2. 江苏远山生物技术有限公司, 盐城 224001
2. Jiangsu Yuanshan Biological Technology Co., Ltd., Yancheng 224001, China
断奶仔猪的消化和免疫系统尚未完全成熟,容易受外界刺激发生腹泻与病原微生物感染,从而影响生长性能[1]。虽然在断奶仔猪饲粮中添加促生长抗生素(antibiotic growth promoters,AGPs)能够起到促进生长、降低腹泻和提高饲料转化率的作用,但细菌耐药问题与畜产品抗生素残留问题也在严重威胁着人们的健康[2]。因此,我国在2016年颁布《遏制细菌耐药国家行动计划》,以规范抗生素的管理与应用[3]。2020年7月1日,我国实行饲料端全面禁用AGPs。
金霉素是一种广谱抑菌的四环素类抗生素,其具备降低腹泻、提高饲料转化率和促进生长的作用,是禁抗以前养猪业使用最多的AGPs[4]。益生元是一类能被宿主微生物选择性利用并赋予宿主健康益处的底物[5]。乳果糖是由1分子半乳糖和1分子果糖组成的二糖,可以完整地到达后肠,供肠道微生物发酵[6],改善宿主消化道内环境与优化消化道菌群组成[7]。Guerra-Ordaz等[8]研究发现,饲喂含10 g/kg乳果糖的饲粮能显著提高断奶仔猪平均日增重和饲料转化率,并能同时降低肠杆菌和乳球菌的比例。Rycroft等[9]研究发现,相比于其他低聚糖,乳果糖具有促进双歧杆菌增殖的作用。益生菌是一类在补充足够量时能给宿主带来健康益处的活微生物[10]。凝结芽孢杆菌是乳酸生成菌,它可以产生多种酶(如阿拉伯糖酶、α-淀粉酶和纤维素酶等),以提高饲料利用率,促进动物生长[11]。此外,凝结芽孢杆菌还能以孢子形式在胃酸中存活,并在肠道中出芽发挥益生作用[11]。Wu等[12]研究发现,在断奶仔猪饲粮中补充2×106或2×107 CFU/g凝结芽孢杆菌能有效地降低仔猪腹泻,改善肠道屏障功能及消化道微生物组成。合生素是益生菌和益生元的混合物,兼具益生菌和益生元的益生效果,被广泛地用作饲料添加剂[13]。本课题组前期研究发现,在无抗饲粮中添加乳果糖和凝结芽孢杆菌合生素可降低断奶仔猪腹泻率,显著提高总能和粗脂肪表观消化率,降低血清总胆红素(total bilirubin,TBIL)含量,对断奶仔猪料重比有一定改善作用[14],但是其潜在的作用机制尚不明确。
代谢组学技术可以允许同时测量1个样品中成千上万个代谢产物与营养物,为研究营养效应和代谢产物间相互作用开辟了更多的可能性[15]。此外,代谢组学已经成为其他组学学科(尤其是基因组学、转录组学和蛋白质组学)的补充技术,能够为更好地解答科学问题提供帮助。因此,在前期研究的基础上,本试验旨在评估乳果糖-凝结芽孢杆菌合生素对断奶仔猪生长的影响,并同时借助代谢组学技术和气相色谱法初探乳果糖-凝结芽孢杆菌合生素的可能益生机制。虽然,本研究中基础饲粮为无抗饲粮,但为了更好地评价乳果糖-凝结合生素作为潜在替抗添加剂的作用,还设定了金霉素添加组(在基础饲粮中添加75 mg/kg金霉素)作为阳性对照。
1 材料与方法 1.1 试验设计及饲粮选取18头健康且体重相近的27~28日龄“杜×长×大”阉公猪,初始体重(9.08±0.59) kg,随机分成3组,每组6个重复,每个重复1头猪。预试期7 d(饲喂基础无抗饲粮),正试期29 d。对照组(CTR组)饲喂基础无抗饲粮,抗生素组(ANT组)饲喂基础无抗饲粮+75 mg/kg金霉素,合生素组(SYN组)饲喂基础无抗饲粮+合生素(10 g/kg乳果糖+2×109 CFU/kg凝结芽孢杆菌)。试验期间自由采食和饮水,漏缝地板,单栏单饲,定期通风与清理,室内温度保持在22~23 ℃。断奶仔猪饲养期间,未与处理外的其他抗生素接触。本试验所用饲粮参考NRC(2012)断奶仔猪营养需要进行配制,饲粮组成及营养水平同赵祖艳等[14]。
1.2 试验材料乳果糖为杜密克乳果糖口服液(667 mg/mL,Abbott Biologicals B.V.,荷兰);凝结芽孢杆菌(活菌数1×109 CFU/g)由江苏某生物技术有限公司提供。
1.3 测定指标和方法 1.3.1 相对生长速率(relative growth rate,RGR)从试验期开始第1天起,每周06:00对每头仔猪进行空腹称重并记录,计算断奶仔猪的RGR。RGR描述为初始权重和终末权重的自然对数之差除以权重之间的时间,而后将该比率乘以105,来规避缩放问题,计算公式[16]如下:
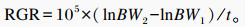
|
式中:BW1为初始体重;BW2为终末体重;t为时间间隔。
1.3.2 粪便代谢组学检测对第29天采集到的仔猪粪便,采用UHPLC-QTOF-MS/MS系统进行非靶代谢组学分析。样品准备与仪器参数详见文献[17]。原始数据经标准化后,用SIMCA-P 13.0多维偏最小二乘判别分析(partial square-discriminate analysis,PLS-DA)进行分析。
