抗生素的弊端在动物生产中日益显著,寻找有效、绿色的抗生素替代物以成为必然趋势。植物及其提取物有天然的抗菌、促生长作用且无残留、无抗药性、无毒副作用,日渐成为研究的热点。大蒜素(allicin)是大蒜中的一种防御分子,因其具有提高动物生长性能,杀菌抑菌和抵抗氧化应激等多种生物活性引了起研究学者的关注。本文综述了大蒜素的生物学功能及其在反刍动物中的作用效果,旨在为进一步研究大蒜素在反刍动物上的应用提供理论参考依据。
1 大蒜素的定义及其理化性质大蒜素化学名为二烯丙基硫代亚磺酸盐,分子式为C6H10S3,相对分子质量为178.33。大蒜素纯品具有大蒜异味,状态为无色油状,比重1.112(20 ℃),折射率1.561,无旋光性,易溶于苯、乙醇、醚等有机溶剂,稍溶于水,在碱性和热环境下不稳定,在酸性环境下较稳定[1-2]。新鲜大蒜中含有大蒜素的前体物质——蒜氨酸(alciin),即S-烯丙茎-L-半胱胺酸亚砜,而不含有大蒜素。当大蒜经切片或捣碎等物理因素影响时,分布在不同部位或不同细胞中的蒜氨酸和蒜酶相结合生成大蒜素[3]。大蒜素可由水蒸气蒸馏法、浸出法、超临界二氧化碳萃取法等直接从大蒜中获得;也可经由二烯丙基二硫醚进一步氧化反应化学合成而得[4]。大蒜素具有不稳定性,即使在常温环境中也容易分解, 其分解产物的生物活性较其自身低[5]。
2 大蒜素的生物学功能 2.1 抑菌杀菌大蒜素具有广谱的抗菌特性[6-7],对多种革兰氏阴性菌和革兰氏阳性菌具有抗菌活性,如金黄色葡萄杆菌和沙门氏菌等[8-9]。此外,大蒜素还具有抗真菌性(如白色念珠菌)、抗寄生虫以及抗病毒的活性[10]。大蒜素会导致菌体细胞壁和细胞膜不可逆的超微结构变化,破坏细胞膜的完整性,导致细胞内容物外流最终实现杀菌活性[11-12]。研究表明,大蒜素还具有高度脂溶性,很容易穿过细胞膜系统[13]。大蒜素渗透到细胞和细胞器内,与含巯基的蛋白质(包括各种酶)和氨基酸反应[14]。DNA促旋酶是原核生物必不可少的酶之一,是临床上抗生素的重要靶标。大蒜素在体外通过抑制DNA促旋酶活性进而抑制细菌DNA的复制[15]。β-半乳糖苷酶是细菌、酵母、真核细胞生理代谢过程中的关键酶,使用大蒜素处理β-半乳糖苷酶会使其活性降低[11]。大蒜素可以通过促进白念球菌菌丝生长负向调控基因磷酸二酯酶2(PDE2)的表达,抑制白念球菌菌丝生长正向调控基因念珠菌菌丝壁蛋白1(HWP1)、凝集素样黏附素1(ALS1)和增强菌丝生长基因1(EFG1)的表达,进而抑制白念球菌菌丝的生长,降低白念球菌的毒性[16]。
除此之外大蒜素还可以抑制菌体蛋白质的合成[17],抑制真菌孢子[18]和被膜的形成[19]。大蒜素可抑制蛋白质功能是因为大蒜素与蛋白质中的巯基发生反应形成二硫键,进而导致蛋白解折叠,破坏了蛋白质正常的结构[20]。值得注意的是,一些不含巯基的酶也被大蒜素抑制,而一些含巯基的酶则不能,这说明大蒜素与蛋白质反应的决定因素不是巯基而是蛋白质内的微环境[21]。大蒜素对蛋白质合成的影响如图 1所示。图 1-A所示,正常条件下蛋白质的合成。翻译后蛋白质折叠成其最终结构。图 1-B所示,易于攻击的半胱氨酸残基(红色表示)通过二硫键交换反应与大蒜素反应。在空间上受阻的半胱氨酸残基(以蓝色表示)不会与大蒜素反应。图 1-C所示,空间上受阻的半胱氨酸残基在蛋白质合成早期会成为大蒜素攻击的潜在靶标。结果会导致蛋白质翻译失败,功能降低甚至没有功能。虽然大蒜素的抑菌机制尚未充分阐明,但是大蒜素通过攻击菌体细胞生存不可或缺的原始材料和抑制基础代谢,使得细菌不能通过突变或代谢适应来克服大蒜素带来的损伤[22]。
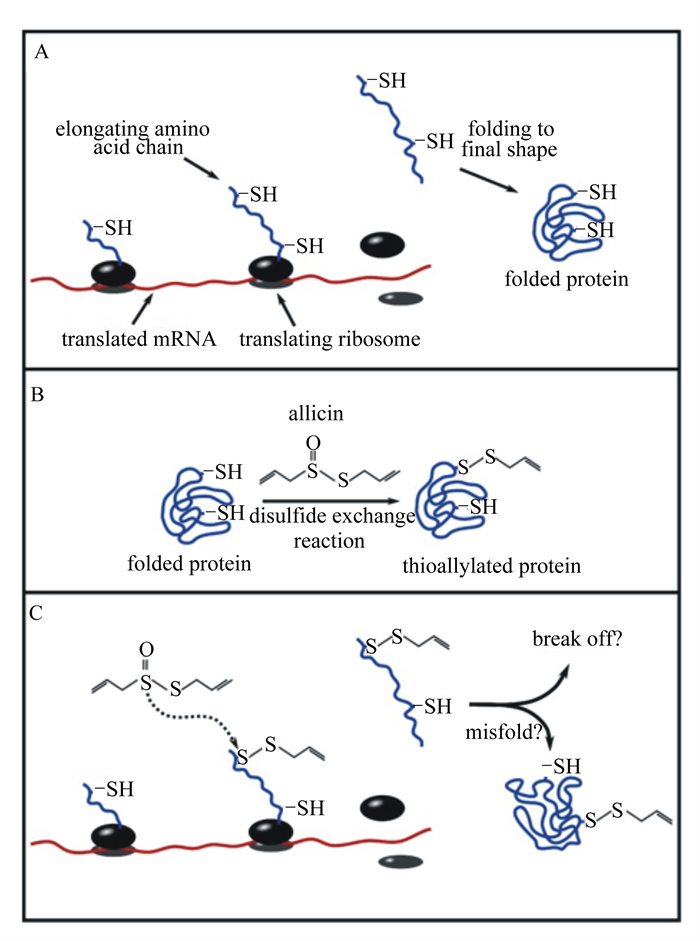
|
-SH:巯基sulfydryl;translated mRNA:被翻译的mRNA;elongating amino acid chain:正在延伸的氨基酸链;folding to final shape:折叠成最终形状;translating ribosome:翻译中的核糖体;folded protein已折叠完成的蛋白质;allicin:大蒜素;disulfide exchange reaction:二硫键交换反应;thioallylated protein:硫酰化蛋白;break off:崩解;misfold:错误折叠。 图 1 大蒜素对蛋白质合成的影响 Fig. 1 Effects of allicin on protein synthesis[23] |
虽然大蒜素在化学水平上是氧化剂,但在生理水平上,低剂量的大蒜素可充当抗氧化剂[23]。大蒜素可减少细胞中氧自由基(ROS)含量[24-25],从而可以减少ROS大量堆积对细胞DNA和蛋白质的损伤[26],保护了线粒体膜的通透性和膜电位,进而保护细胞免于凋亡[27]。ROS是丝裂原活化蛋白激酶(MAPK)有效的激活剂[28]。而大蒜素可以通过抑制p38丝裂原活化蛋白激酶(p38-MAPK)通路的激活来减轻ROS诱导的氧化应激和细胞线粒体凋亡[29]。叉头框转录因子O1(FOXO1)是抗氧化应激、代谢调节以及细胞增殖和凋亡等生物过程的关键转录因子[30]。在抵抗铅氧化应激诱导小鼠骨质流失的研究中,发现大蒜素组FOXO1的活性提高,抗氧化酶如超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)的活性增强,而丙二醛(MDA)和ROS含量则降低[31-32],小鼠的抗氧化应激功能得到提高。核因子E2相关因子2(NF-E2-related factor 2,Nrf2)是一种细胞内的转录因子,可调节许多抗氧化酶和解毒因子基因的表达[33]。大蒜素可以通过提高转录因子Nrf2表达来诱导血红素氧合酶-1(HO-1)和谷氨酸-半胱氨酸连接酶修饰物(GCLM)亚单位等抗氧化酶的表达,从而保护细胞免受氧化应激。此外大蒜素可以通过显著抑制一氧化氮合酶(iNOS)和烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADPH)、氧化酶-4(NOX-4)的表达来保护机体免受氧化应激损伤[34]。
