2. 中国科学院亚热带农业生态研究所, 动物营养生理与代谢过程实验室, 亚热带农业生态过程重点实验室, 畜禽生产污染控制与废物利用国家工程实验室, 长沙 410125
2. National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Key Laboratory of Agro-Ecological Processes in Subtropical Region, Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China
仔猪肠道上皮细胞对能量的需求较高,在细胞分裂、胞质分裂、细胞运动、物质运输以及信号转导等方面均需要消耗能量[1]。仔猪从出生时起就具有较高的脂肪酶活性,在未断奶时可以很好地利用母乳提供能量[2]。断奶后,仔猪的饲粮由较高消化率的母乳替换成消化率相对较低的淀粉类饲粮[3-4],这导致能量摄入不足,进而影响仔猪维持肠上皮的结构和功能。由于早期断奶下调了仔猪肠上皮细胞的三羧酸循环、脂肪酸氧化和糖酵解等途径[5],而正常的黏膜细胞能量状态是缓解断奶应激、提高仔猪生长性能的重要保证[6]。中链脂肪酸甘油三酯(medium-chain fatty acid triglyceride,MCT)作为一种能量来源,在治疗脂肪代谢疾病和糖尿病等方面具有良好的作用效果[7-8]。MCT能够快速氧化供能,可以补偿仔猪断奶后能量不足等缺陷,并且对机体产生抗菌、免疫等作用。在脂肪酶的作用下,MCT在体内被催化水解,随后释放出具有生物活性的中链脂肪酸(medium-chain fatty acid,MCFA)和单甘酯衍生物,能够支持猪的生长发育[9-10]。本文就MCT的消化吸收特性、抗菌机制和改善肠道屏障功能以及在仔猪生产上的应用进行综述,旨在为MCT作为新型饲粮添加剂调控仔猪肠道健康提供理参考。
1 MCT的消化吸收特性MCFA是一种具备6~12个碳原子的单羧酸,一般以MCT的形式广泛存在于乳脂、棕榈油、椰子油和菜籽油等物质中[11]。纯的MCFA由于具有较难闻的不良气味难被畜禽接受[12],在动物饲料中一般是以MCT的形式存在。与长链脂肪酸甘油三酯(long-chain fatty acid triglyceride,LCT)相比,MCT的碳链相对较短、熔点较低且分子质量相对较小,在室温下常呈现为液态,水解速率更快。MCT有更高的水溶性,能形成粗乳状颗粒,进入水溶液的速度和程度更高,这增加了其与胃肠道脂肪酶相互作用的有效表面积[13]。肠道可以快速地吸收由脂肪酶水解产生的MCFA,MCFA的分子和解离常数(pK)值较小,再酯化的可能性低,可以不形成乳糜微粒直接通过门静脉系统运输到肝脏中[2, 14]。大多数MCFA以游离形式被动扩散吸收,但也表现出以酰基酯的形式被吸收[11]。MCFA和长链脂肪酸(long-chain fatty acid,LCFA)进入线粒体内膜的方式不一致。在细胞液中,活化的LCFA(脂酰辅酶A)不能直接通过线粒体内膜。在酯键作用下,脂酰辅酶A连接在肉碱分子的羟基上,以脂酰基的形式跨入线粒体内膜,从而进入到基质中。相反地,被肠道吸收的MCFA不依赖肉碱系统,直接进入到细胞的线粒体中进行β-氧化[15]。
MCFA在进行β-氧化前需要进行ω-氧化,使脂肪酸的末端甲基发生氧化形成羟甲基,再次氧化形成羧基,最终形成二羧酸,在二羧酸的2侧末端开始进行β-氧化。在β-氧化的过程中不仅会释放ATP,并且能够产生乙酰辅酶A和酮体。乙酰辅酶A作为三羧酸循环的主要底物[16],其进入三羧酸循环后被完全氧化成二氧化碳和水,同时释放出能量。图 1为MCT的消化吸收代谢途径[17]。
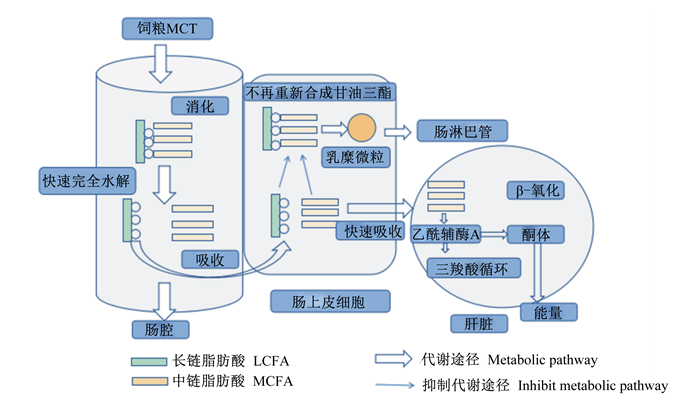
|
图 1 MCT的消化吸收代谢途径 Fig. 1 Digestion, absorption and metabolism pathways of MCT[17] |
