肠黏膜上皮细胞终身不断自我更新,其增殖和分化是一个动态变化的过程,新生的上皮细胞沿着隐窝绒毛轴向上迁移,从未分化的细胞到活跃增殖的隐窝细胞,直到成熟的具有吸收功能的绒毛上皮细胞[1]。前期研究证明,仔猪肠道黏膜氨基酸的利用,在黏膜代谢和损伤修复中具有关键作用[2-9]。多胺(腐胺、亚精胺和精胺)及其主要前体物质(精氨酸、谷氨酰胺和脯氨酸等)在仔猪肠道黏膜细胞增殖分化和损伤修复中发挥重要的作用[2, 6-9]。多胺与DNA、RNA以及蛋白质的生物代谢有关,在细胞分化、细胞周期的调节中起关键作用;对新生仔猪细胞增殖和分化至关重要,是小肠黏膜生长、发育、成熟所必需的物质[10],可促进肠腺隐窝细胞的增殖、分化和移行,促进肠黏膜的损伤修复[11]。仔猪肠道多胺代谢与肠黏膜的生长密切相关,可作为肠道损伤修复的指征[12]。但是,多胺作为氨基酸的代谢产物,如何被肠黏膜感应利用从而调控黏膜增殖的机制尚不清楚。
研究表明,氨基酸转运载体不仅具有转运氨基酸的功能,并且可以通过感受细胞内外氨基酸含量等环境变化对胞内一系列代谢活动进行调节[12-13]。某些氨基酸转运载体能通过转运过程调节细胞内氨基酸含量,从而调控细胞内氨基酸感受体下游信号,发挥氨基酸转运载体和受体的双重功能[14-15]。Na+依赖性中性氨基酸转运载体2(SNAT2)是氨基酸转运感受体的典型代表,前期已克隆得到猪SNAT2的编码基因,发现在仔猪十二指肠、空肠和回肠黏膜中均有表达,而且许多调控多胺代谢的前体氨基酸均能调控SNAT2的表达[16]。进一步证实,SNAT2通过哺乳动物雷帕霉素靶蛋白(mTOR)信号通路调控肠上皮细胞增殖和蛋白质合成;且抑制SNAT2可改变哺乳动物雷帕霉素靶蛋白C1(mTORC1)的上游调控因子基因的表达[17]。因此,本试验选用腐胺(多胺的一种)以及能代谢生成多胺的前体物质脯氨酸和N-氨甲酰谷氨酸(NCG),通过灌服哺乳仔猪,探讨其调控SNAT2表达及其营养感应信号通路的作用,以期为仔猪肠道氨基酸利用和健康调控提供理论依据。
1 材料与方法 1.1 试验设计选取遗传背景相近(精液来源相同)、胎次相同的4窝“杜×长×大”三元杂交仔猪32头,按照同窝和体重相近的原则配对分成4组,每组8头猪,从出生当日开始分别灌服同体积生理盐水(对照组)、腐胺(5 mg/kg BW)、脯氨酸(25 mg/kg BW)和NCG(8 mg/kg BW),每天2次。腐胺的用量是基于Grant等[18]报道结果,脯氨酸的用量是母乳2倍[19],NCG的用量是基于Wu等[20]的报道结果。母猪在相同饲养条件下饲喂同一饲粮(玉米-豆粕型基础饲粮),仔猪正常哺乳。仔猪14日龄断奶,饲喂相同的乳猪料,断奶后3 d,屠宰采样。
1.2 样品采集试验结束当天早晨空腹,试验猪称取体重,颈静脉处穿刺采血5 mL,静置30 min后3 000×g离心15 min,分离血清,-80 ℃冻存,待测胆囊收缩素(cholecystokinin,CCK)含量。试猪放血屠宰,分离空肠和回肠,生理盐水冲洗后,刮取黏膜,迅速放入液氮速冻后,-80 ℃保存,用于游离氨基酸含量检测以及RNA和蛋白质的提取。
1.3 检测指标 1.3.1 生长性能测定试验开始和结束时,所有试验猪空腹称重,计算平均日增重(ADG)。
1.3.2 肠黏膜游离氨基酸含量测定称取空肠黏膜组织0.5 g,加入5 mL 0.1 mol/L HCl匀浆,5 000×g离心10 min,取上清液0.5 mL,并加入等体积的8%磺基水杨酸,混匀,4 ℃静止过夜。12 000×g离心10 min,吸取上清,再次12 000×g离心10 min,取上清过滤膜后用LC-6A型高效液相色谱仪(日本岛津公司)测定氨基酸含量。
1.3.3 血清CCK含量测定血清中CCK含量采用CCK酶联免疫试剂盒(EIA-CCK-1,RayBiotech)测定。所有试剂在25 ℃室温下平衡30 min后制备,血清样品避免反复冻融,按照说明书操作步骤检测。
1.3.4 mRNA相对表达量测定采用Trizol试剂(Invitrogen,美国)提取空肠、回肠黏膜样品中的总RNA。使用无菌、无RNase的DNase Ⅰ(Promega公司,德国)处理,去除总RNA样品中存在的痕量基因组DNA。根据逆转录试剂盒(TaKaRa公司,大连)说明书进行操作, 将RNA逆转录为cDNA。根据GenBank中登入的基因序列,依据引物设计原则,采用Primer5.0软件进行引物的设计(表 1)。引物序列由生工生物工程(上海)股份有限公司合成。以反转录得到的cDNA作为模板,采用SYBR-Green Ⅰ染料(Molecular Probes,Eugene,OR)法,使用ABI 7900HT-PCR仪(ABI Biotechnology,美国)对样品进行扩增,并运用配套软件(Applied Biosystem,SDS2.3)进行数据分析。以最小循环阈值(Ct)和最高荧光值为标准,分别对循环条件、退火温度、引物浓度进行优化。分别检测空肠、回肠黏膜组织中相关基因表达量。
|
|
表 1 引物序列 Table 1 Primer sequence |