2.3 抗炎核因子-κB(NF-κB)和MAPK参与细胞的各种生理反应和病理反应的调控,包括凋亡、炎性损伤和应激[35],核苷酸结合寡聚结构域样受体蛋白3(NLRP3)是激活炎症的关键媒介[36]。大蒜素可以通过抑制炎性诱导因子如丙烯酰胺和氧化应激等对MAPK/NF-κB/NLRP3信号通路的激活,来减少炎性因子白细胞介素-1β(IL-1β)、白细胞介素-18(IL-18)、白细胞介素-6(IL-6)和肿瘤坏死因子(TNF)-α的释放,进而减轻炎症反应,此外大蒜素还可以通过抑制关键酶的磷酸化来抑制MAPK和NF-κB信号通路[37-38]。大蒜素除了通过影响通路来抑制炎症因子的表达外,还可以通过控制细胞自噬蛋白的表达,防止细胞过度自噬,以及抑制中性粒细胞黏附于内皮细胞来抑制和改善炎症[39-40]。由此可见,大蒜素除具有杀菌抑菌、抗氧化的功能,还具有抗炎活性。
2.4 改善机体健康在动物机体中,氧化应激和炎症反应不是孤立的病理生理过程,而是存在紧密联系的联动链,且多出现叠加效应[41]。氧化应激会加重炎症反应,炎症因子同时也会促进氧化应激的发生,因此会出现“氧化应激-炎症反应-免疫失衡”的联动反应,对动物健康带来较大危害[42]。当转录因子Nrf2被激活会抑制氧化应激和炎症反应[43]。研究表明大蒜素可以激活核因子Nrf2,促进抗氧化酶基因表达,抵抗机体氧化应激[34],抑制脂多糖介导的炎症反应[39]。由此可见,大蒜素可有效抑制“氧化应激-炎症反应-免疫失衡”的联动反应,改善机体健康。
3 大蒜素在反刍动物上的饲用效果 3.1 提高反刍动物生产性能提高动物生产性能是畜牧生产与科研工作的重要目标之一。金萍等[44]在荷斯坦奶牛的饲粮中分别添加30、80、120 mg/kg的大蒜素,3个试验组产奶量均增加,乳脂率分别提高0.089%、0.151%、0.050%。大蒜素添加量为80 mg/kg最佳,过多时会造成乳气味劣化。刘敏等[45]添加大蒜素干粉饲喂荷斯坦奶牛,结果表明试验组非脂固形物和乳蛋白含量相比对照组显著提高。胡永杰[46]把大蒜素添加到秦川牛的饲粮中,结果显示与对照组相比,大蒜素组日增重提高13.6%,饲料转化率提高1%,利润提高14.7%。井明艳等[47]在绵羊的饲粮中分别添加浓度为25%的大蒜素1.6、1.2、0.8 g/(只·d),结果表明大蒜素的投放量在1.3~1.5 g/(只·d)增重明显。赵健康等[48]在湖羊的精料中添加2 g/(只·d)大蒜素,显著提高了湖羊的日增重,增重利润较对照组提高了21.2元,经济效益提高了14.7%,同时腹泻率较对照组降低了26.7%。以上研究表明添加适量的大蒜素或含有大蒜素的副产品可以提高反刍动物的生产性能。大蒜素促进动物采食及其具有的生物学功能来改善动物的免疫功能是其提高反刍动物生产性能的关键因素[45, 48]。
3.2 提高动物营养物质消化率消化代谢是反映反刍动物营养物质消化情况的重要指标。Ma等[49]在杜泊×细尾寒羊杂交F1母羊的饲粮中添加2 g/(只·d)大蒜素,发现大蒜素可有效提高母羊的有机物、氮、中性洗涤纤维和酸性洗涤纤维的表观消化率。秦龙等[50]在绵羊的基础饲粮中添加300 mg/kg的大蒜素,结果表明,大蒜素可以提高瘤胃内营养物质的表观消化率和可消化氮含量。Zhong等[51]在感染了胃肠线虫的羔羊基础饲粮中添加50 g/kg的大蒜粉,结果显示大蒜粉组的羔羊干物质和粗蛋白质消化率较对照组有所提高,同时大蒜粉还降低了羔羊粪便中线虫虫卵的数量。以上研究表明,在反刍动物的饲粮中添加适量的大蒜素有助于提高饲粮消化率。大蒜素提高饲粮消化率主要有以下几个原因:1)由于大蒜素气味可以提高消化液的分泌,增强肠胃蠕动。2)大蒜素可以促进多种消化酶的分泌[52-53]。3)大蒜素抑制甲烷菌和原虫的生长,使得更多的能量供给其他细菌,如纤维素分解菌,来达到提高反刍动物饲粮消化率的目的[49]。
3.3 改善瘤胃发酵瘤胃发酵产生的挥发性脂肪酸(VFA)可为机体提供70%~80%的能量,合成微生物蛋白占小肠吸收总蛋白量的50%~80%[54]。在反刍动物体外试验中,Busquet等[55]研究报道,添加不同量的大蒜素对总挥发性脂肪酸(TVFA)含量均无显著影响,当大蒜素的添加量为300 mg/L时,pH显著升高;为3 000 mg/L时,丙酸含量显著增加,支链脂肪酸含量降低。Cardozo等[56]在研究天然植物提取物对瘤胃蛋白质降解和发酵特性影响的试验中添加含有0.7%大蒜素的大蒜提取物,结果发现TVFA含量未受影响,而氨含量显著降低。Busquet等[57]研究表明,添加300和3 000 mg/kg大蒜油,降低乙酸的含量,增加了丙酸和丁酸含量,降低了TVFA含量。
反刍动物在体研究发现,大蒜素添加量为0.2、0.4 g/(只·d)时,显著提高肉牛瘤胃TVFA和丙酸含量,降低乙丙比[58]。秦龙等[50]研究报道,饲粮中添加300 mg/kg的大蒜素,提高绵羊瘤胃氨态氮和VFA含量,增加了菌体蛋白产量,促进了瘤胃发酵。Zhong等[51]在感染了胃肠线虫的羔羊基础饲粮中添加50 g/kg的大蒜粉,结果显示添加大蒜粉增加了瘤胃液中VFA含量,降低了乙酸/丙酸值。VFA的在瘤胃中增多会导致pH降低[59]。然而刘敏等[60]在绵羊上的研究结果表明大蒜干粉提高了瘤胃pH。杨坤等[61]在绵羊的基础饲粮中添加30 mg/kg的大蒜素,也发现大蒜素会提高瘤胃pH的结论。以上不同结果可能是由于添加剂量和饲养环境不同所致,有待进一步研究探讨。另外,杨坤等[61]的试验还发现大蒜素组在氨态氮和菌体蛋白的含量上较对照组有所提高,可能是由于大蒜素具有抑制和杀死甲烷菌的生物活性,从而使更多的碳源用于微生物蛋白的合成。
综上所述,大蒜素影响瘤胃发酵效果不尽相同,并且体外与在体研究结果迥异。因此,大蒜素对瘤胃发酵的影响仍需进一步深入探究。
3.4 降低甲烷产量甲烷是大气重要的温室气体。据科学家估计,2030年甲烷排放量在总温室气体排放量的占比将达到50%,成为首要的温室气体[62]。反刍动物每年甲烷排放量为1.6千兆t二氧化碳当量至2.7千兆t二氧化碳当量,占全球总排放量的3%~5%[63]。瘤胃中甲烷的产生代表着饲粮能量的大量损失,损失达到2%~12%,具体取决于饲粮的类型[64]。因此降低甲烷排放对环境的保护和提高反刍动物饲粮转化率等方面有重要意义。
体外研究发现,在分别添加300 mg/L大蒜素、二烯丙基二硫化物(DAD)、烯丙硫醇(ALM)作为甲烷抑制剂的试验中,甲烷排放量分别降低了73.6%、68.5%、19.5%,其中大蒜素作为甲烷抑制剂的效果最佳[55]。薛艳锋等[65]以4期牧草为底物在体外模拟瘤胃发酵试验,结果表明大蒜素的添加量为1.5%和2.0%时甲烷产量显著降低。Patra等[66]在体外模拟瘤胃发酵试验中添加0.25、0.50、1.00 g/L的大蒜油,甲烷产量随其添加量增加而线性下降;当添加量为1.0 g/L时甲烷产量降低了87%,同时各组添加量都使瘤胃中古细菌和原虫数量降低。
