肠上皮由大量重复的隐窝-绒毛轴单元组成,其更新和再生依赖于肠道干细胞(intestinal stem cell,ISC)持续的增殖和分化[1-2]。ISC位于隐窝基底部,受隐窝及其周边细胞组成的生态位提供的包括表皮生长因子(epidermal growth factor,EGF)在内的多种信号的调控[3-4]。此外,肠内营养素,包括谷氨酸(glutamate,Glu)和谷氨酰胺(glutamine,Gln)等功能性营养素,也可通表皮生长因子受体(epithelial growth factor receptor,EGFR)加速ISC分裂,从而促进肠上皮发育,改善肠道健康[5-7]。随着类肠团培养技术的建立,ISC生态位成分和功能被深入剖析。借助重组菌表达EGF等生长因子,可能为新型饲料添加剂的开发和畜禽生产效率的提升提供新思路。
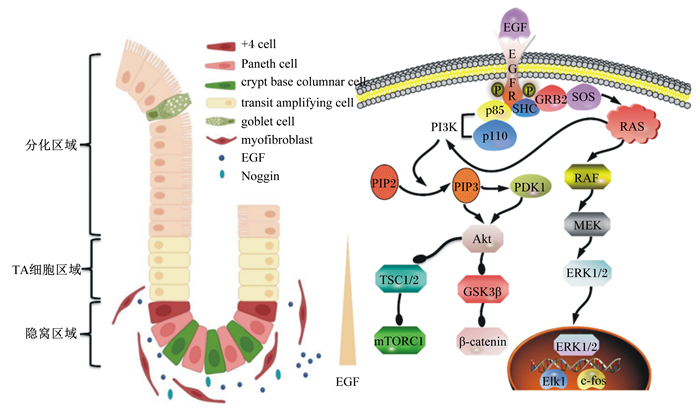
1 EGF信号与ISC生态位ISC生态位由隐窝中的潘氏细胞、邻近隐窝的肠间质细胞和上皮下成纤维细胞等组成[8]。这些细胞不仅在隐窝基底部产生细胞外基质蛋白以固定ISC,而且通过提供EGF、Wnt、Notch和骨形成蛋白(bone morphogenetic protein,BMP)等信号协同调节ISC的增殖和分化过程[9-11]。将ISC或含有干细胞的隐窝放在含有EGF、R-spondin 1(Wnt信号增强剂)和Noggin(BMP抑制剂)等生长因子的基础培养基中培养(模拟体内ISC生态位),或与成纤维细胞共培养的条件下,均能形成具有类似肠上皮结构的类肠团[10]。ISC和潘氏细胞定植在类肠团隐窝状出芽顶部,而ISC的子细胞——短暂扩增细胞(transient amplifying cell,TA细胞)则延伸汇入由肠吸收细胞、杯状细胞和肠内分泌细胞等组成的类似肠绒毛的中央空腔中[12-13]。
ISC生态位中EGF主要由潘氏细胞产生,此外,上皮下成纤维细胞衍生的胞外囊泡(extracellular vesicles,EV)也能传递EGF[14-15]。EGF通过与ISC膜上受体EGFR结合,激活其下游的信号网络,从而刺激ISC增殖,抑制其凋亡[16-19]。
2 ISC生态位中EGF信号的转导EGF对ISC的调控作用主要是通过与EGFR相互作用实现的[20]。当EGF与跨膜EGFR的胞外区结合后,导致后者构象改变,形成催化活性不对称的受体二聚体[16, 21-23]。此时,EGFR胞内区C末端的3个酪氨酸残基发生磷酸化,诱导受体酪氨酸激酶活化,从而使得耦联酪氨酸磷酸化的接头蛋白质SHC(含有SH2结构域的蛋白质)和生长因子受体结合蛋白质2(growth factor receptor-bound protein 2,GRB2)在受体周围聚集。随后,一种双特异性鸟苷酸交换因子(son of sevenless,SOS)与GRB2结合,激活锚定在膜上的小GTP结合蛋白(RAS),进而诱导丝/苏氨酸蛋白激酶(serine/threonine protein kinase,RAF)活化;最终通过丝裂原活化蛋白激酶的激酶(mitogen-activated protein kinase kinase,MEK)磷酸化细胞外信号调节激酶1/2(extracellular regulated protein kinases 1/2,ERK1/2),促使其转位到核内,介导转录激活因子ETS样蛋白质1(ETS-like 1 transcription factor,Elk-1)和原癌基因c-fos等转录因子的活化[24-28],提高基因表达水平。同时,具有磷酸化酪氨酸残基的EGFR可募集磷脂酰肌醇3-激酶(phosphatidy-linositol 3-kinases,PI3K)的调节亚基p85,直接激活PI3K的催化亚单位p110,或通过RAS与p110结合而使后者激活。被活化的p110磷酸化磷酸肌醇(inositol phosphate,PI)肌糖环上的D3位点,从而将底物磷脂酰肌醇4, 5-二磷酸(phosphatidylinositol-4, 5-bisphosphate,PIP2)转化为磷脂酰肌醇(3, 4, 5)-三磷酸(phosphatidylinositol-3, 4, 5-trisphosphate,PIP3)[26, 29]。PIP3作为第二信使,与3-磷酸肌醇依赖性蛋白激酶1(3-phosphoinositide dependent kinase 1,PDK1)和蛋白激酶B(protein kinase B,Akt)结合,促使PDK1磷酸化Akt蛋白308位的丝氨酸残基位点,引起Akt的活化。被活化的Akt通过磷酸化作用抑制其下游靶蛋白——结节性硬化1/2(tuberous sclerosis 1/2,TSC1/2)和糖原合酶激酶3β(glycogen synthase kinase 3β,GSK3β)等,从而激活哺乳动物雷帕霉素靶蛋白复合体1(mammalian target of rapamycin complex 1,mTORC1)和Wnt/β-连环蛋白(β-catenin)信号通路(图 1),促进ISC增殖分化[26-27, 30]。
|