采用Western blot检测空肠和回肠黏膜组织SNAT2蛋白表达水平。取500~1 000 mg空肠和回肠黏膜组织样品置于放有液氮的研钵中磨碎,装至放有600 μL蛋白裂解液(RIPA)的1.5 mL离心管中,剧烈振荡,在4 ℃冰箱中静置1~2 h,4 ℃、12 000×g离心15 min,取上清。以牛血清蛋白(BSA)为标准品,采用双氯喹酸法测定蛋白浓度。用5×十二烷基硫酸钠(SDS)样品缓冲液稀释上清液(含组织蛋白),在沸水中加热5 min。将溶液冷冻后,采用Western blot检测空肠和回肠黏膜组织SNAT2蛋白表达水平。以下一抗用于蛋白定量: SNAT2(1 ∶ 1 000,Santa Cruz Biotechnology);β-肌动蛋白(β-actin, 1 ∶ 1 000,Cell Signaling Technology)。
1.4 数据统计各基因表达量以β-actin为内参,对获得的信号、数据进行处理,采用2-ΔΔCt法对定量结果进行计算分析。试验数据采用SPSS 21.0统计软件中的单因素方差(ANOVA)分析进行统计,差异显著者采用Duncan氏法进行多重比较。以P < 0.05作为差异显著性判断标准,结果用平均值±标准误表示。
2 结果与分析 3.1 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪生长性能的影响由表 2可知,哺乳仔猪初始体重一致,14日龄断奶3 d后,哺乳期灌服腐胺和脯氨酸的仔猪终末体重显著高于对照组(P < 0.05)。与对照组相比,灌服腐胺、脯氨酸和NCG均显著提高了仔猪的平均日增重(P < 0.05)。
|
|
表 2 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪生长性能的影响 Table 2 Effects of N-carbamyl glutamate, proline and putrescine on growth performance of piglets (n=8) |
由表 3可知,与对照组相比,腐胺组仔猪空肠黏膜精氨酸含量显著降低(P < 0.05);NCG组仔猪空肠黏膜精氨酸含量显著升高(P < 0.05);此外,灌服脯氨酸和NCG显著降低了空肠黏膜丙氨酸含量(P < 0.05)。
|
|
表 3 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪空肠黏膜游离氨基酸含量的影响 Table 3 Effects of N-carbamyl glutamate, proline and putrescine on contents of free amino acids in jejunal mucosa of piglets (n=8) |
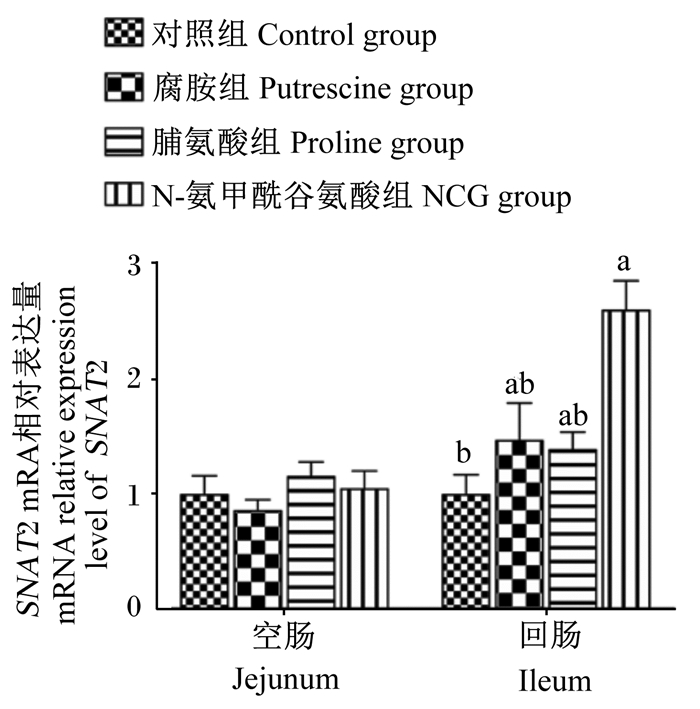
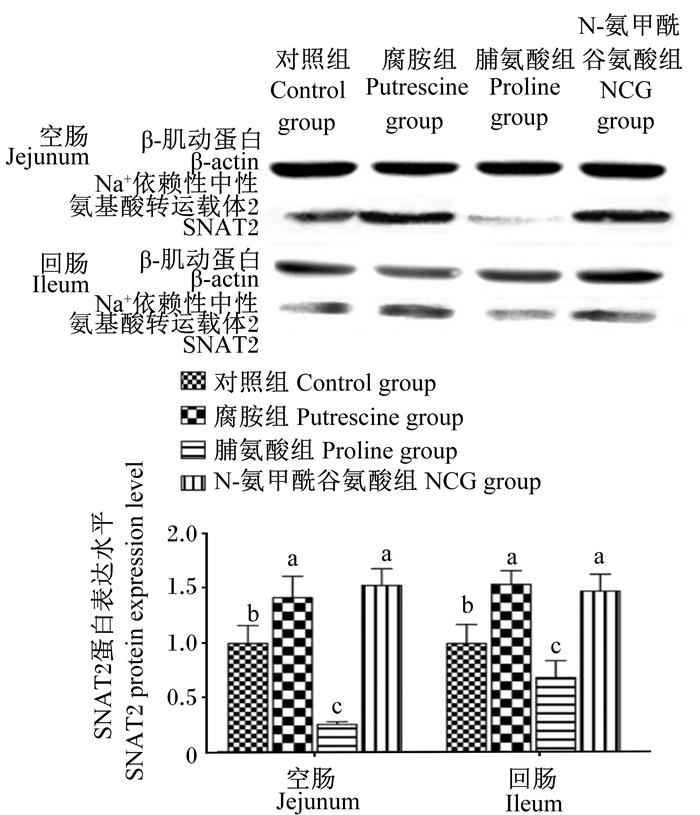
由图 1和图 2可知,与对照组相比,哺乳期灌服NCG显著提高了断奶后第3天仔猪回肠黏膜SNAT2 mRNA相对表达量(P < 0.05);灌服腐胺和NCG均显著提高了空肠和回肠黏膜SNAT2蛋白表达水平(P < 0.05),但灌服脯氨酸显著降低了空肠和回肠黏膜SNAT2蛋白表达水平(P < 0.05)。
|
数据柱标注不同字母表示差异显著(P < 0.05)。下同图。 Data bars with different letters mean significant difference (P < 0.05). The same as below. 图 1 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪小肠黏膜SNAT2 mRNA相对表达量的影响 Fig. 1 Effects of N-carbamyl glutamate, proline and putrescine on mRNA relative expression level of SNAT2 in small intestinal mucosa of piglets (n=8) |
|
图 2 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪小肠黏膜SNAT2蛋白表达水平的影响 Fig. 2 Effects of N-carbamyl glutamate, proline and putrescine on expression level of SNAT2 protein in small intestinal mucosa of piglets (n=8) |
由表 4可知,在空肠黏膜中,与对照组相比,灌服脯氨酸和NCG均显著提高仔猪PAT1、CasR、PLCβ2和T1R3 mRNA相对表达量(P < 0.05);腐胺组PAT1 mRNA相对表达量显著升高(P < 0.05)。
|
|
