-0160-6 |
| [7] |
FARZI A, FRÖHLICH E E, HOLZER P. Gut microbiota and the neuroendocrine system[J]. Neurotherapeutics, 2018, 15(1): 5-22. DOI:10.1007/s13311-017-0600-5 |
| [8] |
LYTE M. Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior[J]. Gut Microbes, 2014, 5(3): 381-389. DOI:10.4161/gmic.28682 |
| [9] |
TILG H, CANI P D, MAYER E A. Gut microbiome and liver diseases[J]. Gut, 2016, 65(12): 2035-2044. DOI:10.1136/gutjnl-2016-312729 |
| [10] |
TRIPATHI A, DEBELIUS J, BRENNER D A, et al. Publisher correction: the gut-liver axis and the intersection with the microbiome[J]. Nature Reviews: Gastroenterology & Hepatology, 2018, 15(7): 785. |
| [11] |
TERATANI T, MIKAMI Y, NAKAMOTO N, et al. The liver-brain-gut neural arc maintains the Treg cell niche in the gut[J]. Nature, 2020, 585(7826): 591-596. DOI:10.1038/s41586-020-2425-3 |
| [12] |
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome[J]. Nature, 2012, 486(7402): 207-214. DOI:10.1038/nature11234 |
| [13] |
DEVLIN A S, FISCHBACH M A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids[J]. Nature Chemical Biology, 2015, 11(9): 685-690. DOI:10.1038/nchembio.1864 |
| [14] |
JAROCKI P, TARGOŃSKI Z. Genetic diversity of bile salt hydrolases among human intestinal bifidobacteria[J]. Current Microbiology, 2013, 67(3): 286-292. DOI:10.1007/s00284-013-0362-1 |
| [15] |
SAYIN S I, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metabolism, 2013, 17(2): 225-235. DOI:10.1016/j.cmet.2013.01.003 |
| [16] |
FIORUCCI S, DISTRUTTI E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders[J]. Trends in Molecular Medicine, 2015, 21(11): 702-714. DOI:10.1016/j.molmed.2015.09.001 |
| [17] |
BIAGIOLI M, CARINO A, CIPRIANI S, et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis[J]. Journal of Immunology, 2017, 199(2): 718-733. DOI:10.4049/jimmunol.1700183 |
| [18] |
RIDLON J M, BAJAJ J S. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics[J]. Acta Pharmaceutica Sinica B, 2015, 5(2): 99-105. DOI:10.1016/j.apsb.2015.01.006 |
| [19] |
ZHANG Y C, LIMAYE P B, RENAUD H J, et al. Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice[J]. Toxicology and Applied Pharmacology, 2014, 277(2): 138-145. DOI:10.1016/j.taap.2014.03.009 |
| [20] |
ESLAM M, SANYAL A J, GEORGE J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014. DOI:10.1053/j.gastro.2019.11.312 |
| [21] |
TOLEDO-ARANA A, DUSSURGET O, NIKITAS G, et al. The Listeria transcriptional landscape from saprophytism to virulence[J]. Nature, 2009, 459(7249): 950-956. DOI:10.1038/nature08080 |
| [22] |
MULLISH B H, MCDONALD J A K, PECHLIVANIS A, et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection[J]. Gut, 2019, 68(10): 1791-1800. DOI:10.1136/gutjnl-2018-317842 |
| [23] |
JONES B V, BEGLEY M, HILL C, et al. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(36): 13580-13585. DOI:10.1073/pnas.0804437105 |
| [24] |
RIDLON J M, KANG D J, HYLEMON P B. Bile salt biotransformations by human intestinal bacteria[J]. Journal of Lipid Research, 2006, 47(2): 241-259. DOI:10.1194/jlr.R500013-JLR200 |
| [25] |
THOMAS C, PELLICCIARI R, PRUZANSKI M, et al. Targeting bile-acid signalling for metabolic diseases[J]. Nature Reviews Drug Discovery, 2008, 7(8): 678-693. DOI:10.1038/nrd2619 |
| [26] |
ALAVI S, MITCHELL J D, CHO J Y, et al. Interpersonal gut microbiome variation drives susceptibility and resistance to cholera infection[J]. Cell, 2020, 181(7): 1533-1546. DOI:10.1016/j.cell.2020.05.036 |