在体研究同样发现,大蒜素添加量为2.0 g/(只·d)时可以显著降低杜泊羊×小尾寒羊杂交F1代6.38%的甲烷排放量[67]。Ma等[49]在杜泊×细尾寒羊杂交F1母羊的饲粮中添加2 g/(只·d)大蒜素,结果表明大蒜素有效地降低了母羊的甲烷日排放量0.16 L/kg。杨坤等[61]在绵羊的基础饲粮中添加30 mg/kg的大蒜素,与对照组相比,大蒜素组绵羊瘤胃氨态氮和菌体蛋白的含量有所提高,甲烷产量显著降低,降低幅度最大为38.28%。以上研究表明,大蒜素对反刍动物甲烷排放量有显著的抑制作用,主要由于大蒜素降低了瘤胃中甲烷菌和原虫的数量。
4 小结与展望综上所述,由天然植物提取的大蒜素是绿色饲料添加剂,可以提高反刍动物生产性能、饲料转化率,同时还能通过抑制甲烷菌活性来降低甲烷产量。但是其作用机理尚未充分阐明,亟需探讨最终为大蒜素在反刍动物生产的研究与应用提供理论支持,实现反刍动物的高效健康养殖。同时,为了使大蒜素更好地在畜牧生产中推广应用,如何提高大蒜素的稳定性也是亟需解决问题。另外,植物提取的大蒜素除含有主要成分大蒜素外还会附带大蒜中含有的其他营养活性成分,不同提取方法得到的附带营养活性成分是否相同以及不同提取方法得到的大蒜素生物活性是否有差异?有待进一步研究讨论。
| [1] |
梅四卫, 朱涵珍. 大蒜素的研究进展[J]. 中国农学通报, 2009, 25(9): 97-101. MEI S W, ZHU H Z. Research advances in allicin[J]. Chinese Agricultural Science Bulletin, 2009, 25(9): 97-101 (in Chinese). |
| [2] |
BORLINGHAUS J, FOERSTER NÉE REITER J, KAPPLER U, et al. Allicin, theodor of freshly crushed garlic: a review of recent progress in understanding allicin's effects on cells[J]. Molecules, 2021, 26(6): 1505. DOI:10.3390/molecules26061505 |
| [3] |
BAHARE S, PAOLO Z, ILKAY E O, et al. Allicin and health: a comprehensive review[J]. Trends in Food Science and Technology, 2019, 86: 502-516. DOI:10.1016/j.tifs.2019.03.003 |
| [4] |
李文清. 大蒜素的合成、转化及其稳定性研究[D]. 硕士学位论文. 广州: 暨南大学, 2015. LI W Q. Study on the synthesis, transformation and stability of allicin[D]. Master's Thesis. Guangzhou: Jinan University, 2015. (in Chinese) |
| [5] |
ILIĆD P, STOJANOVIĆ S, NAJMAN S, et al. Biological evaluation of synthesized allicin and its transformation products obtained by microwaves in methanol: antioxidant activity and effect on cell growth[J]. Biotechnology, Biotechnological Equipment, 2015, 29(1): 189-194. DOI:10.1080/13102818.2014.994267 |
| [6] |
LIU Q, MENG X, LI Y, et al. Antibacterial and antifungal activities of spices[J]. International Journal of Molecular Sciences, 2017, 18(6): 1283. DOI:10.3390/ijms18061283 |
| [7] |
LIU Q, MENG X, LI Y, et al. Natural products for the prevention and management of helicobacter pylori infection[J]. Comprehensive Reviews in Food Science and Food Safety, 2018, 17(4): 937-952. DOI:10.1111/1541-4337.12355 |
| [8] |
JIA Y C, WU X M. In vitro activity of allicin combined with two antibiotics on intestinal Shigella[J]. Infection International, 2017, 6(1): 25-29. DOI:10.1515/ii-2017-0152 |
| [9] |
EL-AZZOUNY M M, EL-DEMERDASH A S, SEADAWY H G, et al. Antimicrobial effect of garlic (Allium sativum) and thyme (Zataria multiflora Boiss) extracts on some food borne pathogens and their effect on virulence gene expression[J]. Cellular and Molecular Biology, 2018, 64(10): 79-86. DOI:10.14715/cmb/2018.64.10.13 |
| [10] |