表 4 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪空肠黏膜氨基酸转运感应相关基因mRNA相对表达量的影响 Table 4 Effects of N-carbamyl glutamate, proline and putrescine on mRNA relative expression levels of genes related to amino acid transport and sensing in jejunal mucosa of piglets (n=8) |
由表 5可知,在回肠黏膜中,与对照组相比,脯氨酸组和NCG组y+LAT2 mRNA相对表达量显著降低(P < 0.05),而EAAC1 mRNA相对表达量显著升高(P < 0.05),腐胺组y+LAT2 mRNA相对表达量显著降低(P < 0.05),而T1R3 mRNA相对表达量显著升高(P < 0.05)。
|
|
表 5 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪回肠黏膜氨基酸转运感应相关基因mRNA相对表达量的影响 Table 5 Effects of N-carbamyl glutamate, proline and putrescine on mRNA relative expression levels of genes related to amino acid transport and sensing in ileal mucosa of piglets (n=8) |
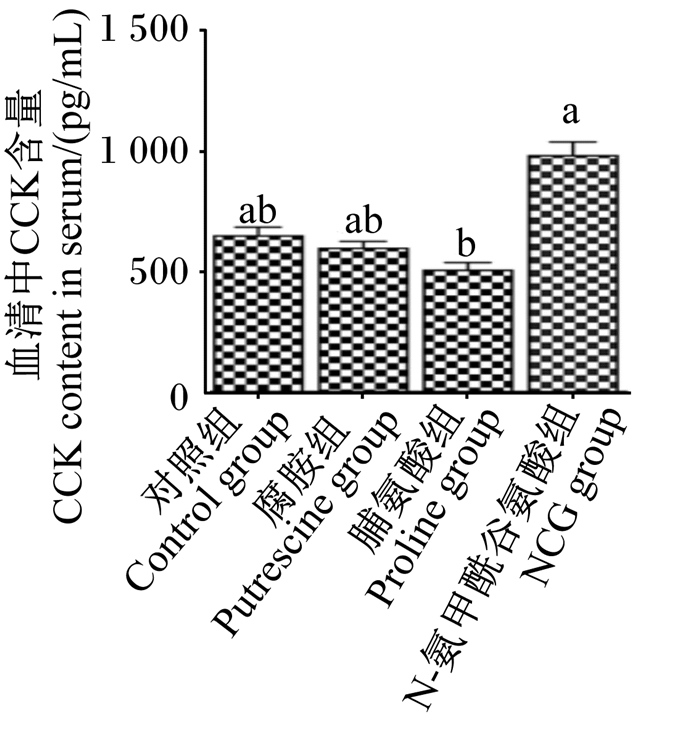
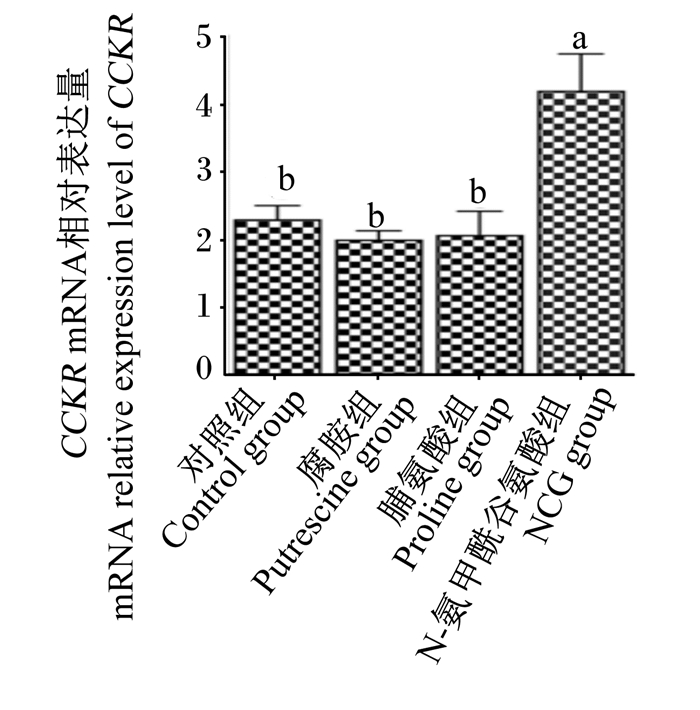
由图 3和图 4可知,与对照组相比,NCG组仔猪血清中CCK含量显著高于脯氨酸组(P < 0.05),且NCG组仔猪空肠黏膜CCKR mRNA相对表达量显著高于其他各组(P < 0.05)。
|
图 3 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪血清中CCK的含量的影响 Fig. 3 Effects of N-carbamyl glutamate, proline and putrescine on contents of CCK in serum of piglets (n=8) |
|
图 4 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪空肠黏膜CCKR mRNA相对表达量的影响 Fig. 4 Effects of N-carbamyl glutamate, proline and putrescine on mRNA relative expression level of CCKR in jejunal mucosa of piglets (n=8) |
由表 6可知,在空肠黏膜中,与对照组相比,哺乳期灌服NCG显著提高了仔猪MAP4K3和mTOR的mRNA相对表达量(P < 0.05)。
|
|
表 6 N-氨甲酰谷氨酸、脯氨酸及腐胺对仔猪空肠黏膜mTOR信号通路相关基因的mRNA相对表达量的影响 Table 6 Effects of N-carbamyl glutamate, proline and putrescine on mRNA relative expression levels of mTOR related genes in jejunal mucosa of piglets (n=8) |
研究表明,多胺对正常生理条件下的肠道上皮细胞完整性的维持具有重要意义;在肠道上皮细胞损伤后,需要多胺作为瞬时能量来源协助修复[21]。虽然母猪初乳中多胺的含量很高,但随着日龄的增长逐渐降低[22]。因此,外源添加多胺物质对肠道黏膜的生长发育和修复损伤具有重要作用[21]。精氨酸被认为是多胺合成的主要前体,然而仔猪小肠中精氨酸酶活性较低,不足以催化精氨酸合成充足的多胺[23]。脯氨酸可经鸟氨酸脱羧酶分解代谢生成多胺,且仔猪小肠中鸟氨酸脱羧酶活性较高,故脯氨酸被认为是仔猪肠道多胺合成的主要前体物质[24]。NCG作为N-乙酰谷氨酸的类似物,能促进内源性精氨酸及精氨酸代谢产物的合成,如通过尿素循环产生多胺[25]。有研究认为,NCG可能有助于克服精氨酸向幼龄动物机体中传递的实际限制[26-27]。多胺之间可以互相转化,腐胺可转化为精胺和亚精胺[28]。因此,本试验选用多胺或促进多胺生成的前体物,通过灌服哺乳仔猪,以期提高仔猪肠道氨基酸的利用,促进提早成熟,缓解断奶应激。多胺调控仔猪肠道发育的作用在我们已发表的研究中得到证实[20]。本试验中,哺乳期灌服腐胺、脯氨酸和NCG均显著提高了仔猪平均日增重,这与多胺和NCG促进仔猪生长速率的前期研究相符[5, 29],同时下文重点讨论的血清氨基酸含量与氨基酸感应信号通路的研究结果也支持这一结论。