动物肠道是一个错综复杂的微生物系统,其微生物菌群的组成和变化与仔猪的生长发育密切相关。MCFA表现出对病原菌强大的抑制活性,其抗菌机制主要为以下2种:1)大多数的MCFA都是以脂溶性的形式存在体内,因此能够直接穿透细菌的细胞膜进入细胞质。MCFA进入细菌细胞质后通过破坏其内部结构,从而使细菌发生代谢障碍和衰竭的现象[2]。Wang等[18]研究发现,癸酸单甘酯能破坏金黄色葡萄球菌和枯草芽孢杆菌的细胞膜,使其通透性发生改变,导致蛋白质、核酸、金属离子等细胞成分外泄,阻碍大分子或变性蛋白质的合成[19]。Bergsson等[20]的研究与上述研究结果一致,其通过双色荧光和电子显微镜检测表明,癸酸单甘酯是通过破坏金黄色葡萄菌的细胞膜使其死亡,而细胞壁始终保持完整状态。辛酸和月桂酸具有同样的抗菌效果。用辛酸处理过的大肠杆菌和用月桂酸处理过的产气荚膜杆菌,通过透射电镜显示胞质结构受损并且产气荚膜杆菌的内外膜受到了分离[21]。通过破坏梭状芽胞杆菌的细胞膜和细胞质,初榨椰子油中的月桂酸能显著抑制梭状芽胞杆菌的生长[22]。同时,MCFA可以通过破坏细胞壁而产生抗菌作用。癸酸单甘酯能降低绿脓杆菌和大肠杆菌的脂多糖(lipopolysaccharide,LPS)吸光度[18]。LPS是绿脓杆菌和大肠杆菌细胞壁的主要成分,在维持细胞正常功能和代谢方面起着重要作用。LPS吸光度的降低提示癸酸单甘酯破坏细菌的细胞壁从而使细胞生长受到抑制。2)MCFA的抗菌机制与其降低细胞的pH有关。MCFA在进入细菌细胞后产生氢离子(H+),为维持细胞体内的酸碱平衡,细胞通过消耗能量泵出多余的H+从而导致自身能量代谢紊乱,造成细胞衰竭。且随着H+的积累,细胞内外会形成pH高低差,酸敏感型细菌则被抑制杀死[23]。然而,本身产酸的乳酸杆菌具有抗酸能力,对乙酸、丙酸、丁酸和乳酸的耐酸效果良好[24],在较低pH时不易造成损伤。
近年来,在禁止使用抗生素之后,研究人员开始对MCFA的抗菌活性进行了深入研究。脂肪酸碳链长度的不同导致了脂肪酸抗菌的效果不一致,从C8到C12,每增加2个碳原子相应的抑菌效果增加3.5倍,但C4、C6、C16和C18对细菌生长的抑制作用不明显[25]。芽孢杆菌和梭状芽孢杆菌具有很强的抗杀伤能力,然而月桂酸单甘酯能够杀死需氧的炭疽芽孢杆菌、枯草芽孢杆菌、蜡样芽孢杆菌以及厌氧产气荚膜梭菌等有害菌[26]。同时,在Batovska等[27]的研究中发现,月桂酸单甘酯对葡萄球菌和链球菌的抑制作用最明显,抗菌效果强于辛酸、癸酸及其他酯类;但是辛酸通过降低大肠杆菌的数量,对仔猪空肠和回肠中的大肠杆菌也表现出较强的抗菌作用[28]。
2.2 MCT对仔猪肠道屏障的作用在仔猪断奶过程中容易引起肠道屏障的损伤,包括绒毛高度的降低和隐窝深度的升高等[29-30]。MCT由于可以迅速提供能量,首先可以通过改善肠道形态结构和紧密连接蛋白的表达促进对肠道的保护作用。补充MCT能够提高空肠的重量和促进空肠中上皮细胞的更新,并加快上皮细胞沿隐窝-绒毛轴的迁移速率进而促进肠道的发育[31]。此外,小肠绒毛高度和隐窝深度影响肠上皮细胞的发育和功能。MCT促使肠道绒毛高度提高和隐窝深度降低,并且提高仔猪对干物质、氮和能量的消化率以及蛋白质的消化率[32-34],这表明MCT可通过改善肠道形态结构促进肠上皮细胞对营养物质的吸收,进一步为肠道提供能量保障。紧密连接作为上皮细胞与内皮细胞板细胞之间的一种黏附方式,是机体溶质通过细胞间隙向肠静脉扩散的主要屏障[35],对于维持肠上皮细胞的结构完整性、保护肠道屏障功能、防止细菌内毒素和有毒大分子进入体内是必不可少的[36]。肠道屏障的重要组成部分包括了闭合蛋白-1(claudin-1)、封闭蛋白(occludin)和紧密连接蛋白-1(ZO-1),MCFA能调节Caco-2细胞的occludin和ZO-1的分布以促进细胞间隙的稳定[37]。同时,MCT通过提高claudin-1、occludin和ZO-1的表达,使仔猪肠道屏障得到改善[38]。
此外,MCT可以促进肠细胞分泌黏蛋白(mucin,MUC)和宿主防御肽(host defense peptide,HDP)等保护肠道健康。肠MUC的主要功能是形成覆盖肠上皮表面的黏液层,其在保护肠上皮屏障的完整性方面起着重要作用。MCT能够显著提高小鼠空肠中MUC2的表达水平,从而改善大肠杆菌诱导的小鼠肠道屏障功能障碍[39]。HDP主要由潘氏细胞产生,具有抗菌、免疫和保护肠道屏障等功能[40]。人源抗菌肽LL-37通过介导P2X嘌呤能受体、表皮因子受体和p38丝裂原活化蛋白激酶,从而诱导肠上皮细胞产生黏蛋白[41]。此外,MCFA对HDP的诱导作用虽然不及短链脂肪酸强烈,但是含有6~8个碳原子的MCFA对猪肠道的J2细胞以及鸡的巨噬细胞、原代单核细胞中HDP的表达具有明显的诱导作用[42-43],这提示MCFA能够通过调节HDP诱导MUC的产生进而保护肠道屏障。