1.3.3 粪便短链脂肪酸(SCFAs)浓度检测用GC-14B型气相色谱仪(岛津公司,日本)测定粪便SCFAs浓度,毛细管柱(No.34292-07B,Supelco公司,美国):30.00 m×0.32 mm×0.25 μm,柱温130 ℃,汽化室和检测器温度均为180 ℃,载气为氮气,压力为60 kPa,氢气压力为50 kPa,氧气压力为50 kPa。样品处理参照秦为琳[18]。
1.4 数据统计与分析所有数据经Excel 2016进行初步整理后,用SPSS 25.0进行单因素方差分析(one-way ANOVA)和Duncan氏法多重比较检验。非靶代谢组学数据通过PLS-DA的变量投影重要性(variable important in projection,VIP)值与t检验的P值进行差异代谢产物筛选,其中,将VIP>1且P < 0.10的代谢产物定义为差异代谢产物,将VIP>1且P < 0.05的代谢产物定义为显著差异代谢产物。代谢产物KEGG富集分析过程中,以差异代谢产物作为富集对象[19],Fisher精确检验分析各个通路代谢产物富集度的显著水平。相关性分析采用Pearson相关。P < 0.05为差异显著,0.05≤P < 0.10为有显著趋势。
2 结果与分析 2.1 乳果糖-凝结芽孢杆菌合生素对断奶仔猪RGR的影响由表 1可知,第1周、第2周和第4周时,3组之间断奶仔猪RGR无显著差异(P>0.05);但在第3周时,ANT组的断奶仔猪RGR显著高于CTR组(P < 0.05),SYN组与其他2组的断奶仔猪RGR无显著差异(P>0.05)。此外,3组断奶仔猪RGR在第1~4周存在差异显著趋势(P=0.052),ANT组和SYN组比CTR组断奶仔猪RGR分别提高了7.08%和6.00%,ANT组比SYN组断奶仔猪RGR提高了1.01%。
|
|
表 1 乳果糖-凝结芽孢杆菌合生素对断奶仔猪RGR的影响 Table 1 Effects of synbiotic with lactulose and Bacillus coagulans on RGR of weaned piglets |
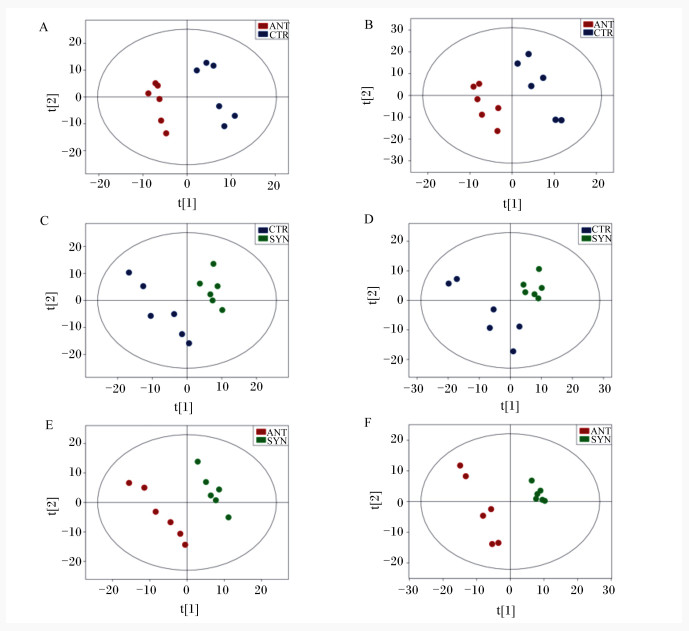
由图 1可知,在正离子或负离子模式下,ANT组与CTR组、ANT组与SYN组、SYN组与CTR组粪便代谢产物在组成上均出现明显区分。
|
图A、图C和图E分别为正离子模式下ANT组与CTR组、SYN组与CTR组和SYN组与ANT组代谢产物聚类结果。图B、图D和图F分别为负离子模式下ANT组与CTR组、SYN组与CTR组和SYN组与ANT组代谢产物聚类结果。CTR:对照组;ANT:抗生素组;SYN:合生素组。 Figure A, figure C and figure E are the metabolite clustering results of ANT group and CTR group, SYN group and CTR group, and SYN group and ANT group in positive ion mode, respectively. Figure B, figure D and figure F are the metabolite clustering results of ANT group and CTR group, SYN group and CTR group, and SYN group and ANT group in negative ion mode, respectively. CTR: control group; ANT: antibiotic group; SYN: synbiotic group. 图 1 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便代谢产物聚类的影响(PLS-DA) Fig. 1 Effects of synbiotic with lactulose and Bacillus coagulans on clustering of fecal metabolites of weaned piglets (PLS-DA) |
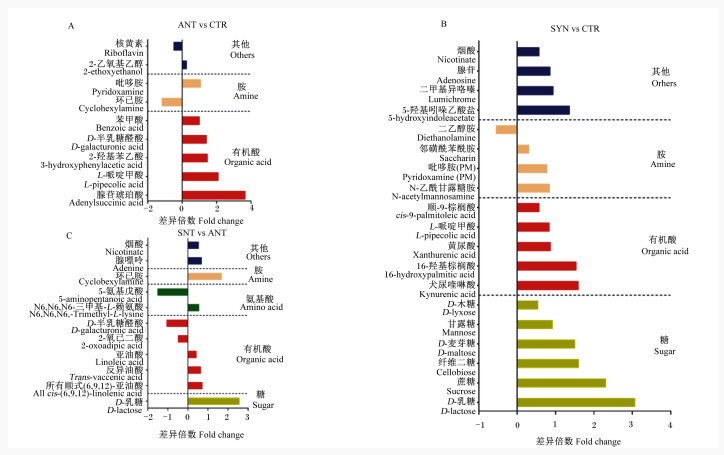
通过非靶代谢组学方法,所有仔猪粪便样品正离子模式下共检测到232种代谢产物,在负离子模式下共检测到189种代谢产物。基于显著差异代谢产物的筛选标准(VIP>1,P < 0.05),在ANT组和CTR组之间共检测到9种显著差异代谢产物(图 2-A),其中ANT组有7种代谢产物(腺苷琥珀酸、L-哌啶甲酸、3-羟基苯乙酸、D-半乳糖醛酸、吡哆胺、苯甲酸和2-乙氧基乙醇)含量显著高于CTR组(P < 0.05),2种代谢产物(核黄素和环己胺)含量显著低于CTR组(P < 0.05)。在SYN组和CTR组之间共检测到19种显著差异代谢产物(图 2-B),其中SYN组有18种代谢产物(D-乳糖、蔗糖、纤维二糖、犬尿喹啉酸、16-羟基棕榈酸、D-麦芽糖、5-羟基吲哚乙酸盐、二甲基异咯嗪、甘露糖、黄尿酸、腺苷、N-乙酰甘露糖胺、L-哌啶甲酸、吡哆胺、烟酸、顺-9-棕榈酸、D-木糖和邻磺酰苯酰胺)含量显著高于CTR组(P < 0.05),1种代谢产物(二乙醇胺)含量显著低于CTR组(P < 0.05)。在SYN组与ANT组之间共检测到11种显著差异代谢产物(图 2-C),其中SYN组有8种代谢产物[D-乳糖、环己胺、所有顺式(6, 9, 12)-亚油酸、腺嘌呤、反异油酸、N6, N6, N6-三甲基-L-赖氨酸、烟酸和亚油酸]含量显著高于ANT组(P < 0.05),3种代谢产物(2-氧己二酸、D-半乳糖醛酸和5-氨基戊酸)含量显著低于ANT组(P < 0.05)。