EGF:表皮生长因子epidermal growth factor;EGFR:表皮生长因子受体epidermal growth factor receptor;GRB2:生长因子受体结合蛋白质2 growth factor receptor-bound protein 2;SOS:鸟苷酸交换因子son of sevenless;RAS:小GTP结合蛋白small GTP binding protein;RAF:丝氨酸/苏氨酸蛋白激酶serine/threonine protein kinase;MEK:丝裂原活化蛋白激酶的激酶mitogen-activated protein kinase kinase;ERK1/2:细胞外信号调节激酶1/2 extracellular regulated protein kinases 1/2;Elk1:转录激活因子ETS样蛋白质1 ETS-like 1 transcription factor;c-fos:原癌基因c-fos;p85:PI3K的调节亚基regulatory subunit of PI3K;p110:PI3K的催化亚基catalytic subunit of PI3K;PI3K:磷脂酰肌醇3-激酶phosphatidy-linositol 3-kinases;PIP2:磷脂酰肌醇4, 5-二磷酸phosphatidylinositol-4, 5-bisphosphate;PIP3:磷脂酰肌醇(3, 4, 5)-三磷酸phosphatidylinositol(3, 4, 5)-trisphosphate;PDK1:3-磷酸肌醇依赖性蛋白激酶1 3-phosphoinositide dependent kinase 1;Akt:蛋白激酶B protein kinase B;TSC1/2:结节性硬化1/2 tuberous sclerosis 1/2;GSK3β:糖原合酶激酶3β glycogen synthase kinase 3β;mTORC1:哺乳动物雷帕霉素靶蛋白复合体1 mammalian target of rapamycin complex 1;β-catenin:β-连环蛋白;+4 cell:+4位干细胞;Paneth cell:潘氏细胞;crypt base columnar cell:隐窝基部柱状细胞;transit amplifying cell:短暂扩增细胞;goblet cell:杯状细胞;myofibroblast:肌成纤维细胞;Noggin:BMP抑制剂BMP inhibitor。箭头表示激活,圆点表示抑制the arrow indicates activation and the dot indicates inhibition。 图 1 ISC生态位中的EGF信号转导 Fig. 1 EGF signal transduction in ISC niche[8, 30] |
EGF信号主要在隐窝区和TA细胞区中表达,其信号强度沿小肠隐窝绒毛轴向上衰减[31]。EGF信号促进ISC有丝分裂,并通过调节Wnt信号诱导ISC向潘氏细胞分化,而潘氏细胞又会分泌EGF促进ISC自更新、增殖和分化,由此形成一种正反馈机制。研究表明,EGFR基因缺失或用其抑制剂Tyrphostin处理小鼠会显著抑制ISC增殖和隐窝分裂[32];相反,在应激条件下,EGFR的激活有助于损伤后的ISC再生和隐窝分裂,从而加速伤口愈合和屏障功能重建[33-36]。体外试验同样发现,阻断类肠团中的EGF信号会导致具有增殖分化活性的ISC进入静息状态,类肠团停止生长;一旦恢复EGF信号,ISC重新进入增殖状态,驱动类肠团形成隐窝样芽状结构[27]。此外,EGF信号还能激活mTORC1,诱导吸收型细胞和分泌型细胞的终末成熟[37-38]。Chen等[39]研究发现,断奶应激会激活促肾上腺皮质激素释放因子(coforticotrophin-releasing factor,CRF),促使ISC向未成熟杯状细胞和肠吸收细胞分化,而断奶后EGF的缺乏则抑制了杯状细胞成熟因子的表达,导致杯状细胞处于未成熟状态。成熟的杯状细胞可分泌三叶因子3(trefoil factor 3,TFF3),其与膜上Nogo结合蛋白2(Nogo-interaction protein 2,LINGO2)结合,释放EGFR,增强了EGF信号传导,抑制细胞凋亡,促进ISC再生[40]。同样,具有保护分化细胞稳定作用的肠上皮细胞硫酸乙酰肝素(heparan sulfate,HS)耗竭会引起细胞死亡,这一过程会反馈调节EGF的表达,进而促进ISC的增殖和分化,以维持完整的肠上皮结构[41]。Ye等[42]将间充质干细胞诱导转变为ISC样细胞,这些细胞再经EGF诱导后可分化为肠上皮细胞,进而缓解葡聚糖硫酸钠诱导的结肠炎。
