2. 中国水产科学研究院淡水渔业研究中心, 农业农村部淡水渔业和种质资源利用重点实验室, 无锡 214081
2. Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China
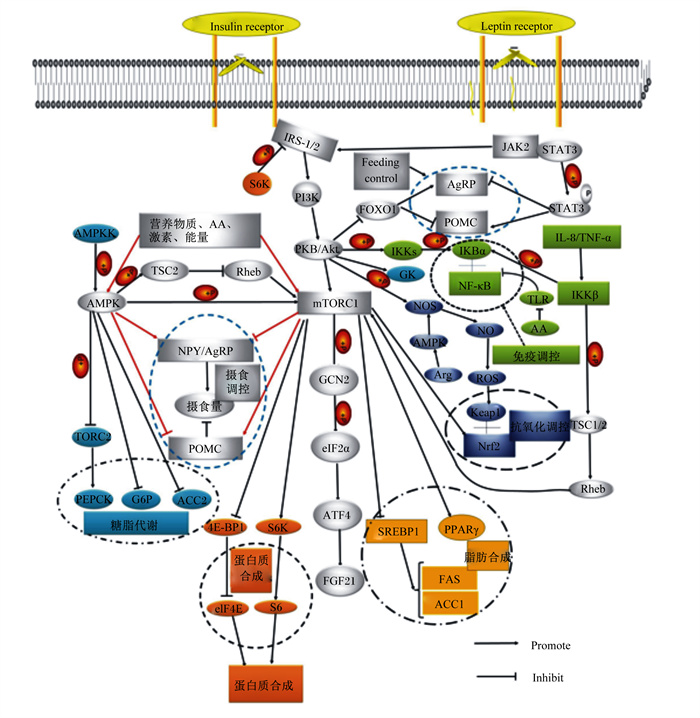
雷帕霉素靶蛋白(target of rapamycin,TOR)是一种高度保守的蛋白激酶,属于磷酸肌醇相关激酶(phosphatidylinositol kinase-related kinases,PIKKs)家族成员[1]。TOR通常与其他蛋白相互作用,形成2个不同的蛋白复合物雷帕霉素靶蛋白复合体1(target of rapamycin complex 1,TORC1)和雷帕霉素靶蛋白复合体2(target of rapamycin complex 2,TORC2),以调节下游效应因子[2]。TORC1由TOR、TOR调节相关蛋白Raptor和mLST8组成,除此之外,TORC1的激活还涉及一系列调节蛋白如Rag GTP酶(Rag GTPases)[3]。TORC1被认为是营养物质和营养相关信号对细胞生长和代谢的调控中心,它整合了包括生长因子、氨基酸和能量等在内的一系列上游信号[4]。同时,TOR在调节细胞生长和细胞周期中起中枢作用。它能够通过激活下游真核起始因子4结合蛋白1(eukaryotic translation initiation factor 4E binding protein 1,4E-BP1)和核糖体蛋白S6激酶1(ribosomal protein S6 kinase1,S6K1),进而调控细胞生长、增殖、凋亡和自噬。本文将对鱼类TOR信号通路生物学功能的研究进展进行综述,以期为进一步研究TOR信号通路在水产动物中的作用提供参考。
1 鱼类TOR信号通路生物学功能相比于哺乳动物,有关鱼类中TOR生物学功能的研究较少,大多集中在对营养物质的代谢调控上,如蛋白质合成代谢、糖代谢、脂代谢等。研究表明,动物机体营养物质的代谢调控均是由TOR形成的复合体TORC1来参与的[5]。在免疫调控方面,Weichhart等[6]报道,TOR能够通过调节核因子-κB(nuclear factor-κB,NF-κB)的活性进而调控机体免疫,相似的结果在草鱼[7]中也有发现。此外,在哺乳动物中发现,TOR还可以通过调节食欲肽的表达进而调控摄食[8-9]。梁晓芳[10]据此对花鲈(Lateolabrax japonicus)的摄食调控进行了研究,发现TOR通过调控S6K1启动食欲肽的表达,最终调控花鲈的摄食。
1.1 摄食调控机体能量状态可由胃肠道、肝脏等外周组织器官所感应,下丘脑可以对这些传递至中枢神经系统的相关内分泌信号进行整合,通过神经信号来调控食欲相关因子,并进一步对摄食进行动态调节。下丘脑部分信号通路如哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)和腺苷酸活化蛋白激酶(adenosine monophosphate activated protein kinase,AMPK)信号通路可以参与摄食调控[11-12]。
下丘脑作为能量代谢调控中心,是mTOR主要的表达场所。pmTOR是mTOR的磷酸化形式,主要定位于室旁核和弓状核(arcuate nucleus,ARC)的神经元中。ARC中含有促食欲神经元神经肽Y(neuropeptide Y,NPY)和刺鼠相关蛋白(agouti-related peptide,AgRP)以及厌食欲神经元黑素皮质素(proopiomelanocortin,POMC)和可卡因-苯丙胺调节转录肽(cocaine-and amphetamine-regulated transcript,CART)。其中mTOR/S6K1-NPY是最直接的摄食调控信号通路。在大鼠中的研究表明,摄食后ARC神经元中mTOR活性升高,而饥饿时活性降低[11]。研究表明小鼠AgRP和NPY的表达均因NPY/AgRP神经元中mTORC1特异性受体AgRP-raptor基因的敲除有所降低,但这并未影响其摄食行为以及代谢平衡,这可能是由于mTORC1信号通路并非是调控摄食行为的必要途径[13]。作为mTOR信号通路的下游效应器,S6K1同样在中枢神经系统中广泛表达,并参与能量平衡的调节。但Smith等[14]通过敲除POMC和AgRP中的S6K1发现小鼠的采食量并未发生改变。这可能表明S6K1缺失时可能有其他机制来弥补相关功能。目前,梁晓芳[10]已在花鲈的研究中明确了mTOR和S6K1的磷酸化对于鱼类摄食调控的关键作用。
Cota等[11]研究表明,饲粮中的氨基酸对于摄食也有一定的影响,精氨酸(Arg)和亮氨酸(Leu)能够激活下丘脑mTOR,进而降低NPY和AgRP的表达,抑制动物的摄食。胰岛素可以激活mTOR调控摄食,这种调控主要是通过磷脂酰肌醇-3-激酶(phosphatidylinositol 3-kinase,PI3K)来实现的。也有研究表明食物中蛋白质含量也可以促进mTOR的磷酸化[15]。研究发现AMPK能够通过Raptor抑制mTOR的活性[16]。还可以通过结节性硬化复合物2(tuberous sclerosis complex 2,TSC2) 调节mTOR的活性[17-18]。因此,在下丘脑中,AMPK可能通过调节mTOR的活性来调控摄食量。
除上述食欲调控因子以外,也有研究表明由肝脏分泌的成纤维细胞因子21(fibroblast growth factor 21,FGF21)在摄食调控中也发挥着重要的作用[19-20]。FGF21在肝脏、胰腺、白色脂肪和棕色脂肪等多组织中均有表达[21]。如图 1所示,mTOR可以通过一般性调控阻遏蛋白激酶2(general control nonderepressible 2,GCN2)进一步调控FGF21,最终调控摄食量。
|
AMPK:腺苷酸活化蛋白激酶adenosine monophosphate activated protein kinase;TORC2:雷帕霉素靶蛋白复合体2 target of rapamycin complex 2;PEPCK:磷酸烯醇式丙酮酸羧激酶phosphoenolpyruvate carboxykinase;G6P:葡萄糖6磷酸glucose-6-phosphate;ACC:乙酰辅酶A羧酸酶acetyl-CoA carboxylate;GK:葡萄糖激酶glucose kinase;TSC2:结节性硬化复合物2 tuberous sclerosis complex 2;NPY:神经肽Y neuropeptide Y;AgRP:刺鼠相关蛋白agouti-related peptide;POMC:厌食欲神经元黑素皮质素proopiomelanocortin;PI3K:磷脂酰肌醇-3-激酶phosphatidylinositol 3-kinase;Akt:磷酸化蛋白激酶protein kinase B;GCN2:一般性调控阻遏蛋白激酶2 general control nonderepressible 2;eIF2α:真核起始因子2α eukaryotic initiation factor 2α;ATF4:转录激活子4 activating transcription factor 4;FGF21:成纤维细胞因子-21 fibroblast growth factor 21;PKB:蛋白激酶B protein kinase B;STAT3:信号传导与转录激活因子3 signal transducer and activator of transcription 3;JAK2:酪氨酸激酶Janus kinase;IRS:胰岛素受体底物insulin receptor substrate;S6K:核糖体蛋白S6激酶ribosomal protein S6 kinase;4E-BP1:真核起始因子4结合蛋白1 eukaryotic translation initiation factor 4E binding protein 1;eIF4E:真核翻译起始因子4E eukaryotic translation initiation factor 4E;SREBP-1:固醇调节元件结合蛋白-1 sterol regulatory element binding protein-1;PPARγ:过氧化物酶体增殖物激活受体γ peroxisome proliferators-activated receptor γ;FAS:脂肪酸合成酶fatty acid synthase;NF-κB:核因子-κB nuclear factor-κB;IL-8:白细胞介素-8 interleukin-8;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;AA:氨基酸amino acids;Nrf2:核因子相关因子2 nuclear related factor 2;Keap1:Kelch样环氧氯丙烷相关蛋白1 Kelch-like ECH-associated protein 1;ROS:活性氧reactive oxygen species;Insulin receptor:胰岛素受体;Leptin receptor: 瘦素受体;Feeding control:饮食控制;Promote:促进;Inhibit:抑制。 图 1 TOR信号通路与蛋白质合成代谢调控 Fig. 1 TOR signaling pathway and protein anabolism regulation |