熊延靖, 吴艳红, 陈京. 大蒜素对白色念珠菌毒力因子作用机制的研究[J]. 中成药, 2020, 42(11): 2964-2970. XIONG Y J, WU Y H, CHEN J. Mechanism of allicin on the virulence factors of Candida albicans[J]. Chinese Traditional Patent Medicine, 2020, 42(11): 2964-2970 (in Chinese). DOI:10.3969/j.issn.1001-1528.2020.11.026 |
| [11] |
姜慧. 大蒜素-乳清分离蛋白结合物的制备、表征及其消化特性和抑菌活性研究[D]. 硕士学位论文. 镇江: 江苏大学, 2020. JIANG H. Preparation, characterization, digestive properties and antibacterial activity of allicin-whey protein isolate conjugates[D]. Master's Thesis. Zhenjiang: Jiangsu University, 2020. (in Chinese) |
| [12] |
EL-SABER BATIHA G, MAGDY BESHBISHY A, G WASEFL, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review[J]. Nutrients, 2020, 12(3): 872. DOI:10.3390/nu12030872 |
| [13] |
MIRON T, RABINKOV A, MIRELMAN D, et al. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity[J]. Biochimica et Biophysica Acta: Biomembranes, 2000, 1463(1): 20-30. DOI:10.1016/S0005-2736(99)00174-1 |
| [14] |
RANITHA M, NURLIDIA M, BESHR A K, et al. Evaluation of allicin as soil urease inhibitor[J]. Procedia Engineering, 2017, 184: 449-459. DOI:10.1016/j.proeng.2017.04.116 |
| [15] |
REITER J, HVBBERS A M, ALBRECHT F, et al. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria[J]. International Journal of Medical Microbiology, 2020, 310(1): 151359. DOI:10.1016/j.ijmm.2019.151359 |
| [16] |
熊延靖, 厉荣玉, 吴艳红. 大蒜素对白念珠菌形态转换的影响研究[J]. 中国真菌学杂志, 2021, 16(1): 14-18. XIONG Y J, LI R Y, WU Y H. The effect of allicin on the morphological transition of Candida albicans[J]. Chinese Journal of Mycology, 2021, 16(1): 14-18 (in Chinese). DOI:10.3969/j.issn.1673-3827.2021.01.004 |
| [17] |
BIRBEN E, SAHINER U M, SACKESEN C, et al. Oxidative stress and antioxidant defense[J]. The World Allergy Organization Journal, 2012, 5(1): 9-19. DOI:10.1097/WOX.0b013e3182439613 |
| [18] |
ROSHAN N, RILEY T V, HAMMER K A. Effects of natural products on several stages of the spore cycle of Clostridium difficile in vitro[J]. Journal of Applied Microbiology, 2018, 125(3): 710-723. DOI:10.1111/jam.13889 |
| [19] |
熊延靖, 吴艳红. 大蒜素对白色念珠菌生物被膜形成的作用[J]. 菌物学报, 2020, 39(2): 343-351. XIONG Y J, WU Y H. The inhibitory effects of allicin on biofilm formation of Candida albicans[J]. Mycosystema, 2020, 39(2): 343-351 (in Chinese). |
| [20] |
MVLLER A, ELLER J, ALBRECHT F, et al. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines[J]. The Journal of Biological Chemistry, 2016, 291(22): 11477-11490. DOI:10.1074/jbc.M115.702308 |
| [21] |
ROSHAN N, RILEY T V, KNIGHT D R, et al. Natural products show diverse mechanisms of action against Clostridium difficile[J]. Journal of Applied Microbiology, 2019, 126(2): 468-479. DOI:10.1111/jam.14152 |
| [22] |
FUJISAWA H, WATANABE K, SUMA K, et al. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds[J]. Bioscience Biotechnology and Biochemistry, 2009, 73(9): 1948-1955. DOI:10.1271/bbb.90096 |