然而,不同情况下,EGFR介导下游信号不尽相同。Xiang等[43]在果蝇上的研究证实,PI3K/雷帕霉素靶蛋白(TOR)信号对于肠道稳态是必需的,但是当肠道上皮损伤后,EGFR/RAS/MEK信号会以一种独立于PI3K/TOR信号的机制触发果蝇ISC产生中间过渡型细胞(enteroblasts,EBs;哺乳动物中又称TA细胞)以及肠上皮细胞(enterocytes,ECs),从而修复肠道损伤。Zhang等[44]进一步揭示细胞分裂周期蛋白42(cell division control 42,Cdc42)与EGFR结合是EGF刺激受体介导的内吞作用所必需的,并且能促进MEK下游的丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号转导,在小鼠ISC中高表达Cdc42变异体2能促进损伤后的肠道再生,提示上调Cdc42/EGFR/MAPK信号能够启动对肠上皮的保护性应答。而在小鼠急性炎症过程中,ISC中PI3K/Akt信号下调导致β-catenin水平降低,隐窝细胞增殖受限[45]。这些结果说明,应激状态下会存在不同的EGF信号应答机制以驱动ISC有丝分裂。
4 EGF信号调节肠上皮结构和功能完整性 4.1 EGF促进肠上皮发育EGF能促进肠细胞增殖分化,改善肠道结构。研究表明,饲粮中添加200 μg/kg EGF,14 d时能显著提高断奶仔猪小肠中段绒毛高度和隐窝深度[46-47]。Cheung等[48]给19~21日龄断奶小鼠连续灌胃50 μg/kg BW的EGF 9 d,发现不仅十二指肠、空肠和回肠绒毛高度显著增加,而且增殖细胞标志物——增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)阳性细胞比例明显上升。Wang等[49-50]同样发现,21日龄断奶仔猪连续采食含60 μg/kg BW EGF的饲粮21 d或在大鼠饮食中添加50 g/kg BW的EGF 15 d,均显著增加了小肠总蛋白、DNA和RNA含量,促进肠道上皮细胞的增殖。EGF还可通过“母子一体化”促进胎儿肠道发育。Cellini等[51]在母兔妊娠期的第24天,利用微渗透泵连续7 d往羊膜注入300 μg/kg EGF,发现宫内发育迟缓胎儿的小肠绒毛高度和5-溴脱氧尿嘧啶核苷(5-bromodeoxyuridine,BrdU)阳性小肠隐窝细胞均接近正常胎儿的水平。此外,饲粮中添加200 μg/kg BW的EGF还能增加断奶仔猪小肠杯状细胞数和内分泌细胞数量,提高溶菌酶mRNA和蛋白质表达水平,提示EGF促进ISC向分泌型肠细胞分化[52]。
EGF主要通过提高消化酶的活性及营养物质转运蛋白的表达,促进肠上皮对营养物质的吸收。大量研究表明,EGF能增强仔猪小肠(尤其是空肠)乳糖酶、蔗糖酶、麦芽糖酶、碱性磷酸酶的mRNA表达[53-55]。同时,EGF还能促进肠上皮细胞对葡萄糖和氨基酸的吸收。Xu等[56]和王定越[57]研究表明,灌服1.8 μg/mL的EGF 100 mL显著提高了仔猪小肠钠/葡萄糖协同转运蛋白1(sodium/glucose cotransporter 1,SGLT1)、葡萄糖转运蛋白2(glucose transporter 2,GLUT2)和寡肽转运蛋白1(peptide transporter 1,PEPT1)的表达。黄骞等[58]用100 μg/L EGF处理Caco-2细胞发现,主要负责转运肠腔内Gln、丙氨酸(alanine,Ala)、丝氨酸(serine,Ser)和半胱氨酸(cysteine,Cys)等中性氨基酸的Na+-依赖性中性氨基酸载体丙氨酸-丝氨酸- 半胱氨酸转运载体2(alanine-serine-cysteine transporter 2,ASCT2)的mRNA表达水平、转运蛋白表达水平和转运活性均显著提高(表 1)。
|
|
表 1 EGF在肠道中的主要作用 Table 1 Main role of EGF in intestine |
EGF具有增强肠道屏障的功能。EGF不仅能通过促进杯状细胞成熟、分泌黏蛋白形成化学屏障、诱导潘氏细胞生成、分泌防御素和抗菌肽等形成免疫屏障,还能提高肠上皮紧密连接蛋白的表达,建立完善的物理屏障[59-61]。研究表明,EGF能上调结肠炎大鼠回肠中封闭蛋白-3(claudin-3)和咬合蛋白(occludin)的蛋白表达[62]。同时,EGF体外预处理Caco-2细胞可通过提高occludin、闭合小环蛋白-1(zonula occludens protein-1,ZO-1)、上皮型钙黏蛋白(E-cadherin)和β-catenin的表达和分布,逆转乙醛引起的单层细胞跨膜电阻值降低和菊糖通透性增加,且该过程依赖ERK1/2的活性,而不依赖于MAPK的活性[63]。
4.2 EGFR整合氨基酸信号促进隐窝-绒毛轴更新肠腔中的营养素与肠细胞密切接触,并通过EGF信号调节ISC功能,维持肠上皮的完整性[64]。Glu及与Glu代谢有关的Gln被认为是小肠黏膜的主要能量来源,研究表明,饲粮来源98%的Glu和66%的Gln在肠道被分解代谢。它们不仅为黏膜提供能量,也是细胞蛋白质和核酸物质等合成的前体和促进剂,对维持黏膜的正常形态、结构和功能具有重要作用[65]。Glu缺乏显著抑制肠上皮细胞增殖和肠上皮发育。Deng等[66]发现,在果蝇饲粮中添加Glu可提高ISC活性。本课题组研究发现,胞外Glu通过EGFR/MEK/ERK和胰岛素受体(IR)/PI3K/Akt通路激活mTORC1信号,增加仔猪ISC活性,促进隐窝-绒毛轴更新[5, 67]。虽然,分子对接预测EGFR可能是胞外Glu的潜在感应体,然而Glu如何激活EGFR还需要进一步的验证。与Glu作用类似,在培养基中加入Gln能激活mTORC1信号通路,促进类肠团出芽,而Gln缺乏会导致类肠团萎缩,增殖细胞数量减少[68]。Momcilovic等[69]研究发现,Gln通过EGFR依赖的方式防止乙醛诱导的小鼠肠道损伤,该过程需要EGFR的胞外配体结合域,然而Gln与EGFR的结合同样需要进一步证实。
