2. 兰州大学草地农业科技学院, 兰州 730020
2. College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou 730020, China
饲料中添加抗生素可以促进畜禽生长,提高养殖效率,但饲用抗生素的使用导致抗生素残留和环境污染等问题,严重制约我国畜牧业的健康可持续发展。植物多酚因具有抗氧化、抗炎、抗菌等多种生物学功能,可作为潜在的畜禽饲料添加剂[1-3]。黄酮类化合物是植物多酚的一个主要亚群,包括黄酮、异黄酮以及黄烷酮醇等[4],多为植物的次生代谢产物,常存在于蔬菜和水果中[5]。二氢槲皮素(dihydroquercetin,DHQ)是一种典型的黄烷酮醇类植物多酚,存在于花旗松[6]、洋葱[7]等植物中,具有抗氧化、抗炎、抗纤维化、抗癌、抗病毒等多种生物学功能。目前,DHQ作为食品添加剂和医药主功效成分,在食品健康和医疗等领域发挥着重要作用,但在畜禽饲料添加剂领域的应用还处于起步阶段。本文就DHQ的分子结构、理化性质、生物学功能及其可能的作用机制进行综述,并总结了DHQ在畜牧业生产中的研究现状,旨在为新型饲料添加剂的开发和应用提供理论依据。
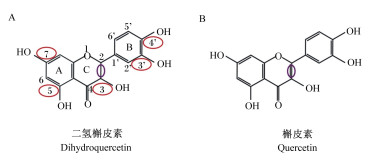
1 DHQ的理化性质DHQ又称紫杉叶素、花旗松素或黄杉素,最早由日本学者Fukui于1965年从花旗松(Pseudotsugamenziesii)的树皮中提取分离[8],而后发现洋葱[7]、松树皮[9]、水飞蓟[10]以及柑橘类水果[11]中均含有DHQ。DHQ属于黄烷酮醇类多酚化合物,化学名称为3,5,7,3’,4’-五羟基二氢黄酮,分子式为C15H12O7,相对分子质量为304.25,属于维生素P族,主要存在于植物的根部、茎干以及果实中,其中,在花旗松树皮中的含量可高达7%[12]。DHQ含有2个芳香族结构和1个杂环结构,能以反式和顺式2种结构形式存在,且反式结构活性更强[13]。DHQ的生物学活性与其分子结构密切相关,其A环C5、C7以及B环C3’、C4’位置上存在羟基,这种特殊分子结构决定了DHQ易成为供氢体,是其发挥螯合过渡金属离子及清除自由基的主要原因[14](图 1-A)。
2 DHQ的生物学功能 2.1 抗氧化DHQ具有极强的抗氧化性能,且来源安全。根据欧洲议会和理事会法规(EU)2015/2283,DHQ可作为食品添加剂应用于牛奶、奶酪等食品中[15]。DHQ和槲皮素(quercetin,QE)均具有较强的抗氧化活性[16]。DHQ具有4个酚羟基,C2和C3骨架之间为单键(图 1-A),而QE为不饱和的双键[16](图 1-B)。研究表明,QE存在光毒性、致突变性和致瘤性,但DHQ无任何毒性[16-17]。因此,相比QE,DHQ是一种安全无毒、具备较强应用潜力的抗氧化剂[18-19]。
DHQ的抗氧化性能表现在2方面:一方面,具备直接清除氧自由基的能力,且这种能力与化合物中所含羟基数量及分子骨架的平面度和离域程度有关[20];另一方面,DHQ可直接阻止活性氧(ROS)的产生。铁作为一种过渡金属,可催化过氧化氢(H2O2)生成氧自由基(Fenton反应)产生细胞毒性[21]。病理条件下,肝细胞对铁的吸收增加,过量的铁在肝细胞中积累,引起机体产生大量ROS,随后诱导细胞凋亡和组织损伤[22]。研究发现,Fe2+与DHQ络合后失去催化Fenton反应的活性,这在一定程度上阻止了ROS的产生[23]。同时,DHQ不仅能够显著抑制HBZY-1细胞、HK2细胞和视网膜色素上皮细胞中ROS的产生,还能有效缓解细胞活力的下降[24-25]。对乙酰氨基酚(acetaminophen,APAP)能诱导小鼠氧化还原失衡,造成急性肝脏损伤,这种损伤在添加DHQ后能够得到明显改善[26],可能是因为DHQ增强了肝脏中谷胱甘肽(GSH)的含量[27]。另外,DHQ还能通过降低丙二醛(MDA)水平,增强超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷胱甘肽过氧化物酶(GSH-Px)等抗氧化酶的活性,发挥抗氧化作用。Shu等[28]对缺氧/复氧(hypoxia/reoxygenation,H/R)诱导的H9C2大鼠心肌细胞氧化应激模型进行研究,发现DHQ能够抑制MDA生成,提高SOD、CAT以及GSH-Px等抗氧化酶的活性,改善细胞脂质过氧化。同时,DHQ还能够缓解缺血/再灌注(ischemia/reperfusion,I/R)诱导的心脏组织氧化损伤,提高其抗氧化能力。高温饲养环境下,肉鸡血清中GSH-Px的活性降低,而在饲粮中添加DHQ可显著提高血清中GSH-Px活性,该研究提示DHQ对肉鸡的抗氧化能力起到了增强作用[29]。此外,DHQ还可以通过降低一氧化氮合酶(nitric oxide synthase,NOS)的转录表达发挥其抗氧化功能[30]。