| [23] |
RLINGHAUS J, ALBRECHT F, GRUHLKE M C, et al. Allicin: chemistry and biological properties[J]. Molecules, 2014, 19(8): 12591-12618. DOI:10.3390/molecules190812591 |
| [24] |
JANG H J, LEE H J, YOON D K, et al. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents[J]. Food Science and Biotechnology, 2018, 27(1): 219-225. DOI:10.1007/s10068-017-0246-4 |
| [25] |
MORSY K, GHAMDI A A, DAJEM S B, et al. The oil ofgarlic, Alliumsativum L. (Amaryllidaceae), as a potential protectant against Anisakis spp.Type Ⅱ (L3) (Nematoda) infection in Wistar rats[J]. RevistaBrasileira de Parasitologia Veterinaria, 2021, 30(1): e015920. DOI:10.1590/s1984-296120201086 |
| [26] |
SHIELDS H J, TRAA A, VAN RAAMSDONK J M. Beneficial and detrimental effects of reactive oxygen species on lifespan: a comprehensive review of comparative and experimental studies[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 628157. DOI:10.3389/fcell.2021.628157 |
| [27] |
TAN Y J, JIN Y, WANG Q, et al. Perilipin 5 protects against cellular oxidative stress by enhancing mitochondrial function in HepG2 cells[J]. Cells, 2019, 8(10): 1241. DOI:10.3390/cells8101241 |
| [28] |
ZENG K W, SONG F J, WANG Y H, et al. Induction of hepatoma carcinoma cell apoptosis through activation of the JNK-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-ROS self-driven death signal circuit[J]. Cancer Letters, 2014, 353(2): 220-231. DOI:10.1016/j.canlet.2014.07.022 |
| [29] |
XIANG Q, CHENG Z R, WANG JT, et al. Allicin attenuated advanced oxidation protein product-induced oxidative stress and mitochondrial apoptosis in human nucleus pulposus cells[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 6685043. |
| [30] |
TIA N, SINGH A K, PANDEY P, et al. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases[J]. Gene, 2018, 648: 97-105. DOI:10.1016/j.gene.2018.01.051 |
| [31] |
LI D, LIANG H, LI Y, et al. Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis via SIRT1/FOXO1 pathway in mice[J]. Biological Trace Element Research, 2021, 199(1): 237-243. DOI:10.1007/s12011-020-02136-5 |
| [32] |
KLOTZ L O, SÁNCHEZ-RAMOS C, PRIETO-ARROYO I, et al. Redox regulation of FoxO transcription factors[J]. Redox Biology, 2015, 6: 51-72. DOI:10.1016/j.redox.2015.06.019 |
| [33] |
SAJADIMAJD S, KHAZAEIM. Oxidative stress and cancer: the role of Nrf2[J]. Current Cancer Drug Targets, 2018, 18(6): 538-557. DOI:10.2174/1568009617666171002144228 |
| [34] |
YANG J J, SONG X B, FENG Y, et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway[J]. Free Radical Biology & Medicine, 2020, 152: 854-864. |
| [35] |
LI Y, XU S, XU QQ, et al. Clostridium difficile toxin B induces colonic inflammation through the TRIM46/DUSP1/MAPKs and NF-κB signalling pathway[J]. Artificial Cells, Nanomedicine, and Biotechnology, 2020, 48(1): 452-462. DOI:10.1080/21691401.2019.1709856 |