5 小结与展望EGF信号是ISC生态位网络的重要组成部分,其通过调控ISC增殖和分化驱动肠上皮细胞更新和再生。外源供给EGF或靶向EGF信号的营养素可能是调节动物肠道健康的一个新策略。然而,目前对EGF信号的研究多集中在细胞水平及果蝇和小鼠等少数几种模式生物上。在家畜,尤其是家禽上的研究较少,而且各生长因子和营养素缺少精准的剂量学和互作效应的研究,EGF信号如何整合所有信息,并将信息有序传递至各细胞器尚不清楚。因此,如何将现有的研究成果应用于动物生产以及医疗和保健仍需进一步探讨。借助类肠团,并结合成簇的规律间隔的短回文重复序列(CRISPR)/成簇的规律间隔的短回文重复序列相关蛋白9(Cas9)基因编辑、微流控和显微注射等技术,充分解析EGF信号及其与其他信号协同调控ISC增殖和分化的机制,将有助于构建基于EGF信号的传导体系,或是逐步实现其应用的前景。
| [1] |
王运星, 吴承堂. 肠道干细胞标记物的研究现状与进展[J]. 中国普通外科杂志, 2008, 17(10): 1013-1015. WANG Y X, WU C T. Research status and progress of intestinal stem cell markers[J]. Chinese Journal of General Surgery, 2008, 17(10): 1013-1015 (in Chinese). |
| [2] |
朱秋杰, 周加义, 梁少杰, 等. Wnt/β-连环蛋白信号驱动小肠上皮更新和再生机制的研究进展[J]. 动物营养学报, 2019, 31(11): 4995-5002. ZHU Q J, ZHOU J Y, LIANG S J, et al. Research progress in Wnt/β-catenin signaling drives small intestinal epithelial renewal and regeneration[J]. Chinese Journal of Animal Nutrition, 2019, 31(11): 4995-5002 (in Chinese). |
| [3] |
CHEN C L, YANG J, JAMES I O A, et al. Heparin-binding epidermal growth factor-like growth factor restores Wnt/β-catenin signaling in intestinal stem cells exposed to ischemia/reperfusion injury[J]. Surgery, 2014, 155(6): 1069-1080. DOI:10.1016/j.surg.2014.01.013 |
| [4] |
LI J L, DEDLOFF M R, STEVENS K, et al. A novel group of secretory cells regulates development of the immature intestinal stem cell niche through repression of the main signaling pathways driving proliferation[J]. Developmental Biology, 2019, 456(1): 47-62. DOI:10.1016/j.ydbio.2019.08.005 |
| [5] |
ZHU M, QIN Y C, GAO C Q, et al. L-glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway[J]. Food & Function, 2020, 11(3): 2714-2724. |
| [6] |
MEENA A S, SHUKLA P K, SHETH P, et al. EGF receptor plays a role in the mechanism of glutamine-mediated prevention of alcohol-induced gut barrier dysfunction and liver injury[J]. The Journal of Nutritional Biochemistry, 2019, 64: 128-143. DOI:10.1016/j.jnutbio.2018.10.016 |
| [7] |
RHOADS M. Glutamine signaling in intestinal cells[J]. Journal of Parenteral and Enteral Nutrition, 1999, 23(5S): S38-S40. DOI:10.1017/S0007485300027292 |
| [8] |