|
图A为ANT组与CTR组之间显著差异代谢产物,图B为SYN组与CTR组之间显著差异代谢产物,图C为SYN组与ANT组之间显著差异代谢产物。CTR:对照组;ANT:抗生素组;SYN:合生素组。 Figure A shows the significantly different metabolites between ANT group and CTR group, figure B shows the significantly different metabolites between SYN group and CTR group, and figure C shows the significantly different metabolites between SYN group and ANT group. CTR: control group; ANT: antibiotic group; SYN: synbiotic group. 图 2 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便差异代谢产物的影响 Fig. 2 Effects of synbiotic with lactulose and Bacillus coagulans on different metabolites in feces of weaned piglets |
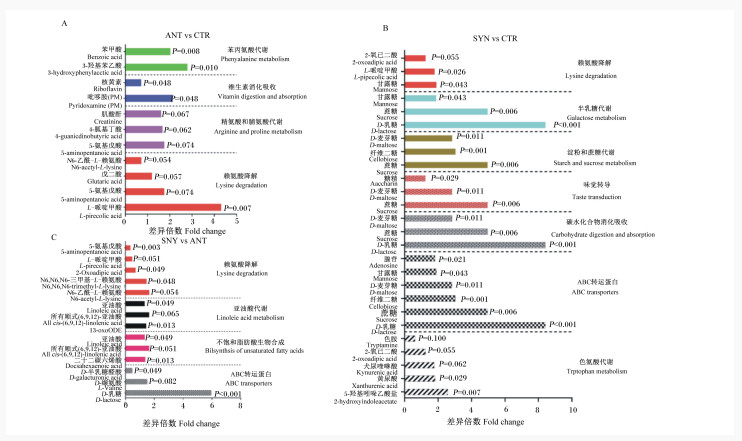
ANT组和CTR组之间的差异代谢产物共4条通路出现显著富集(P < 0.05),分别是赖氨酸降解、精氨酸和脯氨酸代谢、苯丙氨酸代谢和维生素消化吸收通路(图 3-A)。在赖氨酸降解通路中,ANT组L-哌啶甲酸(P<0.05)、5-氨基戊酸(P>0.05)和戊二酸(P>0.05)含量高于CTR组,N6-乙酰-L-赖氨酸含量低于CTR组(P>0.05);在精氨酸和脯氨酸代谢通路中,ANT组5-氨基戊酸、肌酸酐、4-胍基丁酸含量高于CTR组(P>0.05);在维生素消化吸收通路中,ANT组吡哆胺含量高于CTR组(P>0.05),核黄素含量显著低于CTR组(P<0.05);在苯丙氨酸代谢通路中,ANT组3-羟基苯乙酸和苯甲酸含量显著高于CTR组(P<0.05)。
|
图A为ANT组与CTR组之间粪便KEGG显著富集通路,图B为SYN组与CTR组之间粪便KEGG显著富集通路,图C为SYN组与ANT组之间粪便KEGG显著富集通路。CTR:对照组;ANT:抗生素组;SYN:合生素组。 Figure A shows the significantly enriched KEGG pathways between ANT group and CTR group, figure B shows the significantly enriched KEGG pathways between SYN group and CTR group, and figure C shows the significantly enriched KEGG pathways between SYN group and ANT group. CTR: control group; ANT: antibiotic group; SYN: synbiotic group. 图 3 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便显著KEGG富集通路的影响 Fig. 3 Effects of synbiotic with lactulose and Bacillus coagulans on significantly enriched KEGG pathways in feces of weaned piglets |
SYN组和CTR组之间的差异代谢产物共7条通路出现显著富集(P < 0.05),分别是色氨酸代谢、ABC转运蛋白、碳水化合物消化吸收、味觉转导、淀粉和蔗糖代谢、半乳糖代谢和赖氨酸降解通路(图 3-B)。在色氨酸代谢代谢通路中,SYN组5-羟基吲哚乙酸盐(P < 0.05)、黄尿酸(P < 0.05)、犬尿喹啉酸(P>0.05)和2-氧己二酸(P>0.05)含量高于CTR组,色胺含量低于CTR组(P>0.05);在ABC转运蛋白通路中,SYN组D-乳糖、蔗糖、纤维二糖、D-麦芽糖、甘露糖和腺苷含量均显著高于CTR组(P < 0.05);在碳水化合物消化吸收通路中,SYN组D-乳糖、蔗糖和D-麦芽糖含量均显著高于CTR组(P < 0.05);在味觉传导、淀粉和蔗糖代谢、半乳糖代谢通路中,SYN组蔗糖、D-麦芽糖、糖精、纤维二糖、D-乳糖、甘露糖含量均显著高于CTR组(P < 0.05)。在赖氨酸降解通路中,SYN组甘露糖(P < 0.05)、L-哌啶甲酸(P < 0.05)、2-氧己二酸(P>0.05)均高于CTR组。