基于上述基因对摄食的调控作用,可应用于鱼类促摄食调控添加剂,以此人为调控鱼类摄食,从而提高饲料利用率,但其最适添加量还有待研究。
1.2 代谢调控 1.2.1 蛋白质合成代谢蛋白质合成是生长反应过程的关键组成部分,其限速步骤是翻译起始[22]。TORC1调控着整体的翻译水平,通过其下游4E-BP1和S6K1的磷酸化调控蛋白质合成与代谢[23]。S6K1和4E-BP1的磷酸化均依赖于Raptor,且相比于S6K1,4E-BP1的磷酸化对Raptor的依赖性更强[24]。真核翻译起始因子4E(eIF4E)可以被4E-BP1所抑制,通过两者的结合可以抑制蛋白质翻译[25]。mTOR磷酸化4E-BP1从而使其失去活性,并与eIF4E发生解离,进而4E-BP1对eIF4E起始翻译的抑制作用降低,使eIF4E的表达增高,进一步促进了蛋白质的翻译[26]。而p70S6K的磷酸化则直接促进翻译的起始过程,主要通过被磷酸化的S6K1使核糖体蛋白S6的5个丝氨酸残基发生磷酸化,增强含嘧啶基因mRNA的翻译功能,从而调节蛋白质的合成[27]。
有研究表明,适量的精氨酸水平可以提高蛋白质含量,主要是由于精氨酸或其代谢产物(谷氨酰胺和一氧化氮)通过激活机体mTOR信号通路进而磷酸化S6K1和真核起始因子4结合蛋白(eukaryotic translation initiation factor 4E binding proteins,4E-BPs),进一步激活启动多肽合成,最终促进蛋白质的合成[28-29]。除了营养素的调控,在最近的研究中,Wu等[30]研究发现在适宜的盐度下养殖吉富罗非鱼(Oreochromis niloticus)幼鱼可以通过刺激TOR信号通路提高鱼体的蛋白质合成。此研究可在吉富罗非鱼幼鱼的养殖生产中为其养殖盐度的选择提供理论指导,同时我国作为盐碱地大国,这对于开发利用盐碱水域开展水产养殖具有重要的现实意义。其他环境因子如温度、pH等对蛋白质合成的影响也有部分研究,但对于环境影响蛋白质合成的具体机制还有待进一步研究。
1.2.2 脂代谢组织中脂肪的形成过程中,mTOR起着至关重要的作用[31]。在体外,通过抑制mTOR的活性可以阻断脂肪生成[32],而mTOR的过度活化则会促进脂肪生成[33]。有研究报道激活mTOR可以抑制脂肪分解,同时刺激脂肪从头合成并促进细胞内的脂肪储存[34]。此外,mTOR下游的S6K1能够通过调节转录因子的表达来调控脂肪形成[35]。
乙酰辅酶A羧酸酶(acetyl-CoA carboxylate,ACC)、脂肪酸合成酶(fatty acid synthase,FAS)和过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptor γ,PPARγ)是机体调控脂肪沉积主要酶和因子[36]。PPARγ参与脂肪的合成,能够激活脂肪细胞的分化并且促进脂肪酸在脂肪组织中沉积,同时还可以调控特殊脂肪基因的表达[37]。mTOR调控脂肪细胞的终末分化主要是通过控制调节因子PPARγ的翻译来完成的[38]。除了通过激活脂肪生成因子直接促进脂肪组织扩张外,mTORC1还通过S6K1介导的胰岛素信号传导抑制脂肪组织中的胰岛素抵抗[39]。
脂肪合成相关酶的表达受固醇调节元件结合蛋白-1(sterol regulatory element binding protein-1,SREBP-1)的影响,同时它还可以调控脂肪和胆固醇的稳态。TORC1上调磷酸戊糖途径相关基因的转录以及脂肪和甾醇的合成主要是通过激活SREBP-1来间接完成的[40]。在其脂质合成作用的研究中发现,进食或胰岛素刺激均能提高SREBP-1的活性[41-42]。除能独立调控SREBP-1活性外,TORC1还可以与磷酸化蛋白激酶(protein kinase B,Akt)协同调节[43]。在脂肪细胞中,脂质合成基因碳水化合物反应元件结合蛋白(carbohydrate response element binding protein,ChREBP)的表达会随着TORC2活性的丧失而下降,进而引起脂质合成以及脂肪生成转录因子的减少[44-45]。有研究表明,TORC2也可以促进肝脏脂肪的合成,这表明TORC2在脂质合成中具有广泛的作用[46]。
在斑马鱼(Brachydanio rerio var)中的研究表明,餐后TOR信号通路被激活,FAS和ACCα基因的表达显著上调;同时,斑马鱼肝胰脏肉碱棕榈酰基转移酶1b(carnitine palmityl transferase 1b,CPT1b)的表达受到显著抑制[47]。而在虹鳟(Oncorhynchus mykiss)腹腔注射雷帕霉素后,脂肪合成途径相关酶基因的表达将显著下调,这些研究进一步说明脂代谢过程受TOR的调控[48]。此外,黄皓琰[49]对N-氨甲酰谷氨酸(NCG)抑制细胞外信号调节蛋白激酶1/2(ERK1/2)-mTOR-S6K1信号通路调节脂代谢来减少花鲈肝脏脂肪蓄积进而改善肝脏健康的分子机制进行了探索。上述脂代谢调控机制的研究结果将有助于鱼类脂肪肝的调节。
1.2.3 糖代谢mTOR的蛋白质复合物mTORC1通过调节众多糖酵解基因的正向调节子缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)的转录和翻译过程,进而增加糖酵解过程[50-52]。一些研究表明,mTORC1通过调节PPARγ的共激活子PGC1α以及正向调节线粒体合成和氧化功能的转录因子Ying-Yang1(YY1)来增加线粒体的DNA含量以及一些氧化基因的表达[53]。此外,mTORC1还可以通过促进核受体共抑制子在核内的聚集来抑制过氧化物酶体增殖物激活受体α(peroxisome proliferators-activated receptor α,PPARα)的活性,进而抑制肝脏内酮体的生成[54],并且研究表明该过程受S6K2的调节[55]。
胰岛素抵抗是指靶组织对正常循环水平的反应性降低,导致葡萄糖耐受不良、肥胖、血脂异常等代谢综合症状[56]。导致胰岛素抵抗的因素众多,如TOR的超活化[57]。同样的,胰岛素受体底物(insulin receptor substrate,IRS)/PI3K/Akt信号通路在胰岛素抵抗中发挥重要作用[58]。IRS作为信使分子激活的信号受体,是胰岛素作用的重要步骤[59]。糖代谢中利用糖的主要途径是糖酵解,此途径有2个重要的限速酶丙酮酸激酶(pyruvate kinase,PK)和葡萄糖激酶(glucokinase,GK)[60]。通过提高胰岛素信号通路中IRS、PI3K和Akt等核心基因表达,可以进一步提高GK基因的表达,从而促进糖酵解。如图 1所示,胰岛素/胰岛素样生长因子、能量状态(AMPK)和氨基酸等营养物质均能激活TOR,进而调控TOR的下游信号S6K1和4EBP,但是S6K1的过度表达会通过负反馈调节机制抑制胰岛素信号通路中IRS1的活性,并进一步抑制PI3K和下游Akt的活性,促进糖异生核心基因葡萄糖6磷酸酶(glucose-6-phosphate,G6P)和磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase,PEPCK)的表达,从而形成一个“负反馈环”,并对机体葡萄糖转运和代谢产生不利影响[61],即胰岛素抵抗。