2.3 MCFA的免疫作用MCFA已被证明具有良好的免疫调节作用。MCFA可通过调节免疫细胞和细胞因子的表达促进机体的免疫功能。G蛋白偶联受体(G protein-coupled receptor,GPR)是一种在免疫细胞中高度表达的信号蛋白,而MCFA能够作为GPR84的配体。单核细胞和巨噬细胞中的GPR84能被MCFA激活[44],且GPR84可通过激活哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路促进自然杀伤细胞(NK细胞)成熟和发育[45]。此外,分离自胸导管淋巴的树突状细胞与MCFA和LCFA进行体外培养时均表现出了吞噬活性[46]。
Toll样受体(Toll-like receptor,TLR)通路和核苷酸结合寡聚化结构域蛋白(nucleotide binding oligomerization domain protein,NOD)通路在机体免疫和发生炎症时发挥重要作用,它们能进一步激活核因子-κB(nuclear factor-κB,NF-κB)通路,进而释放炎症因子参与免疫应答。骨髓分化因子88(myeloid differentiation factor 88,MyD88)、白细胞介素-1受体相关激酶1(interleukin-1 receptor-associated kinase 1,IRAK1)、肿瘤坏死因子受体相关因子6(tumor necrosis factor receptor associated factor 6,TRAF6)是TLR通路的下游信号分子,当TLR通路激活时其表达增加。MCFA通过降低MyD88、IRAK1、TRAF6、NOD1、NF-κB的表达水平,抑制了TLR通路和NOD通路,进而抑制NF-κB通路减少炎症因子的表达[47]。MCT可以提高小鼠分泌型免疫球蛋白A(secretory immunoglobulin A, IgA)和白细胞介素(interleukin,IL)-10的表达,降低促炎因子和肿瘤坏死因子-α(tumor necrosis factor-alpha, TNF-α)、IL-18等的表达进而减少肠道损伤[48]。Lee等[49]研究了在免疫抑制剂环磷酰胺处理后癸酸对仔猪的肠道氧化应激、炎症和屏障功能的影响,发现癸酸能够显著降低炎症因子TNF-α、IL-6和丙二醛(malondialdehyde,MDA)的表达,相反地提高了超氧化物歧化酶(superoxide dismutase, SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPx)的表达,表明癸酸能有效缓解仔猪的氧化应激,促进肠道免疫功能的提高。此外,在体外细胞模型中,辛酸和壬酸通过使经典组蛋白去乙酰化酶途径的活性下降,且能够提高启动子组蛋白-3-赖氨酸-9(histone-3-lysine-9,H3K9)水平,进而诱导内源性HDP(如β-防御素)的表达水平来保护肠道免疫屏障[50]。
2.4 MCT的供能作用MCFA作为一种能量补充剂,可以补充到新生儿和幼小畜禽的饮食中改善他们的能量供应。MCFA在被运输到肝脏中之后进行氧化,在氧化不完全时会产生酮体。酮体溶于水且分子小,能通过肌肉毛细血管壁和血脑屏障;并且心脏、肌肉和大脑等组织中含有降解酮体活性的酶,能够使酮体重新进行氧化,因此酮体是一种很好的能量来源[51]。人体在补充MCT 8 h后血浆中酮体含量显著升高[52];MCT的混合物不仅能诱导酮体的产生,而且能激活GPR40受体和恢复老年大鼠β细胞功能,为糖尿病的治疗提供了新的思路[53]。