SYN组和ANT组之间差异代谢产物共4条通路出现显著富集(P < 0.05),分别是ABC转运蛋白、不饱和脂肪酸生物合成、亚油酸代谢和赖氨酸降解通路(图 3-C)。在ABC转运蛋白通路中,SYN组D-乳糖(P < 0.05)和L-缬氨酸(P>0.05)含量高于ANT组,D-半乳糖醛酸含量显著低于ANT组(P < 0.05);在不饱和脂肪酸生物合成通路中,SYN组所有顺式(6, 9, 12)-亚油酸(P>0.05)、亚油酸(P < 0.05)和二十二碳六烯酸(P < 0.05)含量高于ANT组;在亚油酸代谢通路中,SYN组所有顺式(6, 9, 12)-亚油酸(P>0.05)、亚油酸(P < 0.05)和13-OxoODE(P < 0.05)含量高于ANT组;在赖氨酸降解通路中,SYN组N6-乙酰-L-赖氨酸(P>0.05)和N6, N6, N6-三甲基-L-赖氨酸(P < 0.05)含量高于ANT组,SYN组2-氧己二酸(P < 0.05)、L-哌啶甲酸(P>0.05)和5-氨基戊酸(P < 0.05)含量低于ANT组。
2.3 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便SCFAs浓度和比例的影响由表 2可知,3组之间总SCFAs及乙酸、丙酸、异丁酸、丁酸、异戊酸、戊酸浓度无显著差异(P>0.05);ANT组丙酸比例显著高于SYN组(P < 0.05),CTR组丙酸比例与其他2组差异不显著(P>0.05)。3组之间乙酸、异丁酸、丁酸、异戊酸、戊酸比例无显著差异(P>0.05)
|
|
表 2 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便SCFAs浓度和比例的影响 Table 2 Effects of synbiotic with lactulose and Bacillus coagulans on concentration and ratio of SCFAs in feces of weaned piglets |
将显著差异代谢产物含量、SCFAs浓度与1~4周RGR进行相关性分析,结果见表 3。RGR与D-半乳糖醛酸和甘露糖含量呈显著负相关(P < 0.05),相关系数分别为-0.53和-0.52;RGR与环己胺含量呈显著正相关(P < 0.05),相关系数为0.49。
|
|
表 3 断奶仔猪粪便代谢产物含量与RGR相关性 Table 3 Correlations between contents of metabolites in feces of weaned piglets with RGR |
本试验中,第3周时ANT组断奶仔猪RGR显著高于CTR组,而SYN组与其他2组差异不显著;第1~4周3组之间断奶仔猪RGR存在显著趋势,其中SYN组RGR比CTR组提高了6.00%,ANT组RGR比CTR组提高了7.08%。有研究表明,AGPs可以通过直接抑菌和抑制炎症作用,促进动物生长[20];这与本试验中ANT组第3周结果类似。ANT组与CTR组第1~4周时RGR差异不显著,这可能与微生物对四环素类抗生素高度耐药有关[21]。乳果糖或凝结芽孢杆菌的促生长作用已在多项研究中被报道。Guerra-Ordaz等[8]研究发现,饲喂含10 g/kg乳果糖的饲粮能显著提高断奶仔猪平均日增重和料重比。王乙茹等[22]研究发现,饲粮中添加2×106 CFU/g凝结芽孢杆菌能显著提高育肥猪生长性能。虽然,本研究中乳果糖-凝结芽孢杆菌合生素对断奶仔猪RGR无显著差异,但前期研究数据显示该合生素可降低断奶仔猪腹泻率,显著提高总能和粗脂肪表观消化率,降低血清TBIL含量,对断奶仔猪料重比有一定改善作用[14]。
3.2 乳果糖-凝结芽孢杆菌合生素对断奶仔猪粪便代谢产物的影响肠道作为乳果糖-凝结芽孢杆菌合生素发挥其生物学作用的主要场所,了解肠道内代谢产物的变化对于解析乳果糖-凝结芽孢杆菌合生素的潜在作用机制至关重要。对第29天断奶仔猪粪便代谢组学分析显示,与CTR组相比,ANT组中有9种代谢产物受到显著影响,其中5种有机酸在ANT组均显著升高。前期研究显示,有机酸能够调节肠道pH,对抑制病原微生物、预防感染有重要作用[23]。同样,与CTR组相比,SYN组有19种代谢产物受到显著影响;对上述有差异的代谢产物分析发现,L-哌啶甲酸和吡多胺为SYN组和ANT组共影响的代谢产物。L-哌啶甲酸参与赖氨酸降解途径,而赖氨酸是猪的第一限制性氨基酸,除广泛参与猪体内酶、激素合成和脂肪代谢外,还能增强宿主免疫[24]。L-哌啶甲酸含量在ANT组和SYN组的升高提示金霉素与乳果糖-凝结芽孢杆菌合生素可能均促进断奶仔猪对赖氨酸的利用。此外,与CTR组相比,SYN组和ANT组还显著影响有机酸和胺类物质的含量,其中包括核黄素、烟酸和腺嘌呤;上述代谢产物广泛参与机体能量和脂质代谢过程,是哺乳动物生长代谢不可或缺的营养物质[25]。虽然,SYN组和ANT组相比CTR组影响的具体代谢产物大部分不同,但是富集通路显示:与CTR组相比,SYN组与ANT组均显著影响了多条必需氨基酸代谢通路。除赖氨酸降解通路外,ANT组还显著影响苯丙氨酸代谢、精氨酸和脯氨酸代谢通路;而SYN组显著影响色氨酸代谢通路。这些必需氨基酸在体内承担包含蛋白质和酶合成、能量代谢等重要生理功能[29]。上述结果说明,乳果糖-凝结芽孢乳杆菌合生素也能通过不同的代谢产物显著影响与金霉素相似的代谢通路。