在团头鲂(Megalobrama amblycephala)幼鱼中的研究表明精氨酸过量时会导致S6K1的过表达,从而通过负反馈调控机制来抑制胰岛素信号通路核心基因的表达,同时降低葡萄糖转运体2(glucose transporter 2,GLUT2)和血清胰岛素水平,提高糖异生核心基因(PEPCK和G6P)的表达,从而产生胰岛素抵抗现象,导致血糖水平异常[62]。此外,TOR对葡萄糖转运有着重要的影响,它能够显著抑制葡萄糖转运子GLUT4的表达以及脂肪的糖摄取功能[63-64]。在虹鳟的肝细胞中也有研究表明,氨基酸单独作用时可以调控糖代谢,但它的调控方式与TOR信号通路的作用模式不同,而氨基酸与胰岛素共同发挥作用时,可以产生具有典型TOR信号通路的调控模式,包括脂肪合成以及糖酵解的增强[65-66]。在氨基酸中,亮氨酸调控TOR信号通路的作用比甲硫氨酸和赖氨酸更强,当它和胰岛素共同作用时,可抑制G6P的表达近90%,提高脂肪酸合成酶基因表达4倍以上[67]。这表明胰岛素与氨基酸之间存在协同作用。除此以外,微量元素如镁等也可以影响鱼类机体的糖代谢[68],但与TOR之间的调控还有待研究。
1.3 免疫调控mTOR信号通路的免疫调控主要是通过影响mRNA的稳定性,从而进一步影响T细胞中免疫因子的表达[69-70]。NF-κB的表达在机体免疫中起着非常重要的作用,mTOR是NF-κB的上游信号分子,可以负调控NF-κB信号通路[7]。通过雷帕霉素抑制mTOR后,NF-κB转录活性提高,白细胞介素-1β(interleukin-1β,IL-1β)、白细胞介素-8(interleukin-8,IL-8)和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等促炎症因子的表达也因此上调,进而启动免疫反应,同时抑制信号传导降低抗炎症因子白细胞介素-10(interleukin-10,IL-10)的表达。IL-10和转化生长因子-β(transforming growth factor-β,TGF-β)是重要的抗炎症因子,可以通过抑制促炎症因子的生成进而降低炎症反应对机体的损伤[71]。此外,还有研究发现NF-κB与病毒之间可能存在紧密复杂的关系[72],其机制还需进一步研究。
有研究表明,饲料中精氨酸水平最佳时可以提高黄颡鱼(Pelteobagrus fulvidraco)和斜带石斑鱼(Epinephelus coioides)的免疫应答[73-74]。而饲料中氨基酸不平衡(缺乏或过量)时会降低营养物质利用率,对免疫器官和免疫应答产生直接的负面影响[75]。王标[76]研究表明,精氨酸可以通过上调TOR的表达来抑制NF-κB的转录活性,从而抑制炎症因子的表达;同时,精氨酸还可以通过抑制NF-κB核转位抑制促炎症因子的表达,NF-κB抑制剂α(inhibitor α of NF-κB,IκBα)作为NF-κB p65的一个特异性的抑制蛋白,可以与NF-κB结合抑制其核转位,从而降低下游促炎症因子的表达。
Liang等[77]对团头鲂幼鱼肠道免疫的研究发现,饲料中最佳精氨酸水平时可以通过提高TOR的mRNA表达量进而提高肠道抗氧化能力和免疫功能,并在维持肠道健康方面发挥重要作用。
1.4 抗氧化调控在机体正常生理代谢和发生免疫反应时,机体会产生活性氧(reactive oxygen species,ROS),ROS主要包括超氧阴离子(O2-·)、过氧化氢(H2O2)和一氧化氮(NO)等。当机体内ROS含量过高而超出机体清除范围时,会造成氧化应激,引起蛋白质氧化、脂质氧化、染色体断裂以及DNA碱基损伤。由于养殖鱼类的肌肉中含有大量的不饱和脂肪酸(约33%),容易遭受氧化损伤[78]。为了抵御外界的氧化损伤和ROS的攻击,细胞形成了一套包括抗氧化酶和非酶性抗氧化物在内的抗氧化防御系统[79]。
核因子相关因子2(nuclear related factor 2,Nrf2)是水产动物机体抗氧化调控的重要因子,可以调控抗氧化酶基因的表达。Liang等[66]研究表明,饲料中精氨酸水平最佳时可以通过激活团头鲂Nrf2信号通路提高抗氧化酶或非酶抗氧化物的活性,上调其在肠道中的基因转录。在正常情况下,Nrf2的核转位被Kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein 1,Keap1)所抑制,当Nrf2和Keap1分离后,Nrf2发生转移与抗氧化反应元件(antioxidant response element,ARE)上的位点结合,从而启动ARE调控抗氧化酶基因的表达,增加细胞对氧化应激的抵抗能力[80]。
在草鱼的研究中发现苯丙氨酸可以通过上调TOR的表达进而提高Nrf2的基因表达量,并进一步上调抗氧化酶基因的表达[81]。在Liang等[82]的研究中也发现类似的结果,亮氨酸可以通过激活TOR提高Nrf2的表达,进而调节抗氧化酶基因的表达。Seiliez等[83]研究发现胰岛素可以上调虹鳟TOR基因的表达量,Nuttall等[84]研究发现苯丙氨酸(Phe)可以提高人类血清中胰岛素水平,由此推测苯丙氨酸上调Nrf2的mRNA表达量可能与其调节TOR信号通路有关。在哺乳动物上的研究发现,上调TOR及其下游靶蛋白S6K1基因的表达能够显著提高Nrf2的表达[85]。王标[76]在草鱼(Ctenopharyngodon idella)的研究中发现精氨酸可以通过上调TOR和S6K1的表达,进而上调Nrf2的表达。由此可以看出,上调抗氧化酶基因的表达主要是通过TOR进行调控的。
2 小结与展望营养素、氨基酸以及激素等均可引起信号通路应答,从而间接调节蛋白质合成代谢、糖与脂代谢、摄食与免疫及抗氧化等过程,目前在哺乳动物中有关上述调控机制已有较深入的研究,但在水产动物中相关研究尚浅,且各营养素与TOR之间的联系也需进一步探索,如已有研究发现硫辛酸可影响草鱼脂代谢[86],桑叶黄酮可影响吉富罗非鱼肌肉抗氧化性能[87],但如何通过TOR信号通路介导还不清晰。这些机制的探索将对提升水产动物的品质提供一定的理论依据。尤其对于养殖鱼类摄食的调控,尽管TOR信号通路与摄食调控机制在鱼类中已有部分研究,但关于FGF21等诸多食欲调节因子在鱼类营养中的报道较少,且它们与TOR的调节机制也有待深入研究。此外,我们还可以探索氨基酸、营养物质以及激素在摄食调控方面的最佳需要量,以便应用于水产饲料添加剂,人为调控水产动物的摄食,提高饲料利用率。
目前,关于环境盐度、温度等对于TOR信号通路调节的分子机制研究较少,Wu等[30]研究发现,8‰盐度养殖环境下吉富罗非鱼幼鱼的生长及蛋白质合成均高于淡水中,且在淡水和半咸水中吉富罗非鱼幼鱼的营养代谢也不同。但到目前为止,盐度是如何调节TOR信号通路并进一步调控生长、免疫以及糖与脂代谢的还有待研究,是否像调控4E-BP1和S6K1一样通过盐度的变化使得胰岛素和生长激素发生变化,再由PI3K-AKT-TOR来实现对生长、营养代谢的调控?盐度升高时,生长会通过上述途径受到抑制,那对于等渗点时生长的促进作用又是如何实现的?相同的问题在温度对TOR的调节中也有待深入研究。解决这些问题后,我们可以在实际生产中人为地改变养殖环境,如改变养殖水体盐度、利用仪器加热等,使得养殖鱼类的生长潜能得到更好地发挥。目前,罗非鱼已普遍采取半咸水养殖,但在其他鱼类中的应用还有待探索。总之,随着TOR信号通路各种调节机制的深入研究,并应用于养殖生产中,其养殖效益也将有所提高。
| [1] |
刘宁, 刘国华, 蔡辉益, 等. 营养介导的TOR信号传导研究进展[J]. 中国畜牧兽医, 2010, 37(6): 25-29. LIU N, LIU G H, CAI H Y, et al. Advances in nutrient regulation of tor signal transduction[J]. China Animal Husbandry & Veterinary Medicine, 2010, 37(6): 25-29 (in Chinese). |
| [2] |
SABATINI D M. Twenty-five years of mTOR: uncovering the link from nutrients to growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(45): 11818-11825. DOI:10.1073/pnas.1716173114 |
| [3] |
WOLFSON R L, SABATINI D M. The dawn of the age of amino acid sensors for the mTORC1 pathway[J]. Cell Metabolism, 2017, 26(2): 301-309. DOI:10.1016/j.cmet.2017.07.001 |
| [4] |
CHANTRANUPONG L, WOLFSON R L, SABATINI D M. Nutrient-sensing mechanisms across evolution[J]. Cell, 2015, 161(1): 67-83. DOI:10.1016/j.cell.2015.02.041 |