首先,灌服NCG显著增加了仔猪空肠黏膜中精氨酸的含量,同时提高了回肠黏膜SNAT2的mRNA相对表达量;灌服腐胺和NCG显著提高了空肠和回肠黏膜SNAT2的蛋白表达水平。外源补充NCG能够促进仔猪内源精氨酸的合成[30]。有研究表明,当增加氨基酸供应时,SNAT2的mRNA及蛋白表达水平以一种依赖mTOR通路的途径显著提高[31],这可能是对于增加的胞内氨基酸转运压力的一种适应性机制[32]。但是,脯氨酸作为SNAT2的底物,降低了空肠和回肠黏膜中的SNAT2蛋白表达水平,表现出对SNAT2的抑制作用。这也许与底物和SNAT2转运Km系数相关[33]。腐胺、脯氨酸和NCG分别上调了空肠PAT1及回肠EAAC1的mRNA相对表达量。PAT1是Na+依赖的亚氨基转运系统的氨基酸转运载体,主要负责脯氨酸的转运[34]。外源补充脯氨酸可提高PAT1的表达[35]。EAAC1则主要是Na+依赖的小肠谷氨酸转运载体[36]。NCG可调控谷氨酸向精氨酸的代谢,影响谷氨酸的转运[5, 27]。灌喂脯氨酸并未显著增加空肠黏膜中脯氨酸的含量,这可能由于脯氨酸进入肠道细胞后直接代谢生成多胺[12]。
此外,CasR和T1R1/T1R3是重要的氨基酸感应受体,属于哺乳类GPCRs家族[37]。灌服脯氨酸和NCG显著上调了空肠黏膜中CasR与T1R3的mRNA相对表达量,灌服腐胺显著上调了回肠黏膜中T1R3的mRNA相对表达量。之前的报道显示,底物的刺激,如胞外的Ca2+和氨基酸等,可以使得Ca2+感应受体CasR表达上调[38]。T1R1/T1R3作为直接感受机体能量状态和胞外氨基酸浓度的感受器,有研究表明基因敲除该感受体后会直接影响氨基酸含量作为信号传递到mTORC1的过程[39]。本研究结果提示多胺及其前体氨基酸对CasR和T1R1/T1R3感应受体具有调控作用。并且,NCG与脯氨酸可能通过影响氨基酸含量改变氨基酸感应受体的表达[40]。已有研究表明,L-谷氨酸和L-精氨酸可以通过激活T1R1/T1R3来提高胰岛素的分泌[41-42]。具体机制可能为T1R1/T1R3通过Gg,激活PLCβ2,产生DAG和IP3,IP3与IP3R3结合,使胞内储存的Ca2+被释放出来,进而激活TRPM5通道,Na+流入细胞内,最终导致膜去极化和神经递质释放[15, 32, 38]。
转运载体介导的氨基酸感应机制是其作为生电转运感受体,诱导膜去极化和激素分泌[42]。氨基酸刺激可以转换成化学信号,通过转运载体蛋白构象的改变或氨基酸与转运载体的简单结合引起信号传导[43-44]。感应信号主要通过与味觉受体结合提高胞内游离Ca2+浓度进而引发神经递质的释放[45]。胞外信号也可与G蛋白偶联受体(GPCR)的结合,激活磷脂酶Cβ2(PLCβ2),使4, 5-二磷酸磷脂酰肌醇(PIP2)发生水解作用,导致了位于细胞内钙库膜上的IP3-门控钙离子通道打开,储备的Ca2+释放,进而激活瞬时受体电位离子通道蛋白5(TRPM5)从而促进膜的去极化。通过检测以上营养物质感应信号途径相关的基因表达及激素分泌的水平发现灌服脯氨酸和NCG提高了空肠PLCβ2的表达量,同时NCG促进了CCK的分泌。CCK作为一种肠道激素,能够调节胰岛素的分泌、胃排空和食欲[46]。CCK的分泌受到多种因素影响,氨基酸能够通过激活CasR促进CCK的分泌[47]。本研究结果显示,NCG和脯氨酸能够正向调节营养利用和相关激素的分泌。有研究证实,饲粮中添加NCG 10 d后环江香猪的平均日增重显著高于基础饲粮组,可能是由于NCG可以促进仔猪肠道对营养物质的吸收,进而改善机体的氨基酸平衡和蛋白质代谢,从而提高仔猪的生长性能[48-49]。
氨基酸刺激mTOR信号通路的表达。NCG对mTOR信号通路基因的表达调控主要反映在MAP4K3和mTOR mRNA相对表达量的升高。MAP4K3是一个保守的丝氨酸/苏氨酸激酶,参与到多种信号通路连接,包括免疫缺陷同系物(IMD)、表皮生长因子受体(EGFR)、雷帕霉素受体C1(TORC1)和c-Jun氨基末端激酶(JNK)的信号通路[5, 50]。有研究表明MAP4K3的活性直接受到氨基酸含量的调节,且不是通过胰岛素的作用,进而调节mTORC1的表达[51]。然而,腐胺及脯氨酸对MAP4K3及mTOR信号通路的mRNA相对表达量无显著作用。这可能与NCG,而非腐胺和脯氨酸,显著增加了仔猪肠道黏膜中精氨酸含量有关。
4 结论哺乳期灌服腐胺、脯氨酸和NCG对仔猪肠道氨基酸含量、氨基酸转运载体及感应信号的调控存在差异性。与腐胺和脯氨酸相比,NCG的作用更加明显。NCG可提高仔猪空肠黏膜中精氨酸含量,促进SNAT2的相对表达量,并上调多种氨基酸转运载体和感应受体的相对表达量,刺激胃肠激素CCK的分泌以及mTOR信号通路的相对表达量。脯氨酸的作用主要表现在调控氨基酸转运载体,但可能通过反馈机制抑制了SNAT2的相对表达量。腐胺虽然上调了SNAT2的相对表达量,但对肠道黏膜氨基酸含量、氨基酸转运载体和mTOR信号通路的相对表达量以及CCK的分泌均无显著影响。因此,NCG可通过促进不同氨基酸转运载体的表达,激活相应的营养感应信号,从而改善断奶仔猪的生长性能。
| [1] |