此外,值得关注的是SYN组还显著提高了D-木糖、甘露糖、D-麦芽糖、纤维二糖、蔗糖和D-乳糖在内的6种代谢产物。糖类是哺乳动物尤其是幼龄动物优先利用的能量物质,这一结果提示合生素可能影响了断奶仔猪对肠道能量的利用模式。乳果糖属于能量物质的一种,虽不能被动物消化道酶分解,但却可以被消化道中的微生物发酵产生SCFAs和乳酸[26],间接为宿主供能。而凝结芽孢杆菌不仅能自身分泌多种消化酶(如α-淀粉酶、木聚糖酶和蛋白酶等),还能提高宿主消化道淀粉酶、脂肪酶活性,从而优化饲粮的利用模式[11, 27]。因此,我们推测前期乳果糖-凝结芽孢杆菌合生素降低断奶仔猪腹泻率,显著提高总能和粗脂肪表观消化率的可能原因归结于SYN组提高了断奶仔猪对于能量的利用与吸收效率。
与ANT组相比,SYN组3种有机酸(如亚油酸)、D-乳糖、环己胺、烟酸、腺嘌呤和N6,N6,N6-三甲基-L-赖氨酸含量显著提高;此外,SYN组5-氨基戊酸、D-半乳糖醛酸和2-氧己二酸显著低于ANT组。亚油酸属于不饱和脂肪酸的一种,是具有降胆固醇、降血压和提高机体免疫等效果的必需脂肪酸[28]。与ANT组相比,SYN组可能改善了断奶仔猪的脂质代谢,提示SYN组仔猪可能拥有更强的不饱和脂肪酸生物合成能力和亚油酸代谢能力,这与前期发现乳果糖-凝结芽孢杆菌合生素显著提高粗脂肪表观消化率的结果相符合。富集通路分析表明,与ANT组相比,SYN组赖氨酸降解和ABC转运蛋白途径通路显著改变,提示SYN组在氨基酸利用和营养物质转运方面的影响优于ANT组,具体机制需进一步探究。
在上述代谢组学分析基础上,我们还发现粪便中丙酸比例在SYN组和ANT组之间出现显著差异。SCFAs组成与断奶仔猪肠道菌群密不可分,而金霉素和合生素均具备调整肠道菌群的能力[30-32]。结合代谢组学部分结果,我们推测丙酸比例在SYN组和ANT组之间出差异可能与仔猪对饲粮营养利用模式的变化和菌群产SCFAs模式变化有关。相关性结果证实,D-半乳糖醛酸、环己胺和甘露糖含量与仔猪RGR呈显著相关性;因此,上述代谢产物或许可作为评价断奶仔猪RGR的生物标志物,并且它们对RGR的贡献值得进一步探究。
4 结论① 乳果糖-凝结芽孢杆菌合生素可通过赖氨酸降解、半乳糖代谢、淀粉和蔗糖、碳水化合物消化吸收等代谢通路影响仔猪的生长和代谢。
② 与CTR组相比,乳果糖-凝结芽孢杆菌合生素不仅具有和金霉素相似的促生长效果,还能部分影响粪便中和金霉素相同的代谢产物和代谢通路。此外,乳果糖-凝结芽孢杆菌影响粪便中代谢产物的种类和代谢通路比金霉素更加广泛。
③ 粪便代谢产物D-半乳糖醛酸、环己胺和甘露糖含量与仔猪RGR呈显著相关。
| [1] |
HEO J M, OPAPEJU F O, PLUSKE J R, et al. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds[J]. Journal of Animal Physiology and Animal Nutrition, 2013, 97(2): 207-237. DOI:10.1111/j.1439-0396.2012.01284.x |
| [2] |
PATEL S J, WELLINGTON M, SHAH R M, et al. Antibiotic stewardship in food-producing animals: challenges, progress, and opportunities[J]. Clinical Therapeutics, 2020, 42(9): 1649-1658. DOI:10.1016/j.clinthera.2020.07.004 |
| [3] |
HU Y J, COWLING B J. Reducing antibiotic use in livestock, China[J]. Bulletin of the World Health Organization, 2020, 98(5): 360-361. DOI:10.2471/BLT.19.243501 |
| [4] |
COLLIGNON P, VOSS A. China, what antibiotics and what volumes are used in food production animals?[J]. Antimicrobial Resistance and Infection Control, 2015, 4: 16. DOI:10.1186/s13756-015-0056-5 |