| [27] |
STERN N J, SVETOCH E A, ERUSLANOV B V, et al. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system[J]. Antimicrobial Agents and Chemotherapy, 2006, 50(9): 3111-3116. DOI:10.1128/AAC.00259-06 |
| [28] |
KUMAR R, GROVER S, MOHANTY A K, et al. Molecular cloning and sequence analysis of bile salt hydrolase gene (BSH) from Lactobacillus plantarum MBUL90 strain of human origin[J]. Food Biotechnology, 2010, 24(3): 215-226. DOI:10.1080/08905436.2010.507130 |
| [29] |
JIANG J K, HANG X M, ZHANG M, et al. Diversity of bile salt hydrolase activities in different lactobacilli toward human bile salts[J]. Annals of Microbiology, 2010, 60(1): 81-88. DOI:10.1007/s13213-009-0004-9 |
| [30] |
TARANTO M P, SESMA F, DE VALDEZ G F. Localization and primary characterization of bile salt hydrolase from Lactobacillus reuteri[J]. Biotechnology Letters, 1999, 21(11): 935-938. DOI:10.1023/A:1005652501404 |
| [31] |
TANAKA H, HASHIBA H, KOK J, et al. Bile salt hydrolase of Bifidobacterium longum-biochemical and genetic characterization[J]. Applied and Environmental Microbiology, 2000, 66(6): 2502-2512. DOI:10.1128/AEM.66.6.2502-2512.2000 |
| [32] |
KIM G K, MIYAMOTO C M, MEIGHEN E A, et al. Cloning and characterization of the bile salt hydrolase genes (BSH) from Bifidobacterium bifidum strains[J]. Applied and Environmental Microbiology, 2004, 70(9): 5603-5612. DOI:10.1128/AEM.70.9.5603-5612.2004 |
| [33] |
JAROCKI P. Molecular characterization of bile salt hydrolase from Bifidobacterium animalis subsp.lactis Bi30[J]. Journal of Microbiology and Biotechnology, 2011, 21(8): 838-845. DOI:10.4014/jmb.1103.03028 |
| [34] |
MILANI C, MANGIFESTA M, MANCABELLI L, et al. Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life[J]. The ISME Journal, 2017, 11(12): 2834-2847. DOI:10.1038/ismej.2017.138 |
| [35] |
TRIVEDI P, PANDEY A. Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 C[J]. Journal of Industrial Microbiology and Biotechnology, 2008, 35(3): 205-209. DOI:10.1007/s10295-007-0284-7 |
| [36] |
COLEMAN J P, HUDSON L L. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens[J]. Applied and Environmental Microbiology, 1995, 61(7): 2514-2520. DOI:10.1128/aem.61.7.2514-2520.1995 |
| [37] |
AZIZ R K, BARTELS D, BEST A A, et al. The RAST server: rapid annotations using subsystems technology[J]. BMC Genomics, 2008, 9: 75. DOI:10.1186/1471-2164-9-75 |
| [38] |
RUSHTON-GREEN R, DARNELL R L, TAIAROA G, et al. Agricultural origins of a highly persistent lineage of vancomycin-resistant enterococcus faecalis in New Zealand[J]. Applied and Environmental Microbiology, 2019, 85(13): e00137. |
| [39] |
BERGHOLZ T M, DEN BAKKER H C, KATZ L S, et al. Determination of evolutionary relationships of outbreak-associated Listeria monocytogenes strains of serotypes 1/2a and 1/2b by whole-genome sequencing[J]. Applied and Environmental Microbiology, 2016, 82(3): 928-938. DOI:10.1128/AEM.02440-15 |
| [40] |
YABUUCHI E, KOSEKI M. Morphology of the type strain of Bacillus anthracis EY 3169T=ATCC 14578T grown either aerobically or anaerobically on agar plates-observation by light and laser microscopes[J]. Microbiology and Immunology, 2013, 47(7): 491-497. |
| [41] |
DEGIROLAMO C, RAINALDI S, BOVENGA F, et al. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice[J]. Cell Reports, 2014, 7(1): 12-18. DOI:10.1016/j.celrep.2014.02.032 |