MCFA在肝脏中氧化完全时产生的乙酰辅酶A,通过进一步的三羧酸循环氧化脱氢释放出能量提供给机体。对己酸酯和辛酸酯进行放射性标记,Odle等[54]研究发现,仔猪在摄入MCT 3.5 h后MCT的氧化率是最高的,能满足仔猪48%的能量需求。在仔猪摄入MCT后12 h以内,每隔1 h测定二氧化碳的生成量以估计MCT氧化的速率,结果发现,在喂食后的第12 h吸收速率是最快的,且辛酸比油酸更容易被氧化,可提供初生仔猪维持所需能量的24.2%[55]。此外,MCFA能够改善母猪的繁殖性能和提高仔猪存活率,其中出生时体重<900 g的猪存活率提高最大[56]。MCFA可能通过母乳传递作用,使仔猪充分利用MCFA提供能量,进而保障仔猪的生长需要。
3 MCT在仔猪上的应用近年来,由于能快速提供能量和保护肠道屏障功能,MCT被广泛应用在畜禽养殖业,在仔猪上的研究也越来越多。然而MCT对断奶仔猪的生长性能改善的结果存在差异。饲粮添加10%的MCT对仔猪生长性能无显著影响,采食量、日增重和料重比均无显著变化[57-58]。含有辛酸和癸酸的MCT,在仔猪饲喂前2周能提高仔猪的体重,但是35 d后与对照组之间无显著差异[59]。用MCT代替大豆油,饲喂仔猪在第14天时提高了生长性能(平均日增重和料重比改善),但是整个试验期统计起来差异微小,各组之间差异不显著[60]。相反地,Chwen等[61]研究发现,MCFA能提高生长性能,这有可能是因为MCFA促进了肠道的发育,提高了绒毛高度和降低了隐窝深度。饲粮添加MCFA后能提高营养物质的吸收和提高生长性能,这与MCFA对小肠上皮细胞功能和肠道形态(绒毛长度和隐窝深度)有着直接或者间接的作用有关,其增加的吸收表面可以促进更多的营养物质吸收以使仔猪更有效地利用生长所需的养分。也有学者认为,MCFA能够提高仔猪生长性能主要是因为MCFA的抗菌作用[2]。Kuang等[62]用有机酸和MCFA的混合物研究其替代抗生素的可行性,结果发现含有MCFA的混合物能够显著提高仔猪采食量和体增重,乳酸杆菌的增加促进了对营养物质的发酵,同时回肠中氨基酸的表观消化率得到提高。
4 小结总得来说,仔猪饲粮添加适量的MCT对肠上皮细胞而言是一种良好的能量来源。MCT在肠道中被消化酶分解成脂肪酸后,可快速地为仔猪提供能量,从而缓解断奶应激导致的能量供应不足。此外,MCT具有抗菌活性和免疫促进的作用,可以作为仔猪生产中一种绿色有效的抗生素替代品。MCT在母猪上的应用也有少量研究,其结果可以提高母猪的繁殖性能和通过母乳将MCT传递给仔猪进而提高仔猪的生长性能。但是MCT在母猪上的研究较少,尚缺乏数据材料支撑,因此MCT具有广阔的应用前景,加强其在猪生产上的研究,有利于促进新型饲料的开发与利用。
| [1] |
谷巍. 浅析动物肠粘膜上皮细胞的能量营养[C]//山东畜牧兽医学会禽病学专业委员会(SPDC)第二届禽病学术研讨会论文集. 潍坊: 山东畜牧兽医学会, 2011: 35-37. GU W. Analysis on energy nutrition of animal intestinal mucosal epithelial cells[C]//Proceedings of the second symposium on poultry diseases of Shandong Animal Husbandry and Veterinary Society (SPDC). Weifang: Shandong Animal Husbandry and Veterinary Society, 2011: 35-37. (in Chinese) |
| [2] |
HANCZAKOWSKA E. The use of medium-chain fatty acids in piglet feeding-a review[J]. Annals of Animal Science, 2017, 17(4): 967-977. DOI:10.1515/aoas-2016-0099 |
| [3] |
WILLIAMS I H, PLUSKE J R, DIVIDICH J L, et al. Growth of the weaned pig[M]//PLUSKE J R, LE DIVIDICH J, VERSTEGEN M W A. Weaning the pig concepts and consequences. Wageningen: Wageningen Academic Publishers, 2003: 17-35.