| [5] |
ROBERFROID M, GIBSON G R, HOYLES L, et al. Prebiotic effects: metabolic and health benefits[J]. The British Journal of Nutrition, 2010, 104. |
| [6] |
RUSZKOWSKI J, WITKOWSKI J M. Lactulose: patient- and dose-dependent prebiotic properties in humans[J]. Anaerobe, 2019, 59: 100-106. DOI:10.1016/j.anaerobe.2019.06.002 |
| [7] |
SCHUMANN C. Medical, nutritional and technological properties of lactulose: an update[J]. European Journal of Nutrition, 2002, 41(Suppl.1): I17-I25. |
| [8] |
GUERRA-ORDAZ A A, MOLIST F, HERMES R G, et al. Effect of inclusion of lactulose and Lactobacillus plantarum on the intestinal environment and performance of piglets at weaning[J]. Animal Feed Science and Technology, 2013, 185(3/4): 160-168. |
| [9] |
RYCROFT C E, JONES M R, GIBSON G R, et al. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides[J]. Journal of Applied Microbiology, 2001, 91(5): 878-887. DOI:10.1046/j.1365-2672.2001.01446.x |
| [10] |
BARBA-VIDAL E, MARTÍN-OR Ú E S M, CASTILLEJOS L. Practical aspects of the use of probiotics in pig production: a review[J]. Livestock Science, 2019, 223: 84-96. DOI:10.1016/j.livsci.2019.02.017 |
| [11] |
ZHOU Y H, ZENG Z H, XU Y B, et al. Application of Bacillus coagulans in animal husbandry and its underlying mechanisms[J]. Animals, 2020, 10(3): 454. DOI:10.3390/ani10030454 |
| [12] |
WU T, ZHANG Y, LV Y, et al. Beneficial impact and molecular mechanism of Bacillus coagulans on piglets' intestine[J]. International Journal of Molecular Sciences, 2018, 19(7): 2084. DOI:10.3390/ijms19072084 |
| [13] |
MARKOWIAK P, ŚLIŻEWSKA K. The role of probiotics, prebiotics and synbiotics in animal nutrition[J]. Gut Pathogens, 2018, 10: 21. DOI:10.1186/s13099-018-0250-0 |
| [14] |
赵祖艳, 杨运南, 刘日亮, 等. 乳果糖和凝结芽孢杆菌合生素对断奶仔猪生长性能、养分表观消化率和血液指标的影响[J]. 动物营养学报, 1-10. (2021-03-19). https://kns.cnki.net/kcms/detail/detail.aspx?FileName=DWYX2021031700K&DbName=CAPJ2021. ZHAO Z Y, YANG Y N, LIU R L, et al. Effects of synbiotic containing lactulose and bacillus coagulans on growth performance, nutrient apparent digestibilities and blood indexes of weaned piglets[J/OL]. Chinese Journal of Animal Nutrition: 1-10. (2021-03-19). https://kns.cnki.net/kcms/detail/detail.aspx?FileName=DWYX2021031700K&DbName=CAPJ2021.(in Chinese) |