MOENS E, VELDHOEN M. Epithelial barrier biology: good fences make good neighbours[J]. Immunology, 2012, 135(1): 1-8. DOI:10.1111/j.1365-2567.2011.03506.x |
| [2] |
TAN B, YIN Y L, KONG X F, et al. L-arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells[J]. Amino Acids, 2010, 38(4): 1227-1235. DOI:10.1007/s00726-009-0334-8 |
| [3] |
WANG W C, GENG M M, LI T J, et al. Proteomic-level responses of early-weaned piglets to dietary arginine supplementation[J]. Animal Husbandry and Feed Science, 2009, 1(4/5): 29-33. |
| [4] |
WU X, RUAN Z, GAO Y L, et al. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet[J]. Amino Acids, 2010, 39(3): 831-839. DOI:10.1007/s00726-010-0538-y |
| [5] |
YANG H S, FU D Z, KONG X F, et al. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets[J]. Journal of Animal Science, 2013, 91(6): 2740-2748. DOI:10.2527/jas.2012-5795 |
| [6] |
YAO K, GUAN S, LI T J, et al. Dietary L-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets[J]. British Journal of Nutrition, 2011, 105(5): 703-709. DOI:10.1017/S000711451000365X |
| [7] |
YAO K, YIN Y L, LI X L, et al. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells[J]. Amino Acids, 2012, 42(6): 2491-2500. DOI:10.1007/s00726-011-1060-6 |
| [8] |
ZENG L M, TAN B, YIN Y L, et al. Gene expression profiles in porcine intestinal epithelial cells treated with arginine using a microarray technique[J]. Journal of Food Agriculture and Environment, 2012, 10(3): 834-839. |
| [9] |
ZHANG J, YIN Y L, SHU X G, et al. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets[J]. Amino Acids, 2013, 45(5): 1169-1177. DOI:10.1007/s00726-013-1573-2 |
| [10] |
IGARASHI K, KASHIWAGI K. Polyamines: mysterious modulators of cellular functions[J]. Biochemical and Biophysical Research Communications, 2000, 271(3): 59-64. |
| [11] |
RAO J N, LI J, LI L, et al. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin Ⅱ[J]. The American Journal of Physiology, 1999, 277(6): G1149-G1158. DOI:10.1152/ajpcell.1999.277.6.C1149 |
| [12] |
WANG J, TAN B E, LI G R, et al. Polyamine metabolism in the intestine of piglets is altered by weaning and proline supplementation[J]. Journal of Animal Science, 2016, 94(Suppl.3): 423-428. |