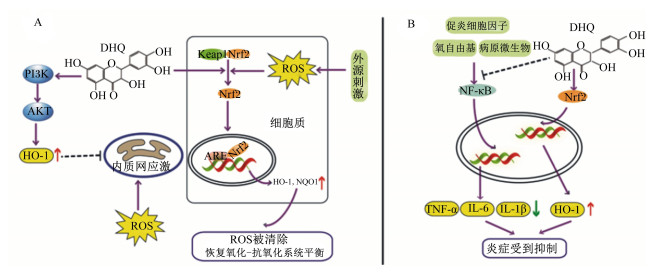
有报道称,多酚通过激活相关信号通路进而促进各种抗氧化酶的表达[31]。DHQ增强机体抗氧化能力的分子机制涉及Kelch样ECH相关蛋白1/细胞核因子E2相关因子2(Kelch-like ECH-associated protein 1/nuclear factor E2-related factor 2,Keap1/Nrf2)和磷脂酰肌醇3激酶/蛋白激酶B(phosphatidylinositol 3 kinase/protein kinase B,PI3K/AKT)通路(图 2-A)。Nrf2是维持氧化还原稳态的重要通路,当机体发生氧化应激时,Nrf2基因表达量上调,Nrf2蛋白从细胞质易位到细胞核,在核内与某些Ⅱ相解毒酶基因转录启动子区的抗氧化反应元件(AREs)相互作用,从而引起血红素加氧酶-1(heme oxygenase-1,HO-1)、NAD(P)H醌脱氢酶1[NAD(P)H quinone dehydrogenase 1,NQO1]等细胞保护相关基因的转录[32-33]。DHQ可以激活Nrf2信号通路,上调Nrf2和HO-1基因的转录表达,发挥抗氧化作用[34]。有研究发现,DHQ对大鼠H9C2心肌细胞的保护作用与Nrf2通路激活及HO-1的表达上调有关[28]。同样,Xie等[25]也发现,DHQ可以激活Nrf2和Ⅱ相抗氧化酶系统,对H2O2诱导的细胞氧化损伤发挥保护作用。关于PI3K/AKT信号通路的机制研究发现,ROS在内质网中积累引发内质网应激,并与PI3K/AKT介导的线粒体凋亡通路共同触发细胞凋亡[35]。Shu等[28]利用Western blot对蛋白质进行半定量分析,发现DHQ对凋亡信号通路的抑制作用主要是通过激活PI3K和AKT的磷酸化来增加HO-1蛋白的表达量,从而阻断ROS诱导的内质网应激。同时,Salama等[36]发现,DHQ调节氧化还原敏感的PI3K/AKT和MAPK信号(p38/c-Fos),并通过降低脂质和蛋白质氧化提高铁诱导的肝脏组织的抗氧化能力。此外,Zai等[37]发现,在APAP诱导的小鼠原代肝细胞损伤模型中,添加DHQ消除了肝细胞中过量的ROS,可能的机制是通过激活蛋白质酪氨酸激酶2/信号转导与转录激活因子3(JAK2/STAT3)信号通路发挥作用。
综上所述,DHQ在清除氧自由基及增强机体抗氧化防御系统中发挥重要作用,主要通过Keap1/Nrf2和PI3K/AKT通路调控HO-1、NQO1等抗氧化酶的表达,以及通过丝裂原活化蛋白激酶(MAPK)和JAK2/STAT3信号平衡机体的氧化还原状态,缓解ROS的累积,进而提高生物体的抗氧化能力。在畜牧业实际生产中,动物往往易受到外界环境刺激引发应激反应,严重的氧化应激会直接影响动物健康,导致畜禽生长缓慢、体重减轻以及饲料转化率降低等问题。为了规避这些问题,积极采取有效的抗氧化措施极其重要。在试验动物及细胞模型中DHQ的抗氧化能力已被反复证实[26, 28],具备较强的科学性和实用性,这为开发抗氧化补充防御系统帮助畜禽维持氧化还原平衡提供了研究基础。
2.2 抗炎除了抗氧化能力,DHQ具有抵抗炎症反应的能力[38],能够通过下调促炎细胞因子的表达,抑制相关信号通路发挥抗炎作用[7, 36, 39]。脂多糖(lipopolysaccharide,LPS)是宿主体内常见的内毒素,可通过多种途经刺激细胞释放炎性介质,引起炎症反应[40-41]。研究表明,在LPS刺激的RAW 264.7小鼠单核巨噬白血病细胞中,添加DHQ显著下调了肿瘤坏死因子-α(TNF-α)、干扰素-γ(IFN-γ)以及Toll样受体-4(TLR-4)基因的表达量,并通过TNF-α和白细胞介素(IL)-6生成受阻缓解了LPS刺激的RAW 264.7细胞炎症反应[7]。Liu等[42]研究发现,在LPS诱导的急性肺损伤模型中,添加高浓度的DHQ(2.5 μg/mL及以上)可以抑制TNF-α、IL-1β和IL-6的基因表达,改善炎症反应。另外,在APAP诱导的小鼠急性肝损伤试验中,与对照组相比,DHQ组小鼠肝脏TNF-α和IL-6的mRNA表达量下调[26]。