| [15] |
KOULMAN A, VOLMER D A. Perspectives for metabolomics in human nutrition: an overview[J]. Nutrition Bulletin, 2008, 33(4): 324-330. DOI:10.1111/j.1467-3010.2008.00733.x |
| [16] |
WINDER J A, BRINKS J S, BOURDON R M, et al. Genetic analysis of absolute growth measurements, relative growth rate and restricted selection indices in red Angus cattle[J]. Journal of Animal Science, 1990, 68(2): 330-336. DOI:10.2527/1990.682330x |
| [17] |
GUO Z L, ZHANG P, XIE H Q, et al. First in vivo evidence for compromised brain energy metabolism upon intranasal exposure to ZnO nanoparticles[J]. Environmental Science & Technology Letters, 2020, 7(5): 315-322. |
| [18] |
秦为琳. 应用气相色谱测定瘤胃挥发性脂肪酸方法的研究改进[J]. 南京农业大学学报, 1982(4): 110-116. QIN W L. Determ nation of rumen volatile fatty acids by means of gas chromatography[J]. Journal of Nanjing Agricultural College, 1982(4): 110-116 (in Chinese). |
| [19] |
WANG J, XU R Y, XIANG X E, et al. Transcriptomic and metabolomic responses in the livers of pigs to diets containing different non-starchy polysaccharides[J]. Journal of Functional Foods, 2020, 64: 103590. DOI:10.1016/j.jff.2019.103590 |
| [20] |
张显东. 促生长抗生素作用机理及其替代方案的重新思考[J]. 饲料工业, 2017, 38(11): 61-64. ZHANG X D. Rethinking about mechanism of antibiotic growth promoter and its alternatives[J]. Feed Industry, 2017, 38(11): 61-64 (in Chinese). |
| [21] |
YANG H, PARUCH L, CHEN X J, et al. Antibiotic application and resistance in swine production in China: current situation and future perspectives[J]. Frontiers in Veterinary Science, 2019, 6: 136. DOI:10.3389/fvets.2019.00136 |
| [22] |
王乙茹, 白华毅, 王桂瑛, 等. 饲料添加凝结芽孢杆菌及枯草芽孢杆菌对生长猪的生长性能影响[J]. 饲料博览, 2018(9): 1-5. WANG Y R, BAI H Y, WANG G Y, et al. Effects of feed probiotics Bacillus coagulans and Bacillus subtilis on the growth performance in growing pigs[J]. Feed Review, 2018(9): 1-5 (in Chinese). DOI:10.3969/j.issn.1001-0084.2018.09.001 |
| [23] |
CARPENTER C E, BROADBENT J R. External concentration of organic acid anions and pH: key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods[J]. Journal of Food Science, 2009, 74(1): R12-R15. DOI:10.1111/j.1750-3841.2008.00994.x |