| [13] |
HE F, WU C L, LI P, et al. Functions and signaling pathways of amino acids in intestinal inflammation[J]. BioMed Research International, 2018, 2018: 9171905. |
| [14] |
BRÖER S, BRÖER A. Amino acid homeostasis and signalling in mammalian cells and organisms[J]. Biochemical Journal, 2017, 474(12): 1935-1963. |
| [15] |
NESTEROV S V, YAGUZHINSKY L S, PODOPRIGORA G I, et al. Amino acids as regulators of cell metabolism[J]. Biochemistry (Moscow), 2020, 85(4): 393-408. DOI:10.1134/S000629792004001X |
| [16] |
LI G R, LI J J, TAN B, et al. Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets[J]. PLoS One, 2015, 10(6): e0128207. DOI:10.1371/journal.pone.0128207 |
| [17] |
TANG Y L, TAN B, LI G R, et al. The regulatory role of MeAIB in protein metabolism and the mTOR signaling pathway in porcine enterocytes[J]. International Journal of Molecular Sciences, 2018, 19(3): 714. DOI:10.3390/ijms19030714 |
| [18] |
GRANT A L, THOMAS J W, KING K J, et al. Effects of dietary amines on small intestinal variables in neonatal pigs fed soy protein isolate[J]. Journal of Animal Science, 1990, 68(2): 363-371. DOI:10.2527/1990.682363x |
| [19] |
WU G Y, KNABE D A. Free and protein-bound amino acids in sow's colostrum and milk[J]. The Journal of Nutrition, 1994, 124(3): 415-424. DOI:10.1093/jn/124.3.415 |
| [20] |
WU X, ZHANG Z, LIU T, et al. Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets[J]. Journal of Animal Science, 2012, 90(Suppl.4): 337-229. |
| [21] |
WANG J, TAN B E, LI J J, et al. Regulatory role of l-proline in fetal pig growth and intestinal epithelial cell proliferation[J]. Animal Nutrition, 2020, 6(4): 438-446. DOI:10.1016/j.aninu.2020.07.001 |
| [22] |
CHENG Z B, LI D F, GE C R, et al. Polyamines in sow colostrum and milk at different stages of lactation[J]. Animal Science, 2006, 82(1): 95-99. DOI:10.1079/ASC20051 |
| [23] |
WU G Y, FLYNN N E, KNABE D A. Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets[J]. American Journal of Physiology-Endocrinology and Metabolism, 2000, 279(2): E395-E402. DOI:10.1152/ajpendo.2000.279.2.E395 |
| [24] |