SAILAJA B S, HE X C, LI L H. The regulatory niche of intestinal stem cells[J]. Journal of Physiology, 2016, 594(17): 4827-4836. DOI:10.1113/JP271931 |
| [9] |
冯伟民, 袁丁, 顿耀艳. 肠道干细胞及其微环境在衰老过程中变化的研究进展[J]. 现代医学, 2016, 44(1): 139-142. FENG W M, YUAN D, DUN Y Y. Research progress of intestinal stem cells and their niche changes during aging[J]. Modern Medicine, 2016, 44(1): 139-142 (in Chinese). |
| [10] |
VISHY C E, SWIETLICKI E A, GAZIT V, et al. Epimorphin regulates the intestinal stem cell niche via effects on the stromal microenvironment[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2018, 315(2): G185-G194. DOI:10.1152/ajpgi.00224.2017 |
| [11] |
SPECA S, GIUSTI I, RIEDER F, et al. Cellular and molecular mechanisms of intestinal fibrosis[J]. World Journal of Gastroenterology, 2012, 18(28): 3635-3661. DOI:10.3748/wjg.v18.i28.3635 |
| [12] |
HALL P A, COATES P J, ANSARI B, et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis[J]. Journal of Cell Science, 1994, 107: 3569-3577. DOI:10.1242/jcs.107.12.3569 |
| [13] |
QI Z, CHEN Y G. Regulation of intestinal stem cell fate[J]. Science China Life Sciences, 2015, 44(10): 570-578. DOI:10.1007/s11427-015-4859-7 |
| [14] |
OSZVALD Á, SZVICSEK Z, SÁNDOR G O, et al. Extracellular vesicles transmit epithelial growth factor activity in the intestinal stem cell niche[J]. Stem Cells, 2020, 38(2): 291-300. |
| [15] |
PASTUŁA A, MARCINKIEWICZ J. Cellular interactions in the intestinal stem cell niche[J]. Archivum Immunologiae et Therapiae Experimentalis, 2019, 67(1): 19-26. DOI:10.1007/s00005-018-0524-8 |
| [16] |
ENDRES N F, ENGEL K, DAS R, et al. Regulation of the catalytic activity of the EGF receptor[J]. Current Opinion in Structural Biology, 2011, 21(6): 777-784. DOI:10.1016/j.sbi.2011.07.007 |
| [17] |
MORIKI T, MARUYAMA H, MARUYAMA I N. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain[J]. Journal of Molecular Biology, 2001, 311(5): 1011-1026. DOI:10.1006/jmbi.2001.4923 |
| [18] |
JARDÉ T, CHAN W H, ROSSELLO F J, et al. Mesenchymal niche-derived neuregulin-1 drives intestinal stem cell proliferation and regeneration of damaged epithelium[J]. Cell stem cell, 2020, 27(4): 646-662. DOI:10.1016/j.stem.2020.06.021 |
| [19] |
WANG Z X. ErbB receptors and cancer[M]//WANG Z X. ErbB receptor signaling. New York: Humana Press, 2017.