|
| [4] |
PLUSKE J R, WILLIAMS I H, AHERNE F X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning[J]. Animal Science, 1996, 62(1): 131-144. DOI:10.1017/S1357729800014417 |
| [5] |
XIONG X, YANG H S, TAN B, et al. Differential expression of proteins involved in energy production along the crypt-villus axis in early-weaning pig small intestine[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2015, 309(4): G229-G237. DOI:10.1152/ajpgi.00095.2015 |
| [6] |
QI M, WANG J, TAN B, et al. Postnatal growth retardation is associated with intestinal mucosa mitochondrial dysfunction and aberrant energy status in piglets[J]. Journal of Cellular and Molecular Medicine, 2020, 24(17): 10100-10111. DOI:10.1111/jcmm.15621 |
| [7] |
宫雪. 中链脂肪酸对糖尿病小鼠糖脂代谢的影响[D]. 硕士学位论文. 北京: 中国人民解放军医学院, 2012. GONG X. Effects of medium chain fatty acid on glucose and lipid metabolism in KKAy diabetic mice[D]. Master's Thesis. Beijing: Chinese People's Liberation Army Military Medical College of Continuing Education, 2012. (in Chinese) |
| [8] |
薛长勇, 吴坚. 生物活性脂类: 中链脂肪酸及其与脂代谢和糖代谢[J]. 临床药物治疗杂志, 2011, 9(4): 4-7. XUE C Y, WU J. Bioactive lipids: medium chain fatty acids and their relationship with lipid metabolism and sugar metabolism[J]. Clinical Medication Journal, 2011, 9(4): 4-7 (in Chinese). DOI:10.3969/j.issn.1672-3384.2011.04.002 |
| [9] |
DECUYPERE J A, DIERICK N A. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: concept, possibilities and limitations.An overview[J]. Nutrition Research Reviews, 2003, 16(2): 193-210. DOI:10.1079/NRR200369 |
| [10] |
DIERICK N A, DECUYPEREA J A, MOLLY K, et al. The combined use of triacylglycerols (TAGs) containing medium chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative to nutritional antibiotics in piglet nutrition: Ⅱ.In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance[J]. Livestock Production Science, 2002, 76(1/2): 1-16. |
| [11] |
LIU Y L. Fatty acids, inflammation and intestinal health in pigs[J]. Journal of Animal Science and Biotechnology, 2015, 6(1): 41. DOI:10.1186/s40104-015-0040-1 |
| [12] |
OPREAN L, IANCU R, STAN R, et al. Comparison between types of feeding on goat milk composition[J]. Scientific Papers: Animal Science and Biotechnologies, 2011, 44(1): 76-79. |
| [13] |
ZENTEK J, BUCHHEIT-RENKO S, FERRARA F, et al. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets[J]. Animal Health Research Reviews, 2011, 12(1): 83-93. DOI:10.1017/S1466252311000089 |
| [14] |
RAMÍREZ M, AMATE L, GIL A. Absorption and distribution of dietary fatty acids from different sources[J]. Early Human Development, 2001, 65(Suppl 2): S95-S101. |
| [15] |
ZULKANAIN I N, AB-RAHIM S, CAMALXAMAN S N, et al. Medium-chain fatty acids in nutritional therapy: a review[J]. Malaysian Journal of Fundamental and Applied Sciences, 2020, 16(3): 318-323. DOI:10.11113/mjfas.v16n3.1610 |
| [16] |
LEI E, VACY K, BOON W C. Fatty acids and their therapeutic potential in neurological disorders[J]. Neurochemistry International, 2016, 95: 75-84. DOI:10.1016/j.neuint.2016.02.014 |
| [17] |
LEE Y Y, TANG T K, CHAN E S, et al. Medium chain triglyceride and medium-and long chain triglyceride: metabolism, production, health impacts and its applicationsa review[J/OL]. Critical Reviews in Food Science and Nutrition, 2021: 1-17. (2021-01-22)[2021-03-12]. https://pubmed.ncbi.nlm.nih.gov/33480262/.DOI: 10.1080/10408398.2021.1873729.