| [5] |
LAPLANTE M, SABATINI D M. mTOR signaling in growth control and disease[J]. Cell, 2012, 149(2): 274-293. DOI:10.1016/j.cell.2012.03.017 |
| [6] |
WEICHHART T, COSTANTINO G, POGLITSCH M A, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response[J]. Immunity, 2008, 29(4): 565-577. DOI:10.1016/j.immuni.2008.08.012 |
| [7] |
邓玉平. 亮氨酸对生长中期草鱼生长、肌肉品质和肠道免疫的影响研究[D]. 硕士学位论文. 雅安: 四川农业大学, 2014. DENG Y P. Effects of graded levels of dietary leucine on growth, flesh quality, intestinal immunity and antioxidant statusin young grass carp (Ctenopharyngodon idella)[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2014. (in Chinese) |
| [8] |
COTA D, MATTER E K, WOODS S C, et al. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity[J]. The Journal of Neuroscience, 2008, 28(28): 7202-7208. DOI:10.1523/JNEUROSCI.1389-08.2008 |
| [9] |
BLOUET C, ONO H, SCHWARTZ G J. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis[J]. Cell Metabolism, 2008, 8(6): 459-467. DOI:10.1016/j.cmet.2008.10.004 |
| [10] |
梁晓芳. 花鲈利用鱼粉和植物蛋白源的选择性摄食调控机制研究[D]. 博士学位论文. 北京: 中国农业科学院, 2017. LIANG X F. Mechanism on feed intake regulation of Lateolabrax japonicas when fishmeal was replaced by plant protein[D]. Ph. D. Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2017. (in Chinese) |
| [11] |
COTA D, PROULX K, SMITH K A B, et al. Hypothalamic mTOR signaling regulates food intake[J]. Science, 2006, 312(5775): 927-930. DOI:10.1126/science.1124147 |
| [12] |
HAO S Z, SHARP J W, ROSS-INTA C M, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex[J]. Science, 2005, 307(5716): 1776-1778. DOI:10.1126/science.1104882 |
| [13] |
ALBERT V, CORNU M, HALL M N. mTORC1 signaling in Agrp neurons mediates circadian expression of AgRP and NPY but is dispensable for regulation of feeding behavior[J]. Biochemical and Biophysical Research Communications, 2015, 464(2): 480-486. DOI:10.1016/j.bbrc.2015.06.161 |
| [14] |
SMITH M A, KATSOURI L, IRVINE E E, et al. Ribosomal S6K1 in POMC and AgRP neurons regulates glucose homeostasis but not feeding behavior in mice[J]. Cell Reports, 2015, 11(3): 335-343. DOI:10.1016/j.celrep.2015.03.029 |
| [15] |
LIU Y Y, LI F N, KONG X F, et al. Signaling pathways related to protein synthesis and amino acid concentration in pig skeletal muscles depend on the dietary protein level, genotype and developmental stages[J]. PLoS One, 2015, 10(9): e0138277. DOI:10.1371/journal.pone.0138277 |
| [16] |
GWINN D M, SHACKELFORD D B, EGAN D F, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint[J]. Molecular Cell, 2008, 30(2): 214-226. DOI:10.1016/j.molcel.2008.03.003 |
| [17] |
INOKI K, ZHU T Q, GUAN K L. TSC2 mediates cellular energy response to control cell growth and survival[J]. Cell, 2003, 115(5): 577-590. DOI:10.1016/S0092-8674(03)00929-2 |
| [18] |
HAISSAGUERRE M, SAUCISSE N, COTA D. Influence of mTOR in energy and metabolic homeostasis[J]. Molecular and Cellular Endocrinology, 2014, 397(1/2): 67-77. |
| [19] |
LAEGER T, REED S D, HENAGAN T M, et al. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein[J]. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 2014, 307(3): R310-R320. DOI:10.1152/ajpregu.00116.2014 |
| [20] |
VON HOLSTEIN-RATHLOU S, BONDURANT L D, PELTEKIAN L, et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver[J]. Cell Metabolism, 2016, 23(2): 335-343. DOI:10.1016/j.cmet.2015.12.003 |
| [21] |
FON TACER K, BOOKOUT A L, DING X S, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse[J]. Molecular Endocrinology, 2010, 24(10): 2050-2064. DOI:10.1210/me.2010-0142 |
| [22] |
MASTER A, NAUMAN A. Molecular mechanisms of protein biosynthesis initiation-biochemical and biomedical implications of a new model of translation enhanced by the RNA hypoxia response element (rHRE)[J]. Postepy Biochemii, 2014, 60(1): 39-54. |