|
| [20] |
DOMAGALA T, KONSTANTOPOULOS N, SMYTH F, et al. Stoichiometry, kinetic and binding analysis of the interaction between epidermal growth factor (EGF) and the extracellular domain of the EGF receptor[J]. Growth Factors, 2000, 18(1): 11-29. DOI:10.3109/08977190009003231 |
| [21] |
FERGUSON K M. Structure-based view of epidermal growth factor receptor regulation[J]. Annual Review of Biophysics, 2008, 37: 353-373. DOI:10.1146/annurev.biophys.37.032807.125829 |
| [22] |
YARDEN Y, SLIWKOWSKI M X. Untangling the ErbB signalling network[J]. Nature Reviews Molecular Cell Biology, 2001, 2(2): 127-137. DOI:10.1038/35052073 |
| [23] |
MACDONALD-OBERMANN J L, PIKE L J. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation[J]. Journal of Biological Chemistry, 2014, 289(38): 26178-26188. DOI:10.1074/jbc.M114.586826 |
| [24] |
TAMIRAT M Z, KURPPA K J, ELENIUS K, et al. Deciphering the structural effects of activating EGFR somatic mutations with molecular dynamics simulation[J]. Journal of Visualized Experiments, 2020, 159: e61125. |
| [25] |
MARMOR M D, SKARIA K B, YARDEN Y. Signal transduction and oncogenesis by ErbB/HER receptors[J]. International Journal of Radiation Oncology Biology Physics, 2004, 58(3): 903-913. DOI:10.1016/j.ijrobp.2003.06.002 |
| [26] |
周平. EGF、TGFα、EGFR在绵羊早期胚胎中的表达和作用研究[D]. 博士学位论文. 呼和浩特: 内蒙古大学, 2007. ZHOU P. The expression and function of EGF, TGFα and EGFR in ovine preimplantation embryos development[D]. Ph. D. Thesis. Hohhot: Inner Mongolia University, 2007. (in Chinese) |
| [27] |
BASAK O, BEUMER J, WIEBRANDS K, et al. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells[J]. Cell Stem Cell, 2017, 20(2): 177-190. DOI:10.1016/j.stem.2016.11.001 |
| [28] |
XING P Y, PETTERSSON S, KUNDU P. Microbial metabolites and intestinal stem cells tune intestinal homeostasis[J]. Proteomics, 2020, 20(5/6): 1800419. |
| [29] |
BEUMER J, CLEVERS H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration[J]. Development, 2016, 143(20): 3639-3649. DOI:10.1242/dev.133132 |
| [30] |
GEHART H, CLEVERS H. Tales from the crypt: new insights into intestinal stem cells[J]. Nature Reviews Gastroenterology & Hepatology, 2019, 16(1): 19-34. |
| [31] |
YANG Y P, MA H T, STARCHENKO A, et al. A chimeric EGFR protein reporter mouse reveals EGFR localization and trafficking in vivo[J]. Cell Reports, 2017, 19(6): 1257-1267. DOI:10.1016/j.celrep.2017.04.048 |
| [32] |
RIEHL T E, ALVARADO D, EE X, et al. Hyaluronic acid promotes Lgr5+ stem cell proliferation and crypt fission through TLR4 and PGE2 transactivation of EGFR[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2020, 319(1): G63-G73. DOI:10.1152/ajpgi.00242.2019 |
| [33] |
陈舟. 外源性表皮生长因子在未成熟肠道粘膜损伤中的作用及其受体表达的研究[D]. 硕士学位论文. 上海: 复旦大学, 2009. CHEN Z. Effects of exogenous epidermal growth factor on repair of premature intestinal mucosa with necrotizing enterocolitis[D]. Master's Thesis. Shanghai: Fudan University, 2009. (in Chinese) |
| [34] |
陈曦, 孙炳伟. 表皮生长因子(EGF)与肠道黏膜损伤的修复[J]. 江苏大学学报(医学版), 2006, 16(2): 177-180. CHEN X, SUN B W. Epidermal growth factor (EGF) and repair of intestinal mucosal injury[J]. Journal of Jiangsu University (Medicine), 2006, 16(2): 177-180 (in Chinese). |
| [35] |
CHOI H J, AHN J H, PARK S H, et al. Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics[J]. Infection and Immunity, 2012, 80(3): 1079-1087. DOI:10.1128/IAI.05820-11 |
| [36] |
赵允召, 黎介寿. 表皮生长因子对胃肠道的作用(文献综述)[J]. 国外医学·外科学分册, 1993(4): 201-204. ZHAO Y Z, LI J S. Effect of epidermal growth factor in gastrointestine[J]. Foreign Medicine·Division of Surgery, 1993(4): 201-204 (in Chinese). |
| [37] |
ZHOU Y, RYCHAHOU P, WANG Q, et al. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium[J]. Cell Death & Disease, 2015, 6(2): e1631. |