|
| [18] |
WANG W Y, WANG R, ZHANG G J, et al. In vitro antibacterial activities and mechanisms of action of fatty acid monoglycerides against four foodborne bacteria[J]. Journal of Food Protection, 2020, 83(2): 331-337. DOI:10.4315/0362-028X.JFP-19-259 |
| [19] |
MAROUNEK M, PUTTHANA V, BENADA O, et al. Antimicrobial activities of medium-chain fatty acids and monoacylglycerols on Cronobacter sakazakii DBM 3157T and Cronobacter malonaticus DBM 3148[J]. Czech Journal of Food Sciences, 2012, 30(6): 573-580. DOI:10.17221/433/2011-CJFS |
| [20] |
BERGSSON G, ARNFINNSSON J, STEINGRÍMSSON Ó, et al. Killing of gram-positive cocci by fatty acids and monoglycerides[J]. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 2001, 109(10): 670-678. DOI:10.1034/j.1600-0463.2001.d01-131.x |
| [21] |
SKŘIVANOVÁ E, MAROUNEK M, BENDA V, et al. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin[J]. Veterinární Medicína, 2006, 51(3): 81-88. |
| [22] |
SHILLING M, MATT L, RUBIN E, et al. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile[J]. Journal of Medicinal Food, 2013, 16(12): 1079-1085. DOI:10.1089/jmf.2012.0303 |
| [23] |
杨金堂. 中链脂肪酸对高温环境下猪饲养和疾病防治的应用与机理研究[D]. 博士学位论文. 南京: 南京农业大学, 2014. YANG J T. Effects of medium-chain fatty acid on pig breeding and disease prevention under high ambient temperature[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2014. (in Chinese). |
| [24] |
HSIAO C P, SIEBERT K J. Modeling the inhibitory effects of organic acids on bacteria[J]. International Journal of Food Microbiology, 1999, 47(3): 189-201. DOI:10.1016/S0168-1605(99)00012-4 |
| [25] |
HASSINEN J B, DURBIN G T, BERNHARDT F W. The bacteriostatic effects of saturated fatty acids[J]. Archives of Biochemistry and Biophysics, 1951, 31(2): 183-189. DOI:10.1016/0003-9861(51)90204-4 |
| [26] |
SCHLIEVERT P M, KILGORE S H, KAUS G M, et al. Glycerol monolaurate (GML) and a nonaqueous five-percent GML gel kill Bacillus and Clostridium spores[J]. mSphere, 2018, 3(6): e00597-18. |
| [27] |
BATOVSKA D I, TODOROVA I T, TSVETKOVA I V, et al. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships[J]. Polish Journal of Microbiology, 2009, 58(1): 43-47. |
| [28] |
HANCZAKOWSKA E, Ś WIATKIEWICZ M, NATONEK-WIŚNIEWSKA M, et al. Medium chain fatty acids (MCFA) and/or probiotic Enterococcus faecium as a feed supplement for piglets[J]. Livestock Science, 2016, 192: 1-7. DOI:10.1016/j.livsci.2016.08.002 |
| [29] |
MONTAGNE L, BOUDRY G, FAVIER C, et al. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning[J]. British Journal of Nutrition, 2007, 97(1): 45-57. DOI:10.1017/S000711450720580X |
| [30] |
SMITH F, CLARK J E, OVERMAN B L, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2010, 298(3): G352-G363. DOI:10.1152/ajpgi.00081.2009 |
| [31] |
CZERNICHOW B, GALLUSER M, CUI S Q, et al. Comparison of enteral or parenteral administration of medium chain triglycerides on intestinal mucosa in adult rats[J]. Nutrition Research, 1996, 16(5): 797-804. DOI:10.1016/0271-5317(96)00072-3 |
| [32] |
DIERICK N A, DECUYPERE J A, DEGEYTER I. The combined use of whole Cuphea seeds containing medium chain fatty acids and an exogenous lipase in piglet nutrition[J]. Archiv Fur Tierernahrung, 2003, 57(1): 49-63. |
| [33] |
DEVI S M, KIM I H. Effect of medium chain fatty acids (MCFA) and probiotic (Enterococcus faecium) supplementation on the growth performance, digestibility and blood profiles in weanling pigs[J]. Veterinární Medicína, 2014, 59: 527-535. |