| [23] |
DENNIS P B, JAESCHKE A, SAITOH M, et al. Mammalian TOR: a homeostatic ATP sensor[J]. Science, 2001, 294(5544): 1102-1105. DOI:10.1126/science.1063518 |
| [24] |
刘新伟. 凡纳滨对虾TOR信号通路及其2个重要成员的功能研究[D]. 硕士学位论文. 青岛: 中国科学院大学, 2018. LIU X W. Study on TOR signaling pathway and functional analysis of two important factors in Litopenaeus vannamei[D]. Master's Thesis. Qingdao: University of Chinese Academy of Sciences, 2018. (in Chinese) |
| [25] |
孟艳梅. 慢性粒细胞白血病患者SHIP基因及4EBP1基因的表达及其意义[D]. 硕士学位论文. 石家庄: 河北医科大学, 2014. MENG Y M. The significance and expression of SHIP gene and 4EBP1 gene in chronic myeloid leukemia patients[D]. Master's Thesis. Shijiazhuang: Hebei Medical University, 2014. (in Chinese) |
| [26] |
孟兰佳. Bufalin对人食管癌细胞4EBP1活化及凋亡的影响[D]. 硕士学位论文. 石家庄: 河北医科大学, 2014. MENG L J. The effect of Bufalin on the activation of 4EBP1 and cell apoptosis in human esophageal cancer cells[D]. Master's Thesis. Shijiazhuang: Hebei Medical University, 2014. (in Chinese) |
| [27] |
INOKI K, OUYANG H J, LI Y, et al. Signaling by target of rapamycin proteins in cell growth control[J]. Microbiology and Molecular Biology Reviews, 2005, 69(1): 79-100. DOI:10.1128/MMBR.69.1.79-100.2005 |
| [28] |
PERVIN S, SINGH R, HERNANDEZ E, et al. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway[J]. Cancer Research, 2007, 67(1): 289-299. DOI:10.1158/0008-5472.CAN-05-4623 |
| [29] |
YUAN C, DING Y, HE Q, et al. L-arginine upregulates the gene expression of target of rapamycin signaling pathway and stimulates protein synthesis in chicken intestinal epithelial cells[J]. Poultry Science, 2015, 94(5): 1043-1051. DOI:10.3382/ps/pev051 |
| [30] |
WU L H, LIANG H L, HAMUNJO C M K, et al. Culture salinity alters dietary protein requirement, whole body composition and nutrients metabolism related genes expression in juvenile genetically improved farmed tilapia (GIFT) (Oreochromis niloticus)[J]. Aquaculture, 2021, 531: 735961. DOI:10.1016/j.aquaculture.2020.735961 |
| [31] |
LAPLANTE M, SABATINI D M. An emerging role of mTOR in lipid biosynthesis[J]. Current Biology, 2009, 19(22): R1046-R1052. DOI:10.1016/j.cub.2009.09.058 |
| [32] |
GAGNON A, LAU S, SORISKY A. Rapamycin-sensitive phase of 3T3-L1 preadipocyte differentiation after clonal expansion[J]. Journal of Cellular Physiology, 2001, 189(1): 14-22. DOI:10.1002/jcp.1132 |
| [33] |
ZHANG H H, HUANG J X, DVVEL K, et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway[J]. PLoS One, 2009, 4(7): e6189. DOI:10.1371/journal.pone.0006189 |
| [34] |
CHAKRABARTI P, ENGLISH T, SHI J, et al. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage[J]. Diabetes, 2010, 59(4): 775-781. DOI:10.2337/db09-1602 |
| [35] |
CARNEVALLI L S, MASUDA K, FRIGERIO F, et al. S6K1 plays a critical role in early adipocyte differentiation[J]. Developmental Cell, 2010, 18(5): 763-774. DOI:10.1016/j.devcel.2010.02.018 |
| [36] |
周招洪. 饲粮能量和精氨酸水平对育肥猪肉品质和脂肪代谢的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2014. ZHOU Z H. Effects of dietary energy and arginine levels on pork quality and lipid metabolism of finishing pigs[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2014. (in Chinese) |
| [37] |
BISPHAM J, GARDNER D S, GNANALINGHAM M G, et al. Maternal nutritional programming of fetal adipose tissue development: differential effects on messenger ribonucleic acid abundance for uncoupling proteins and peroxisome proliferator-activated and prolactin receptors[J]. Endocrinology, 2005, 146(9): 3943-3949. DOI:10.1210/en.2005-0246 |
| [38] |
LE BACQUER O, PETROULAKIS E, PAGLIALUNGA S, et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2[J]. The Journal of Clinical Investigation, 2007, 117(2): 387-396. DOI:10.1172/JCI29528 |