| [36] |
TEJERA D, MERCAN D, SANCHEZ-CARO J M, et al. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome[J]. The EMBO Journal, 2019, 38(17): e101064. |
| [37] |
NAN B, YANG C Y, LI L, et al. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver[J]. Food and Chemical Toxicology, 2021, 148: 111937. DOI:10.1016/j.fct.2020.111937 |
| [38] |
ALAM R T M, FAWZI E M, ALKHALF M I, et al. Anti-inflammatory, immunomodulatory, and antioxidant activities of allicin, norfloxacin, or their combination against Pasteurella multocida infection in male New Zealand rabbits[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 1780956. |
| [39] |
ZHANG M, PAN H C, XU YJ, et al. Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2[J]. Cellular Physiology and Biochemistry, 2017, 41(6): 2255-2267. DOI:10.1159/000475640 |
| [40] |
DAI J J, CHEN Y, JIANG F. Allicin reduces inflammation by regulating ROS/NLRP3 and autophagy in the context of A.fumigatus infection in mice[J]. Gene, 2020, 762: 145042. DOI:10.1016/j.gene.2020.145042 |
| [41] |
卢德勋. 健康养殖的营养技术策略的系统观[J]. 饲料工业, 2019, 40(2): 1-5. LU D X. Systematic view of nutritional technology strategies for healthy breeding[J]. Feed Industry, 2019, 40(2): 1-5 (in Chinese). |
| [42] |
卢德勋. 动物营养学科发展在战略方向上的重大突破: 构建动物健康营养理论和技术体系及其实际应用[J]. 动物营养学报, 2021, 33(1): 1-12. LU D X. A major breakthrough in development of animal nutrition in strategic direction: building an animal health and nutrition theory and technology system and its application[J]. Chinese Journal of Animal Nutrition, 2021, 33(1): 1-12 (in Chinese). |
| [43] |
KOBAYASHI E H, SUZUKI T, FUNAYAMA R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription[J]. Nature Communications, 2016, 7: 11624. DOI:10.1038/ncomms11624 |
| [44] |
金萍, 秦希杰. 乳牛日粮中添加大蒜素对乳品质和产奶率的效果观察[J]. 现代畜牧兽医, 2009(7): 22-24. JIN P, QIN X J. Effect of additionin of garlicin on milk quality and milk production rate in diet of dairy cattle[J]. Modern Journal of Animal Husbandry and Veterinary Medicine, 2009(7): 22-24 (in Chinese). DOI:10.3969/j.issn.1672-9692.2009.07.012 |
| [45] |
刘敏, 张志军, 马光辉, 等. 大蒜素对荷斯坦奶牛生产性能的影响[J]. 新疆农业科学, 2012, 49(3): 555-559. LIU M, ZHANG Z J, MA G H, et al. Effect of allicin on production performance of Holstein cows[J]. Xinjiang Agricultural Sciences, 2012, 49(3): 555-559 (in Chinese). |
| [46] |
胡永杰. 日粮中添加大蒜素饲喂秦川牛的效果试验[J]. 畜牧兽医杂志, 2003, 22(1): 34-35. HU Y J. Effect of adding allicin to diet on Qinchuan cattle[J]. Journal of Animal Science and Veterinary Medicine, 2003, 22(1): 34-35 (in Chinese). |
| [47] |
井明艳, 孙建义, 赵树盛, 等. 沙葱、地椒和大蒜素对绵羊体增重效果的影响[J]. 饲料广角, 2004(8): 4-6. JING M Y, SUN J Y, ZHAO S C, et al. Effects of allium mongolicum regel, thymus mongolicus and allicin on weight gain of sheep[J]. Feed Research, 2004(8): 4-6 (in Chinese). |