| [34] |
HANCZAKOWSKA E, AGNIESZKA S, OKOŃ K. Effects of dietary caprylic and capric acids on piglet performance and mucosal epithelium structure of the ileum[J]. Journal of Animal and Feed Sciences, 2011, 20(4): 556-565. DOI:10.22358/jafs/66213/2011 |
| [35] |
TSUKITA S, FURUSE M, ITOH M. Multifunctional strands in tight junctions[J]. Nature Reviews Molecular Cell Biology, 2001, 2(4): 285-293. DOI:10.1038/35067088 |
| [36] |
TANG Z R, DENG H, ZHANG X L, et al. Effects of orally administering the antimicrobial peptide Buforin Ⅱ on small intestinal mucosal membrane integrity, the expression of tight junction proteins and protective factors in weaned piglets challenged by enterotoxigenic Escherichia coli[J]. Animal Feed Science and Technology, 2013, 186(3/4): 177-185. |
| [37] |
LINDMARK T, KIMURA Y, ARTURSSON P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells[J]. The Journal of Pharmacology and Experimental Therapeutics, 1998, 284(1): 362-369. |
| [38] |
CUI Z J, WANG X Z, HOU Z P, et al. Low-protein diet supplemented with medium-chain fatty acid glycerides improves the growth performance and intestinal function in post-weaning piglets[J]. Animals, 2020, 10(10): 1852. DOI:10.3390/ani10101852 |
| [39] |
WANG J, LU J X, XIE X W, et al. Blend of organic acids and medium chain fatty acids prevents the inflammatory response and intestinal barrier dysfunction in mice challenged with enterohemorrhagic Escherichia coli O157:H7[J]. International Immunopharmacology, 2018, 58: 64-71. DOI:10.1016/j.intimp.2018.03.014 |
| [40] |
易宏波. 抗菌肽CWA对断奶仔猪肠道炎症和肠道屏障功能的作用及其机制[D]. 博士学位论文. 杭州: 浙江大学, 2016. YI H B. Effects and mechanism of antimicrobial peptide CWA on intestinal inflammation and intestinal barrier functions in weaned piglets[D]. Ph. D. Thesis. Hangzhou: Zhejiang University, 2016. (in Chinese) |
| [41] |
OTTE J M, ZDEBIK A E, BRAND S, et al. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity[J]. Regulatory Peptides, 2009, 156(1/3): 104-117. |
| [42] |
ZENG X F, SUNKARA L T, JIANG W Y, et al. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs[J]. PLoS One, 2013, 8(8): e72922. DOI:10.1371/journal.pone.0072922 |
| [43] |
SUNKARA L T, JIANG W Y, ZHANG G L. Modulation of antimicrobial host defense peptide gene expression by free fatty acids[J]. PLoS One, 2012, 7(11): e49558. DOI:10.1371/journal.pone.0049558 |
| [44] |
WANG J H, WU X U, SIMONAVICIUS N, et al. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84[J]. The Journal of Biological Chemistry, 2006, 281(45): 34457-34464. DOI:10.1074/jbc.M608019200 |
| [45] |
韦欢. G蛋白偶联受体GPR84对NK细胞的免疫调控作用及其机制的研究[D]. 硕士学位论文. 上海: 华东师范大学, 2019. WEI H. Immunoregulation of G protein-coupled receptor GPR84 on NK cells and the underlying mechanisms[D]. Master's Thesis. Shanghai: East China Normal University, 2019. (in Chinese) |
| [46] |
TSUZUKI Y, MIYAZAKI J, MATSUZAKI K, et al. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids[J]. Journal of Gastroenterology, 2006, 41(3): 209-216. DOI:10.1007/s00535-005-1747-0 |
| [47] |
陈少魁. 中链脂肪酸对脂多糖诱导的仔猪肠道和肝脏损伤的调控作用[D]. 硕士学位论文. 武汉: 武汉轻工大学, 2016. CHEN S K. Regulative effect of medium-chain fatty acids on intestinal and liver injury of piglets after lipopolysaccharide challenge[D]. Master's Thesis. Wuhan: Wuhan Polytechnic University, 2016. (in Chinese) |
| [48] |