| [39] |
UM S H, FRIGERIO F, WATANABE M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity[J]. Nature, 2004, 431(7005): 200-205. DOI:10.1038/nature02866 |
| [40] |
DVVEL K, YECIES J L, MENON S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1[J]. Molecular Cell, 2010, 39(2): 171-183. DOI:10.1016/j.molcel.2010.06.022 |
| [41] |
TIAN J, GOLDSTEIN J L, BROWN M S. Insulin induction of SREBP-1c in rodent liver requires LXRα-C/EBPβ complex[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(29): 8182-8187. DOI:10.1073/pnas.1608987113 |
| [42] |
HAAS J T, MIAO J, CHANDA D, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression[J]. Cell Metabolism, 2012, 15(6): 873-884. DOI:10.1016/j.cmet.2012.05.002 |
| [43] |
赵迪, 朱燕婷, 史道华. mTOR介导转录因子调控细胞糖脂代谢的研究进展[J]. 基础医学与临床, 2014, 34(11): 1574-1577. ZHAO D, ZHU Y T, SHI D H. Research progress in cell metabolism of glucose and lipid regulated by transcription factors via mTOR[J]. Basic & Clinical Medicine, 2014, 34(11): 1574-1577 (in Chinese). |
| [44] |
KUMAR A, LAWRENCE J C, J r, JUNG D Y, et al. Fat cell-specific ablation of Rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism[J]. Diabetes, 2010, 59(6): 1397-1406. DOI:10.2337/db09-1061 |
| [45] |
TANG Y F, WALLACE M, SANCHEZ-GURMACHES J, et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism[J]. Nature Communications, 2016, 7: 11365. DOI:10.1038/ncomms11365 |
| [46] |
HAGIWARA A, CORNU M, CYBULSKI N, et al. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c[J]. Cell Metabolism, 2012, 15(5): 725-738. DOI:10.1016/j.cmet.2012.03.015 |
| [47] |
SEILIEZ I, MÉDALE F, AGUIRRE P, et al. Postprandial regulation of growth- and metabolism-related factors in zebrafish[J]. Zebrafish, 2013, 10(2): 237-248. DOI:10.1089/zeb.2012.0835 |
| [48] |
DAI W W, PANSERAT S, MENNIGEN J A, et al. Post-prandial regulation of hepatic glucokinase and lipogenesis requires the activation of TORC1 signalling in rainbow trout (Oncorhynchus mykiss)[J]. Journal of Experimental Biology, 2013, 216(23): 4483-4492. |
| [49] |
黄皓琰. N-氨甲酰谷氨酸在花鲈饲料中的有效性和耐受评价及其调控营养代谢的机制研究[D]. 硕士学位论文. 北京: 中国农业科学院, 2019. HUANG H Y. Efficacy and tolerance evaluation of N-carbamylglutamate in Japanese sebass (Lateolabrax japonicas) diet and the related nutrient metabolism regulation mechanism[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2019. (in Chinese) |
| [50] |
BRUGAROLAS J B, VAZQUEZ F, REDDY A, et al. TSC2 regulates VEGF through mTOR-dependent and-independent pathways[J]. Cancer Cell, 2003, 4(2): 147-158. DOI:10.1016/S1535-6108(03)00187-9 |
| [51] |
HUDSON C C, LIU M, CHIANG G G, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin[J]. Molecular and Cellular Biology, 2002, 22(20): 7004-7014. DOI:10.1128/MCB.22.20.7004-7014.2002 |
| [52] |
LAUGHNER E, TAGHAVI P, CHILES K, et al. HER2(neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression[J]. Molecular and Cellular Biology, 2001, 21(12): 3995-4004. DOI:10.1128/MCB.21.12.3995-4004.2001 |
| [53] |
CUNNINGHAM J T, RODGERS J T, ARLOW D H, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex[J]. Nature, 2007, 450(7170): 736-740. DOI:10.1038/nature06322 |
| [54] |
LEFEBVRE P, CHINETTI G, FRUCHART J C, et al. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis[J]. The Journal of Clinical Investigation, 2006, 116(3): 571-580. DOI:10.1172/JCI27989 |
| [55] |
KIM K, PYO S, UM S H. S6 kinase 2 deficiency enhances ketone body production and increases peroxisome proliferator-activated receptor alpha activity in the liver[J]. Hepatology, 2012, 55(6): 1727-1737. DOI:10.1002/hep.25537 |
| [56] |