| [48] |
赵健康, 杨开伦, 张琦智, 等. 大蒜素对生长期湖羊增重和腹泻率的影响[J]. 黑龙江畜牧兽医, 2016(6): 154-155, 158. ZHAO J K, YANG K L, ZHANG Q Z, et al. Effect of allicin on weight gain and diarrhea rate of Hu sheep during the growth period[J]. Heilongjiang Animal Science and Veterinary Medicine, 2016(6): 154-155, 158 (in Chinese). |
| [49] |
MA T, CHEN D D, TU Y, et al. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes[J]. Journal of Animal Science and Biotechnology, 2016, 7: 1. DOI:10.1186/s40104-015-0057-5 |
| [50] |
秦龙, 杨坤, 姜宁, 等. 不同甲烷抑制剂对绵羊瘤胃发酵及消化代谢的影响[C]//中国畜牧兽医学会动物营养学分会第十二次动物营养学术研讨会论文集, 武汉: 中国畜牧兽医学会, 2016: 544. QIN L, YANG K, JIANG N, et al. Effects of different methane inhibitors on rumen fermentation, digestion and metabolism of sheep[C]//Proceedings of the 12th symposium on animal nutrition of animal nutrition branch of Chinese society of animal husbandry and veterinary. Wuhan: Chinese society of animal husbandry and veterinary medicine, 2016: 544. |
| [51] |
ZHONG R Z, XIANG H, CHENG L, et al. Effects of feeding garlic powder on growth performance, rumen fermentation, and the health status of lambs infected by gastrointestinal nematodes[J]. Animals, 2019, 9(3): 102. DOI:10.3390/ani9030102 |
| [52] |
张伟, 张爱忠, 姜宁, 等. 大蒜素对畜牧生产环境的保护作用[J]. 黑龙江畜牧兽医, 2017(8): 171-173. ZHANG W, ZHANG A Z, JIANG N, et al. Protective effect of allicin on animal husbandry production environment[J]. Heilongjiang Animal Science and Veterinary Medicine, 2017(8): 171-173 (in Chinese). |
| [53] |
卓清, 曾丹, 周艺, 等. 大蒜及其提取物在猪生产中的应用[J]. 饲料工业, 2015, 36(11): 18-21. ZHUO Q, ZENG D, ZHOU Y, et al. Application of garlic and its extract in pig production[J]. Feed Industry, 2015, 36(11): 18-21 (in Chinese). |
| [54] |
王玉强, 赵倩明, 沈宇, 等. 日粮中添加米糠和玉米胚芽粕对泌乳奶牛瘤胃发酵及微生物菌群的影响[J]. 扬州大学学报(农业与生命科学版), 2019, 40(3): 65-71. WANG Y Q, ZHAO Q M, SHEN Y, et al. Effects of rice bran and corn germ meal on rumen fermentation and microflora of lactating dairy cows[J]. Journal of Yangzhou University (Agricultural and Life Science Edition), 2019, 40(3): 65-71 (in Chinese). |
| [55] |
BUSQUET M, CALSAMIGLIA S, FERRET A, et al. Effect of garlic oil and four of its compounds on rumen microbial fermentation[J]. Journal of Dairy Science, 2005, 88(12): 4393-4404. DOI:10.3168/jds.S0022-0302(05)73126-X |
| [56] |
CARDOZO P W, CALSAMIGLIA S, FERRET A, et al. Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture[J]. Journal of Animal Science, 2004, 82(11): 3230-3236. DOI:10.2527/2004.82113230x |
| [57] |
BUSQUET M, CALSAMIGLIA S, FERRET A, et al. Plant extracts affect in vitro rumen microbial fermentation[J]. Journal of Dairy Science, 2006, 89(2): 761-771. DOI:10.3168/jds.S0022-0302(06)72137-3 |