| [38] |
KAUR H, MOREAU R. Role of mTORC1 in intestinal epithelial repair and tumorigenesis[J]. Cellular and Molecular Life Sciences, 2019, 76(13): 2525-2546. DOI:10.1007/s00018-019-03085-6 |
| [39] |
CHEN X, YANG Z H, HU H L, et al. Differentiation and proliferation of intestinal stem cells and its underlying regulated mechanisms during weaning[J]. Current Protein & Peptide Science, 2019, 20(7): 690-695. |
| [40] |
DOSSA A Y, ESCOBAR O, GOLDEN J, et al. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2016, 310(2): G81-G92. DOI:10.1152/ajpgi.00065.2015 |
| [41] |
WEI M, SHI L, KONG R Y, et al. Heparan sulfate maintains adult midgut homeostasis in Drosophila[J]. Cell Biology International, 2020, 44(3): 905-917. DOI:10.1002/cbin.11289 |
| [42] |
YE L, SUN L X, WU M H, et al. A simple system for differentiation of functional intestinal stem cell-like cells from bone marrow mesenchymal stem cells[J]. Molecular Therapy-Nucleic Acids, 2018, 13: 110-120. DOI:10.1016/j.omtn.2018.08.017 |
| [43] |
XIANG J Y, BANDURA J, ZHANG P, et al. EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration[J]. Nature Communications, 2017, 8: 15125. DOI:10.1038/ncomms15125 |
| [44] |
ZHANG X, BANDYOPADHYAY S, ARAUJO L P, et al. Elevating EGFR-MAPK program by a nonconventional Cdc42 enhances intestinal epithelial survival and regeneration[J]. JCI Insight, 2020, 5(16): e135923. DOI:10.1172/jci.insight.135923 |
| [45] |
LEE G, GORETSKY T, MANAGLIA E, et al. Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis[J]. Gastroenterology, 2010, 139(3): 869-881. DOI:10.1053/j.gastro.2010.05.037 |
| [46] |
王丽霞. 表皮生长因子对断奶仔猪肠道功能与上皮细胞更新的影响及机制研究[D]. 硕士学位论文. 长沙: 湖南师范大学, 2019. WANG L X. The effect and mechanism of epidermal growth factor on intestinal function and epithelial cell turnover in weaning piglets[D]. Master's Thesis. Changsha: Hunan Normal University, 2019. (in Chinese) |
| [47] |
KANG P, TOMS D, YIN Y L, et al. Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs[J]. The Journal of Nutrition, 2010, 140(4): 806-811. DOI:10.3945/jn.109.114173 |
| [48] |
CHEUNG Q C K, YUAN Z F, DYCE P W, et al. Generation of epidermal growth factor-expressing Lactococcus lactis and its enhancement on intestinal development and growth of early-weaned mice[J]. The American Journal of Clinical Nutrition, 2009, 89(3): 871-879. DOI:10.3945/ajcn.2008.27073 |
| [49] |
WANG S J, GUO C H, ZHOU L, et al. Comparison of the biological activities of Saccharomyces cerevisiae-expressed intracellular EGF, extracellular EGF, and tagged EGF in early-weaned pigs[J]. Applied Microbiology and Biotechnology, 2015, 99(17): 7125-7135. DOI:10.1007/s00253-015-6468-6 |
| [50] |
WANG S J, ZHOU L, CHEN H N, et al. Analysis of the biological activities of Saccharomyces cerevisiae expressing intracellular EGF, extracellular EGF, and tagged EGF in early-weaned rats[J]. Applied Microbiology and Biotechnology, 2015, 99(5): 2179-2189. DOI:10.1007/s00253-014-6044-5 |
| [51] |
CELLINI C, XU J, ARRIAGA A, et al. Effect of epidermal growth factor infusion on fetal rabbit intrauterine growth retardation and small intestinal development[J]. Journal of Pediatric Surgery, 2004, 39(6): 891-897. DOI:10.1016/j.jpedsurg.2004.02.008 |
| [52] |
WANG L X, ZHU F, LI J Z, et al. Epidermal growth factor promotes intestinal secretory cell differentiation in weaning piglets via Wnt/β-catenin signaling[J]. Animal, 2020, 14(4): 790-798. DOI:10.1017/S1751731119002581 |
| [53] |
LEE D N, CHUANG Y S, CHIOU H Y, et al. Oral administration recombinant porcine epidermal growth factor enhances the jejunal digestive enzyme genes expression and activity of early-weaned piglets[J]. Journal of Animal Physiology and Animal Nutrition, 2008, 92(4): 463-470. DOI:10.1111/j.1439-0396.2007.00735.x |