AL-JADA D N, AHMAD M N. Dietary fat and insulin resistance: a connection through leptin and PPARγ activation[J]. Functional Foods in Health and Disease, 2016, 6(6): 306-328. DOI:10.31989/ffhd.v6i6.249 |
| [57] |
栾会玲, 王茉, 翁雨晴, 等. mTOR信号调节糖脂代谢的研究进展[J]. 海峡药学, 2018, 30(5): 5-8. LUAN H L, WANG M, WENG Y Q, et al. Advances in mTOR signal regulation of glycolipid metabolism[J]. Strait Pharmaceutical Journal, 2018, 30(5): 5-8 (in Chinese). DOI:10.3969/j.issn.1006-3765.2018.05.002 |
| [58] |
BRUCE K D, HANSON M A. The developmental origins, mechanisms, and implications of metabolic syndrome[J]. The Journal of Nutrition, 2010, 140(3): 648-652. DOI:10.3945/jn.109.111179 |
| [59] |
CHEATHAM B, KAHN C R. Insulin action and the insulin signaling network[J]. Endocrine Reviews, 1995, 16(2): 117-142. |
| [60] |
唐小红, 樊佳佳, 于凌云, 等. 鱼类糖酵解关键酶的研究进展[J]. 中国农学通报, 2014, 30(2): 69-75. TANG X H, FAN J J, YU L Y, et al. Research advances in glycolytic key enzyme of fish[J]. Chinese Agricultural Science Bulletin, 2014, 30(2): 69-75 (in Chinese). |
| [61] |
WULLSCHLEGER S, LOEWITH R, HALL M N. TOR signaling in growth and metabolism[J]. Cell, 2006, 124(3): 471-484. DOI:10.1016/j.cell.2006.01.016 |
| [62] |
梁化亮. 精氨酸对团头鲂幼鱼生长、营养代谢和免疫功能的影响及作用机制[D]. 博士学位论文. 南京: 南京农业大学, 2019. LIANG H L. Effects of dietary arginine on growth, nutrient metabolism and immune capacity involved in fuctional mechanism in juvenile blunt snout bream, Megalobrama amblycephala[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2019. (in Chinese) |
| [63] |
FUHRMANN A, LOPES P, SERENO J, et al. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model[J]. Biochemical Pharmacology, 2014, 88(2): 216-228. DOI:10.1016/j.bcp.2014.01.020 |
| [64] |
许戈阳, 刘芬婷, 沈哲民, 等. mTOR信号通路在糖代谢中作用[J]. 生理科学进展, 2015, 46(2): 94-98. XU G Y, LIU F T, SHEN Z M, et al. The role of mTOR signaling pathway in glucose metabolism[J]. Progress in Physiological Sciences, 2015, 46(2): 94-98 (in Chinese). |
| [65] |
LANSARD M, PANSERAT S, PLAGNES-JUAN E, et al. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR[J]. Amino Acids, 2010, 39(3): 801-810. DOI:10.1007/s00726-010-0533-3 |
| [66] |
辛芳, 王雷, 刘梅, 等. 水产动物雷帕霉素受体信号通路的研究进展[J]. 海洋科学, 2016, 40(1): 147-154. XIN F, WANG L, LIU M, et al. Mechanistic target of rapamycin signaling in aquatic animals[J]. Marine Sciences, 2016, 40(1): 147-154 (in Chinese). |
| [67] |
LANSARD M, PANSERAT S, PLAGNES-JUAN E, et al. L-leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes[J]. The Journal of Nutrition, 2011, 141(1): 75-80. DOI:10.3945/jn.110.124511 |
| [68] |
汪福保, 罗莉, 文华, 等. 镁对草鱼生长、形体、肝功能和糖代谢的影响[J]. 淡水渔业, 2011, 41(2): 57-62, 68. WANG F B, LUO L, WEN H, et al. Effects of dietary magnesium on the growth, body index, liver function and glucose metabolism of grass carp, Ctenopharyngodon idella[J]. Freshwater Fisheries, 2011, 41(2): 57-62, 68 (in Chinese). DOI:10.3969/j.issn.1000-6907.2011.02.009 |
| [69] |
陈银涛, 于秉治, 武迪迪. PI3K/Akt/mTOR信号通路及临床相关肿瘤抑制剂[J]. 中国生物化学与分子生物学报, 2014, 30(10): 949-956. CHEN Y T, YU B Z, WU D D. PI3K/Akt/mTOR signaling and the related cancer inhibitors[J]. Chinese Journal of Biochemistry and Molecular Biology, 2014, 30(10): 949-956 (in Chinese). |
| [70] |
陈洪菊, 屈艺, 母得志. mTOR信号通路的生物学功能[J]. 生命的化学, 2010, 30(4): 555-561. CHEN H J, QU Y, MU D Z. The progress of study on the biological function of mTOR pathway[J]. Chemistry of Life, 2010, 30(4): 555-561 (in Chinese). |
| [71] |
ROMBOUT J H W M, ABELLI L, PICCHIETTI S, et al. Teleost intestinal immunology[J]. Fish & Shellfish Immunology, 2011, 31(5): 616-626. |
| [72] |
杨冰贞, 张民, 王克坚. NF-κB信号通路在鱼类先天性免疫中的作用[J]. 生物技术通报, 2014(1): 46-52. YANG B Z, ZHANG M, WANG K J. Role of NF-κB signal pathway in the innate immune system of fish[J]. Biotechnology Bulletin, 2014(1): 46-52 (in Chinese). |
| [73] |