| [42] |
WINSTON J A, THERIOT C M. Diversification of host bile acids by members of the gut microbiota[J]. Gut Microbes, 2020, 11(2): 158-171. DOI:10.1080/19490976.2019.1674124 |
| [43] |
ZHENG M M, WANG R F, LI C X, et al. Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques[J]. Process Biochemistry, 2015, 50(4): 598-604. DOI:10.1016/j.procbio.2014.12.026 |
| [44] |
RIDLON J M, HARRIS S C, BHOWMIK S, et al. Consequences of bile salt biotransformations by intestinal bacteria[J]. Gut Microbes, 2016, 7(1): 22-39. DOI:10.1080/19490976.2015.1127483 |
| [45] |
CHIANG J Y L C. Bile acids: regulation of synthesis: thematic review series: bile acids[J]. Journal of Lipid Research, 2009, 50(10): 1955-1966. DOI:10.1194/jlr.R900010-JLR200 |
| [46] |
GRARD P. Metabolism of cholesterol and bile acids by the gut microbiota[J]. Pathogens, 2014, 3(1): 14-24. |
| [47] |
EYSSEN H, DE PAUW G, STRAGIER J, et al. Cooperative formation of omega-muricholic acid by intestinal microorganisms[J]. Applied and Environmental Microbiology, 45(1): 141-147.
|
| [48] |
KISIELA M, SKARKA A, EBERT B, et al. Hydroxysteroid dehydrogenases (HSDs) in bacteria-a bioinformatic perspective[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2012, 129(1/2): 31-46. |
| [49] |
DUBOC H, RAJCA S, RAINTEAU D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases[J]. Gut, 2013, 62(4): 531-539. DOI:10.1136/gutjnl-2012-302578 |
| [50] |
MARTINEZ-MOYA P, ROMERO-CALVO I, REQUENA P, et al. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis[J]. International Immunopharmacology, 2013, 15(2): 372-380. DOI:10.1016/j.intimp.2012.11.017 |
| [51] |
CAMPBELL C, MCKENNEY P T, KONSTANTINOVSKY D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells[J]. Nature, 2020, 581(7809): 475-479. DOI:10.1038/s41586-020-2193-0 |
| [52] |
JOSEFOWICZ S Z, NIEC R E, KIM H Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation[J]. Nature, 2012, 482(7385): 395-399. DOI:10.1038/nature10772 |
| [53] |
HIRANO S, MASUDA N. Transformation of bile acids by Eubacterium lentum[J]. Applied and Environmental Microbiology, 1981, 42(5): 912-915. DOI:10.1128/aem.42.5.912-915.1981 |
| [54] |
BATTA A K, SALEN G, SHEFER S. Transformation of bile acids into iso-bile acids by Clostridium perfringens: possible transport of 3β-hydrogen via the coenzyme[J]. Hepatology, 1985, 5(6): 1126-1131. DOI:10.1002/hep.1840050611 |
| [55] |
VANG S, LONGLEY K, STEER C J, et al. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases[J]. Global Advances in Health and Medicine, 2014, 3(3): 58-69. DOI:10.7453/gahmj.2014.017 |
| [56] |
DE A K, SANA S, DATTA S, et al. Protective efficacy of ursodeoxycholic acid nanoparticles in animal model of inflammatory bowel disease[J]. Journal of Microencapsulation, 2014, 31(8): 725-737. DOI:10.3109/02652048.2014.918666 |
| [57] |
VAN DEN BOSSCHE L, BORSBOOM D, DEVRIESE S, et al. Tauroursodeoxycholic acid protects bile acid homeostasis under inflammatory conditions and dampens Crohn's disease-like ileitis[J]. Laboratory Investigation, 2016, 97(5): 519-529. |
| [58] |
KULLMANN F, ARNDT H, GROSS V, et al. Beneficial effect of ursodeoxycholic acid on mucosal damage in trinitrobenzene sulphonic acid-induced colitis[J]. European Journal of Gastroenterology & Hepatology, 1997, 9(12): 1205-1211. |
| [59] |
SEUNG K, HOON C, HYUK C, et al. Ursodeoxycholic acid attenuates 5-fluorouracil-induced mucositis in a rat model[J]. Oncology Letters, 2018, 16(2): 2585-2590. |