WU G Y, BAZER F W, BURGHARDT R C, et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition[J]. Amino Acids, 2011, 40(4): 1053-1063. DOI:10.1007/s00726-010-0715-z |
| [25] |
ZENG X F, HUANG Z M, ZHANG F R, et al. Oral administration of N-carbamylglutamate might improve growth performance and intestinal function of suckling piglets[J]. Livestock Science, 2015, 181: 242-248. DOI:10.1016/j.livsci.2015.09.004 |
| [26] |
WU X, RUAN Z, HOU Y Q. Function of N-carbamylglutamate for swine nutrition metabolism[J]. Journal of Food Agriculture and Environment, 2013, 11(3): 1088-1092. |
| [27] |
RAO J N, XIAO L, WANG J Y. Polyamines in gut epithelial renewal and barrier function[J]. Physiology, 2020, 35(5): 328-337. DOI:10.1152/physiol.00011.2020 |
| [28] |
WANG J, LI G R, TAN B E, et al. Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets[J]. Journal of Animal Science, 2015, 93(4): 1679-1688. DOI:10.2527/jas.2014-8230 |
| [29] |
VAN WETTERE W H E J, WILLSON N L, PAIN S J, et al. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics[J]. Animal, 2016, 10(10): 1655-1659. DOI:10.1017/S1751731116000446 |
| [30] |
CHACHER B, LIU H Y, WANG D M, et al. Potential role of N-carbamoyl glutamate in biosynthesis of arginine and its significance in production of ruminant animals[J]. Journal of Animal Science and Biotechnology, 2013, 4: 16. DOI:10.1186/2049-1891-4-16 |
| [31] |
JONES H N, ASHWORTH C J, PAGE K R, et al. Expression and adaptive regulation of amino acid transport system A in a placental cell line under amino acid restriction[J]. Reproduction, 2006, 131(5): 951-960. DOI:10.1530/rep.1.00808 |
| [32] |
DRUMMOND M J, GLYNN E L, FRY C S, et al. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle[J]. American Journal of Physiology, Endocrinology and Metabolism, 2010, 298(5): E1011-E1018. DOI:10.1152/ajpendo.00690.2009 |
| [33] |
HYDE R, CWIKLINSKI E L, MACAULAY K, et al. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability[J]. Journal of Biological Chemistry, 2007, 282(27): 19788-19798. DOI:10.1074/jbc.M611520200 |
| [34] |
YANG C B. Expression of porcine intestinal nutrient transporters along crypt-villus axis and during postnatal development[D]. Ph. D. Thesis. Guelph: The University of Guelph, 2011.