ZHOU Q, JIN M, ELMADA Z C, et al. Effects of dietary arginine levels on growth, immune ability and resistance to Aeromonas hydrophilus of juvenile yellow catfish (Pelteobagrus fulvidraco)[J]. Feed Review, 2015(2): 46 (in Chinese). |
| [74] |
韩凤禄, 张琴, 黄国强, 等. 斜带石斑鱼幼鱼的饲料精氨酸需求量[J]. 中国水产科学, 2016, 23(3): 584-593. HAN F L, ZHANG Q, HUANG G Q, et al. Requirement of dietary arginine for juvenile orange-spotted grouper, Epinephelus coioides[J]. Journal of Fishery Sciences of China, 2016, 23(3): 584-593 (in Chinese). |
| [75] |
LI P, YIN Y L, LI D F, et al. Amino acids and immune function[J]. The British Journal of Nutrition, 2007, 98(2): 237-252. DOI:10.1017/S000711450769936X |
| [76] |
王标. 精氨酸对中期草鱼肉质和铜诱导的鳃屏障功能的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2014. WANG B. The effect of dietary arginine supplement on flesh quality and Cu-induced gill barrier function of young grass carp (Ctenopharyngodon idellus)[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2014. (in Chinese) |
| [77] |
LIANG H L, MOKRANI A, JI K, et al. Effects of dietary arginine on intestinal antioxidant status and immunity involved in Nrf2 and NF-κB signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala[J]. Fish & Shellfish Immunology, 2018, 82: 243-249. |
| [78] |
胡欣欣. γ射线照射对蛋白质结构和功能影响的研究[D]. 硕士学位论文. 济南: 山东大学, 2015. HU X X. Studies on the effect of γ-radiation on the conformation and function of protein[D]. Master's Thesis. Jinan: Shandong University, 2015. (in Chinese) |
| [79] |
ZHANG W B, CHEN Q Y, MAI K S, et al. Effects of dietary α-lipoic acid on the growth and antioxidative responses of juvenile abalone Haliotis discus hannai Ino[J]. Aquaculture Research, 2010, 41(11): e781-e787. DOI:10.1111/j.1365-2109.2010.02592.x |
| [80] |
TOKUR B, KORKMAZ K. The effects of an iron-catalyzed oxidation system on lipids and proteins of dark muscle fish[J]. Food Chemistry, 2007, 104(2): 754-760. DOI:10.1016/j.foodchem.2006.12.033 |
| [81] |
李文. 苯丙氨酸对生长中期草鱼生长性能、肌肉品质和肠道黏膜免疫功能的影响研究[D]. 硕士学位论文. 雅安: 四川农业大学, 2014. LI W. The effect of dietary phenylalanine supplement on growth, flesh quality parameters, antioxidant capacity and intestine immune function of young grass carp (Ctenopharyngodon idellus)[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2014. (in Chinese) |
| [82] |
LIANG H L, MOKRANI A, JI K, et al. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala)[J]. Fish & Shellfish Immunology, 2018, 73: 57-65. |
| [83] |
SEILIEZ I, GABILLARD J C, SKIBA-CASSY S, et al. An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss)[J]. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 2008, 295(1): R329-R335. DOI:10.1152/ajpregu.00146.2008 |
| [84] |
NUTTALL F Q, SCHWEIM K J, GANNON M C. Effect of orally administered phenylalanine with and without glucose on insulin, glucagon and glucose concentrations[J]. Hormone and Metabolic Research, 2006, 38(8): 518-523. DOI:10.1055/s-2006-949523 |
| [85] |
WANG X M, PROUD C G. The mTOR pathway in the control of protein synthesis[J]. Physiology, 2006, 21(5): 362-369. DOI:10.1152/physiol.00024.2006 |
| [86] |
黄陈翠, 孙健, 吉红, 等. 硫辛酸对草鱼脂肪细胞脂质含量及脂代谢相关基因表达的影响[J]. 淡水渔业, 2020, 50(1): 87-92. HUANG C C, SUN J, JI H, et al. Effect of α-lipoic acid on lipid content and lipid metabolism related gene expression in Ctenopharyngodon idellus adipocyte[J]. Freshwater Fisheries, 2020, 50(1): 87-92 (in Chinese). DOI:10.3969/j.issn.1000-6907.2020.01.013 |
| [87] |
陈冰, 杨继华, 曹俊明, 等. 桑叶黄酮对吉富罗非鱼肌肉抗氧化指标及营养组成的影响[J]. 淡水渔业, 2018, 48(3): 90-95. CHEN B, YANG J H, CAO J M, et al. Effects of dietary mulberry leaf flavonoids on muscle antioxidant indices and nutritional compositions of GIFT, Oreochromis niloticus[J]. Freshwater Fisheries, 2018, 48(3): 90-95 (in Chinese). DOI:10.3969/j.issn.1000-6907.2018.03.015 |