| [60] |
KULLMANN F, GROSS V, RVSCHOFF J, et al. Effect of ursodeoxycholic acid on the inflammatory activity of indomethacin-induced intestinal inflammation in rats[J]. Ztschrift Für Gastroenterologie, 1997, 35(3): 171-178. |
| [61] |
WARD J B J, LAJCZAK N K, KELLY O B, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon[J]. AJP Gastrointestinal and Liver Physiology, 2017, 312(6): G550-G558. DOI:10.1152/ajpgi.00256.2016 |
| [62] |
SÖNMEZGÖZ E, TAKCI S, GVL A, et al. Ursodeoxycholic acid protects neonatal rats from necrotizing enterocolitis: a biochemical, histopathological, and immunohistochemical study[J]. The Journal of Maternal-fetal & Neonatal Medicine, 2020. DOI:10.1080/14767058.2020.1818210 |
| [63] |
UCHIDA A, YAMADA T, HAYAKAWA T, et al. Taurochenodeoxycholic acid ameliorates and ursodeoxycholic acid exacerbates small intestinal inflammation[J]. American Journal of Physiology, 1997, 272(5 Pt 1): G1249-G1257. |
| [64] |
BARRASA J I, OLMO N, LIZARBE M A, et al. Bile acids in the colon, from healthy to cytotoxic molecules[J]. Toxicology in Vitro, 2012, 27(2): 964-977. |
| [65] |
KOZONI V, TSIOULIAS G, SHIFF S, et al. The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen[J]. Carcinogenesis, 2000, 21(5): 999-1005. DOI:10.1093/carcin/21.5.999 |
| [66] |
HANG S Y, PAIK D, YAO L N, et al. Bile acid metabolites control TH17 and Treg cell differentiation[J]. Nature, 2019, 576(7785): 143-148. DOI:10.1038/s41586-019-1785-z |
| [67] |
SONG X Y, SUN X M, OH S F, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis[J]. Nature, 2020, 577(7790): 410-415. DOI:10.1038/s41586-019-1865-0 |
| [68] |
WANG H, LATORRE J D, BANSAL M, et al. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling[J]. Scientific Reports, 2019, 9: 14541. DOI:10.1038/s41598-019-51104-0 |
| [69] |
BELKAID Y, SEGRE J A. Dialogue between skin microbiota and immunity[J]. Science, 2014, 346(6212): 954-959. DOI:10.1126/science.1260144 |
| [70] |
AGACE W W, MCCOY K D. Regionalized development and maintenance of the intestinal adaptive immune landscape[J]. Immunity, 2017, 46(4): 532-548. DOI:10.1016/j.immuni.2017.04.004 |
| [71] |
KONJAR Š, FERREIRA C, BLANKENHAUS B, et al. Intestinal barrier interactions with specialized CD8 T cells[J]. Frontiers in Immunology, 2017, 8: 1281. DOI:10.3389/fimmu.2017.01281 |
| [72] |
ZEISSIG S, BLUMBERG R S. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces[J]. Current Opinion in Immunology, 2013, 25(6): 690-696. DOI:10.1016/j.coi.2013.09.012 |
| [73] |
COOMBES J L, SIDDIQUI K R R, ARANCIBIA-CÁRCAMO C V, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β-and retinoic acid-dependent mechanism[J]. Journal of Experimental Medicine, 2007, 204(8): 1757-1764. DOI:10.1084/jem.20070590 |
| [74] |
SUZUKI K, OIDA T, HAMADA H, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis[J]. Immunity, 2000, 13(6): 691-702. |
| [75] |
KASER A, BLUMBERG R S. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease[J]. Gastroenterology, 2011, 140(6): 1738-1747. DOI:10.1053/j.gastro.2011.02.048 |
| [76] |
PETERSON L W, ARTIS D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis[J]. Nature Reviews Immunology, 2014, 14(3): 141-153. DOI:10.1038/nri3608 |
| [77] |
HOOPER L V. Epithelial cell contributions to intestinal immunity[J]. Advances in Immunology, 2015, 126: 129-172. |