|
| [35] |
LIU N, DAI Z L, ZHANG Y C, et al. Maternal L-proline supplementation enhances fetal survival, placental development, and nutrient transport in mice[J]. Biology of Reproduction, 2019, 100(4): 1073-1081. DOI:10.1093/biolre/ioy240 |
| [36] |
WANG Q J, CUI Y Z, ZHANG X Y, et al. Effect of early weaning on the expression of excitatory amino acid transporter 1 in the jejunum and ileum of piglets[J]. Molecular Medicine Reports, 2017, 16(5): 6518-6525. DOI:10.3892/mmr.2017.7421 |
| [37] |
YANG L, CUI M, LIU B. Current progress in understanding the structure and function of sweet taste receptor[J]. Journal of Molecular Neuroscience, 2021, 71(2): 234-244. DOI:10.1007/s12031-020-01642-4 |
| [38] |
HOFER A M, BROWN E M. Extracellular calcium sensing and signalling[J]. Nature Reviews Molecular Cell Biology, 2003, 4(7): 530-538. DOI:10.1038/nrm1154 |
| [39] |
WAUSON E M, ZAGANJOR E, LEE A Y, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy[J]. Molecular Cell, 2012, 47(6): 851-862. DOI:10.1016/j.molcel.2012.08.001 |
| [40] |
SAN GABRIEL A, UNEYAMA H. Amino acid sensing in the gastrointestinal tract[J]. Amino Acids, 2013, 45(3): 451-461. |
| [41] |
OYA M, SUZUKI H, WATANABE Y, et al. Amino acid taste receptor regulates insulin secretion in pancreatic β-cell line MIN6 cells[J]. Genes to Cells, 2011, 16(5): 608-616. DOI:10.1111/j.1365-2443.2011.01509.x |
| [42] |
TAKAI S, SHIGEMURA N. Insulin function in peripheral taste organ homeostasis[J]. Current Oral Health Reports, 2020, 7(2): 168-173. DOI:10.1007/s40496-020-00266-2 |
| [43] |
TAKAHARA T, AMEMIYA Y, SUGIYAMA R, et al. Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes[J]. Journal of Biomedical Science, 2020, 27: 87. DOI:10.1186/s12929-020-00679-2 |
| [44] |
FINDLAY G M, YAN L J, PROCTER J, et al. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling[J]. The Biochemical Journal, 2007, 403(1): 13-20. |
| [45] |
BOBRAKOVA A A. Psychophysiological parameters in rosacea patients after complex therapy with cortexin[J]. Research Results in Pharmacology, 2020, 6(4): 59-64. DOI:10.3897/rrpharmacology.6.60161 |
| [46] |
NAKAJIMA S, HIRA T, HARA H. Calcium-sensing receptor mediates dietary peptide-induced CCK secretion in enteroendocrine STC-1 cells[J]. Molecular Nutrition & Food Research, 2012, 56(5): 753-760. |
| [47] |
LIOU A P, SEI Y, ZHAO X L, et al. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal Ⅰ cells[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2011, 300(4): G538-G546. |
| [48] |
UNNO K, SUMIYOSHI A, KONISHI T, et al. Theanine, the main amino acid in tea, prevents stress-induced brain atrophy by modifying early stress responses[J]. Nutrients, 2020, 12(1): 174. |
| [49] |
CHEN M C, WU S V, REEVE J R, Jr., et al. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels[J]. American Journal of Physiology[J]. Cell Physiology, 2006, 291(4): C726-C739.
|
| [50] |
RESNIK-DOCAMPO M, DE CELIS J F. MAP4K3 is a component of the TORC1 signalling complex that modulates cell growth and viability in Drosophila melanogaster[J]. PLoS One, 2011, 6(1): e14528. |
| [51] |
周笑犁, 印遇龙, 孔祥峰, 等. N-氨甲酰谷氨酸对环江香猪生长性能、营养物质消化率及血浆游离氨基酸含量的影响[J]. 动物营养学报, 2011, 23(11): 1970-1975. ZHOU X L, YIN Y L, KONG X F, et al. N-carbamylglutamate affects growth performance, nutrient digestibility and plasma concentrations of free amino acids in Huanjiang mini-pigs[J]. Chinese Journal of Animal Nutrition, 2011, 23(11): 1970-1975 (in Chinese). |