| [24] |
KIM Y W, INGALE S L, KIM J S, et al. Effects of dietary lysine and energy levels on growth performance and apparent total tract digestibility of nutrients in weanling pigs[J]. Asian-Australasian Journal of Animal Sciences, 2011, 24(9): 1256-1267. DOI:10.5713/ajas.2011.11134 |
| [25] |
蒋鹏飞. 中国饲用维生素生产、应用现状及发展方向[D]. 硕士学位论文. 厦门: 集美大学, 2019. JIANG P F. Current situation and development direction of feed grade vitamin production and application in China[D]. Master's Thesis. Xiamen: Jimei University, 2019. (in Chinese) |
| [26] |
KAMPHUES J, TABELING R, STUKE O, et al. Investigations on potential dietetic effects of lactulose in pigs[J]. Livestock Science, 2007, 109(1/3): 93-95. |
| [27] |
LEE N K, KIM W S, PAIK H D. Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier[J]. Food Science and Biotechnology, 2019, 28(5): 1297-1305. DOI:10.1007/s10068-019-00691-9 |
| [28] |
张春娥, 张惠, 刘楚怡, 等. 亚油酸的研究进展[J]. 粮油加工, 2010(5): 18-21. ZHANG C E, ZHANG H, LIU C Y, et al. Research progress of linoleic acid[J]. Cereals and Oils Processing, 2010(5): 18-21 (in Chinese). |
| [29] |
戴求仲, 王康宁, 印遇龙, 等. 生长猪肠道氨基酸代谢研究进展[J]. 家畜生态学报, 2005, 26(2): 63-69. DAI Q Z, WANG K N, YIN Y L, et al. Improvement of study on intestinal amino acid metabolism of growing pig[J]. Acta Ecologiae Animalis Domastici, 2005, 26(2): 63-69 (in Chinese). DOI:10.3969/j.issn.1673-1182.2005.02.017 |
| [30] |
POOLE T L, SUCHODOLSKI J S, CALLAWAY T R, et al. The effect of chlortetracycline on faecal microbial populations in growing swine[J]. Journal of Global Antimicrobial Resistance, 2013, 1(3): 171-174. DOI:10.1016/j.jgar.2013.04.004 |
| [31] |
CUI S M, GU J Y, LIU X M, et al. Lactulose significantly increased the relative abundance of Bifidobacterium and Blautia in mice feces as revealed by 16S rRNA amplicon sequencing[J/OL]. Journal of the Science of Food and Agriculture, 2021. (2021-03-01). https://pubmed.ncbi.nlm.nih.gov/33650140/.
|
| [32] |
KELLER D, VAN DINTER R, CASH H, et al. Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1)[J]. Beneficial Microbes, 2017, 8(3): 491-496. DOI:10.3920/BM2016.0196 |