| [58] |
贾玉山, 格根图, 董占元, 等. 大蒜对肉牛瘤胃VFA及其瘤胃内饲料降解的影响[J]. 中国畜牧杂志, 2003, 39(6): 20-23. JIA Y S, GEGENTU, DONG Z Y, et al. Study on the influence of garlic the rumen VFA and the degradation of fodder in rumen of beef cattle[J]. Chinese Journal of Animal Science, 2003, 39(6): 20-23 (in Chinese). DOI:10.3969/j.issn.0258-7033.2003.06.009 |
| [59] |
胡红莲, 卢德勋, 刘大程, 等. 日粮不同NFC/NDF比对奶山羊瘤胃pH值、挥发性脂肪酸及乳酸含量的影响[J]. 畜牧与饲料科学, 2011, 32(Z1): 41-46. HU H L, LU D X, LIU D C, et al. Effects of different dietary NFC/NDF ratios on rumen pH, VFA and lactate content in dairy goats[J]. Animal Husbandry and Feed Science, 2011, 32(Z1): 41-46 (in Chinese). |
| [60] |
刘敏, 张志军, 米热古丽·伊马木, 等. 大蒜素对哈萨克羊瘤胃发酵的影响[J]. 中国草食动物, 2012, 32(1): 22-25. LIU M, ZHANG Z J, MIREGULI Y, et al. Effect of allicin on rumen fermentation of Kazakh sheep[J]. China Herbivores, 2012, 32(1): 22-25 (in Chinese). DOI:10.3969/j.issn.2095-3887.2012.01.006 |
| [61] |
杨坤, 姜宁, 张爱忠, 等. 添加不同甲烷抑制剂对绵羊瘤胃发酵指标及甲烷产量的影响[J]. 黑龙江八一农垦大学学报, 2016, 28(6): 16-20. YANG K, JIANG N, ZHANG A Z, et al. Effect of different methane inhibitor on indices of rumen fermentation and methane production in sheep[J]. Journal of Heilongjiang August First Land Reclamation University, 2016, 28(6): 16-20 (in Chinese). DOI:10.3969/j.issn.1002-2090.2016.06.003 |
| [62] |
张福凯, 徐龙君. 甲烷对全球气候变暖的影响及减排措施[J]. 矿业安全与环保, 2004, 31(5): 6-9, 38. ZHANG F K, XU L J. Effect of methane on global warming and mitigating measures[J]. Mining Safety & Environmental Protection, 2004, 31(5): 6-9, 38 (in Chinese). DOI:10.3969/j.issn.1008-4495.2004.05.003 |
| [63] |
BUSTAMANTE M, ROBLEDO-ABAD C, HARPER R, et al. Co-benefits, trade-offs, barriers and policies for greenhouse gas mitigation in the agriculture, forestry and other land use (AFOLU) sector[J]. Global Change Biology, 2014, 20(10): 3270-3290. DOI:10.1111/gcb.12591 |
| [64] |
JOHNSON K A, JOHNSON D E. Methane emissions from cattle[J]. Journal of Animal Science, 1995, 73(8): 2483-2492. DOI:10.2527/1995.7382483x |
| [65] |
薛艳锋, 孙红梅, 郝力壮, 等. 大蒜素对藏羊瘤胃发酵及甲烷产量的影响[J]. 吉林农业科学, 2015, 40(4): 72-77, 82. XUE Y F, SUN H M, HAO L Z, et al. Effect of allicin on fermentation parameters and methane emissions of Tibetan sheep[J]. Journal of Jilin Agricultural Sciences, 2015, 40(4): 72-77, 82 (in Chinese). |
| [66] |
PATRA A K, YU Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations[J]. Applied and Environmental Microbiology, 2012, 78(12): 4271-4280. DOI:10.1128/AEM.00309-12 |
| [67] |
陈丹丹, 屠焰, 马涛, 等. 大蒜素和茶皂素对肉羊气体代谢及甲烷排放的影响[J]. 中国畜牧杂志, 2014, 50(11): 57-61. CHEN D D, TU Y, MA T, et al. Effect of allicin and tea saponin on gas metabolism and methane emission in mutton sheep[J]. Chinese Journal of Animal Science, 2014, 50(11): 57-61 (in Chinese). DOI:10.3969/j.issn.0258-7033.2014.11.014 |