KONO H, FUJII H, ASAKAWA M, et al. Medium-chain triglycerides enhance secretory IgA expression in rat intestine after administration of endotoxin[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2004, 286(6): G1081-G1089. DOI:10.1152/ajpgi.00457.2003 |
| [49] |
LEE S I, KANG K S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs[J]. Scientific Reports, 2017, 7(1): 16530. DOI:10.1038/s41598-017-16561-5 |
| [50] |
WANG J, HUANG N N, XIONG J, et al. Caprylic acid and nonanoic acid upregulate endogenous host defense peptides to enhance intestinal epithelial immunological barrier function via histone deacetylase inhibition[J]. International Immunopharmacology, 2018, 65: 303-311. DOI:10.1016/j.intimp.2018.10.022 |
| [51] |
JOHN J, SAPA N K, SHENOY R R. Virgin coconut oil ameliorates colchicine induced cognitive dysfunction-a preclinical study[J]. Pharmaceutical Sciences, 2020, 26(1): 1-12. DOI:10.34172/PS.2019.61 |
| [52] |
ST-PIERRE V, VANDENBERGHE C, LOWRY C M, et al. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day[J]. Frontiers in nutrition, 2019, 6: 46. DOI:10.3389/fnut.2019.00046 |
| [53] |
PUJOL J B, CHRISTINAT N, RATINAUD Y, et al. Coordination of GPR40 and ketogenesis signaling by medium chain fatty acids regulates beta cell function[J]. Nutrients, 2018, 10(4): 473. DOI:10.3390/nu10040473 |
| [54] |
ODLE J, LIN X, WIELAND T M, et al. Emulsification and fatty acid chain length affect the kinetics of[14C]-medium-chain triacylglycerol utilization by neonatal piglets[J]. The Journal of Nutrition, 1994, 124(1): 84-93. DOI:10.1093/jn/124.1.84 |
| [55] |
LEE H F, CHIANG S H. Energy value of medium-chain triglycerides and their efficacy in improving survival of neonatal pigs[J]. Journal of Animal Science, 1994, 72(1): 133-138. DOI:10.2527/1994.721133x |
| [56] |
AZAIN M J. Effects of adding medium-chain triglycerides to sow diets during late gestation and early lactation on litter performance[J]. Journal of Animal Science, 1993, 71(11): 3011-3019. DOI:10.2527/1993.71113011x |
| [57] |
ALLEE G L, ROMSOS D R, LEVEILLE G A, et al. Metabolic consequences of dietary medium-chain triglycerides in the pig[J]. Proceedings of the Society for Experimental Biology and Medicine.Society for Experimental Biology and Medicine, 1972, 139(2): 422-427. DOI:10.3181/00379727-139-36158 |
| [58] |
DIERICK N, MICHIELS J, VAN NEVEL C. Effect of medium chain fatty acids and benzoic acid, as alternatives for antibiotics, on growth and some gut parameters in piglets[J]. Communications in Agricultural and Applied Biological Sciences, 2004, 69(2): 187-190. |
| [59] |
HONG S M, HWANG J H, KIM I H. Effect of medium-chain triglyceride (MCT) on growth performance, nutrient digestibility, blood characteristics in weanling pigs[J]. Asian-Australasian Journal of Animal Sciences, 2012, 25(7): 1003-1008. DOI:10.5713/ajas.2011.11402 |
| [60] |
LI Y, ZHANG H, YANG L, et al. Effect of medium-chain triglycerides on growth performance, nutrient digestibility, plasma metabolites and antioxidant capacity in weanling pigs[J]. Animal Nutrition, 2015, 1(1): 12-18. DOI:10.1016/j.aninu.2015.02.001 |
| [61] |
CHWEN L T, FOO H L, THANH N T, et al. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol[J]. Asian-Australasian Journal of Animal Sciences, 2013, 26(5): 700-704. DOI:10.5713/ajas.2012.12561 |
| [62] |
KUANG Y, WANG Y, ZHANG Y, et al. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs[J]. Animal Feed Science and Technology, 2015, 208: 145-157. DOI:10.1016/j.anifeedsci.2015.07.010 |