| [54] |
JAEGER L A, LAMAR C H, CLINE T R, et al. Effect of orally administered epidermal growth factor on the jejunal mucosa of weaned pigs[J]. American Journal of Veterinary Research, 1990, 51(3): 471-474. DOI:10.2754/avb199059010073 |
| [55] |
JAMES P S, SMITH M W, TIVEY D R, et al. Epidermal growth factor selectively increases maltase and sucrase activities in neonatal piglet intestine[J]. Journal of Physiology, 1987, 393(1): 583-594. DOI:10.1113/jphysiol.1987.sp016842 |
| [56] |
XU S, WANG D, ZHANG P, et al. Oral administration of Lactococcus lactis-expressed recombinant porcine epidermal growth factor stimulates the development and promotes the health of small intestines in early-weaned piglets[J]. Journal of Applied Microbiology, 2015, 119(1): 225-235. DOI:10.1111/jam.12833 |
| [57] |
王定越. 重组猪EGF乳酸乳球菌的构建及其对早期断奶仔猪肠道健康的影响[D]. 博士学位论文. 成都: 四川农业大学, 2013. WANG D Y. Recombinant porcine epidermal growth factor-secreting Lactococcus lactis strain promotes the development of intestine in early-weaned piglets[D]. Ph. D. Thesis. Chengdu: Sichuan Agricultural University, 2013. (in Chinese) |
| [58] |
黄骞, 李宁, 朱维铭, 等. Na+-依赖性谷氨酰胺载体ASCT2的生物学功能及表皮生长因子的调控作用[J]. 肠外与肠内营养, 2006, 13(6): 329-334, 341. HUANG Q, LI N, ZHU W M, et al. The biological function of Na+-dependent glutamine transporter ASCT2 and regulatory effect of epidermal growth factor[J]. Journal of Parenteral and Enteral Nutrition, 2006, 13(6): 329-334, 341 (in Chinese). DOI:10.3969/j.issn.1007-810X.2006.06.003 |
| [59] |
SIGISMUND S, AVANZATO D, LANZETTI L. Emerging functions of the EGFR in cancer[J]. Molecular Oncology, 2018, 12(1): 3-20. DOI:10.1002/1878-0261.12155 |
| [60] |
TERAKADO M, GON Y, SEKIYAMA A, et al. The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells[J]. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2011, 300(1): L56-L63. DOI:10.1152/ajplung.00159.2010 |
| [61] |
BARKER N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration[J]. Nature Reviews Molecular Cell Biology, 2014, 15(1): 19-33. DOI:10.1038/nrm3721 |
| [62] |
CHEN Y L, PENG H C, HSIEH Y C, et al. Epidermal growth factor improved alcohol-induced inflammation in rats[J]. Alcohol, 2014, 48(7): 701-706. DOI:10.1016/j.alcohol.2014.07.008 |
| [63] |
SAMAK G, AGGARWAL S, RAO R K. ERK is involved in EGF-mediated protection of tight junctions, but not adherens junctions, in acetaldehyde-treated Caco-2 cell monolayers[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2011, 301(1): G50-G59. DOI:10.1152/ajpgi.00494.2010 |
| [64] |
BEAUMONT M, BLACHIER F. Amino acids in intestinal physiology and health[M]//WU G. Amino acids in nutrition and health. Cham: Springer, 2020, 1265: 1-20.
|
| [65] |
WU G Y. Intestinal mucosal amino acid catabolism[J]. The Journal of Nutrition, 1998, 128(8): 1249-1252. DOI:10.1093/jn/128.8.1249 |
| [66] |
DENG H S, GERENCSER A A, JASPER H. Signal integration by Ca2+ regulates intestinal stem-cell activity[J]. Nature, 2015, 528(7581): 212-217. DOI:10.1038/nature16170 |
| [67] |
ZHU M, QIN Y C, GAO C Q, et al. Extracellular glutamate-induced mTORC1 activation via the IR/IRS/PI3K/Akt pathway enhances the expansion of porcine intestinal stem cells[J]. Journal of Agricultural and Food Chemistry, 2019, 67(34): 9510-9521. DOI:10.1021/acs.jafc.9b03626 |
| [68] |
MOORE S R, GUEDES M M, COSTA T B, et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2015, 308(10): G831-G839. DOI:10.1152/ajpgi.00422.2014 |
| [69] |
MOMCILOVIC M, BAILEY S T, LEE J T, et al. Targeted inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer[J]. Cell Reports, 2017, 18(3): 601-610. DOI:10.1016/j.celrep.2016.12.061 |