类似的研究,Chen等[43]发现,DHQ可降低促炎细胞因子TNF-α、IFN-γ、IL-2以及IL-4基因mRNA的表达量,抑制伴刀豆球蛋白A诱导的肝脏损伤。其次,DHQ还可通过减少炎性因子IL-1β的转录表达[30]和降低ROS含量,缓解小鼠胰腺炎[44]。除此之外,DHQ通过抑制核苷酸结合寡聚化结构域样受体样蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)、核苷酸结合寡聚化结构域受体家族半胱氨酸天冬氨酸蛋白酶募集域蛋白质4(nucleotide-binding oligomerization domain-like receptor family caspase recruitment domain-containing protein 4,NLRC4)以及黑色素瘤缺乏因子2(absent in melanoma 2,AIM2)炎性小体的生成,抑制H2O2诱导的细胞炎性坏死[45]。同时,Ding等[24]还发现,DHQ对糖尿病肾病的保护作用也与NLRP3的减少有关。
研究发现,DHQ主要通过抑制NF-κB信号通路或激活Nrf2信号通路来实现抗炎作用[7, 46-47](图 2-B)。NF-κB是一种先天和适应性免疫反应的主要调节因子,在急性和慢性炎症的发病机制中发挥重要作用[48-49]。该通路的激活能引起TNF-α、IL-1β和IL-6等促炎细胞因子的释放。研究表明,LPS诱导急性肺损伤会引发炎症反应和细胞凋亡[42],DHQ补充可以缓解该症状,主要作用机制是DHQ诱导miR-132-3p表达上调,通过抑制转录因子叉头框蛋白O3(forkhead box O3,FOXO3)激活NF-κB信号通路发挥抗炎作用[42]。一氧化碳(carbon monoxide,CO)是HO-1的酶促反应产物,能抑制促炎细胞因子的表达[50]。最新的研究发现,在LPS诱导的Raw 264.7细胞的炎症模型中,敲除HO-1基因,减弱了DHQ对促炎细胞因子TNF-α和IFN-γ的抑制,但增强了Nrf2基因mRNA的表达,促进了AMP激活蛋白激酶(AMP-activated protein kinase,AMPK)的磷酸化,该研究认为,DHQ可能通过AMPK/Nrf2/HO-1发挥抗炎作用[7]。Hou等[51]通过给小鼠灌胃DHQ,探究DHQ对葡聚糖硫酸钠(dextran sulfate,DSS)诱导的小鼠溃疡性结肠炎的缓解作用,发现DHQ能够增加结肠紧密连接蛋白的咬合蛋白(Occludin)和闭合蛋白-1(Claudin-1)的表达,进而增强肠道屏障完整性,并通过抑制NF-κB信号通路、调节肠道菌群等缓解小鼠结肠炎症。这一发现是DHQ等多种天然黄酮类物质开发成为治疗肠道炎症的重要理论依据,同时,总结DHQ的抗炎作用,也为猪肺部炎症、奶牛乳房炎等畜禽的炎症性疾病治疗提供了理论支撑。
|
↑:提高increased;↓:降低decreased;ROS:活性氧reactive oxygen species;Keap1:Kelch样ECH相关蛋白1 kelch-like ECH-associated protein 1;Nrf2:细胞核因子E2相关因子2 nuclear factor E2-related factor 2;ARE:抗氧化反应元件antioxidant reaction element;PI3K:磷脂酰肌醇3激酶phosphatidylinositol 3 kinase;AKT:蛋白激酶B protein kinase B;HO-1:血红素加氧酶-1 heme oxygenase-1;NQO1:NAD(P)H醌脱氢酶NAD(P)H quinone dehydrogenase 1;NF-κB:核转录因子-κB nuclear factor-kappa B;TNF-α:肿瘤坏死因子-α tumor necrosis factor-alpha;IL-6:白细胞介素-6 interleukin-6;IL-1β:白细胞介素-1β interleukin-1 beta。 图 2 DHQ发挥抗氧化和抗炎作用的信号通路 Fig. 2 Signal pathways of DHQ exerting anti-oxidative and anti-inflammatory effects[7, 26, 34, 40-42, 51] |
ROS是诱导纤维化发生的介质,通过激活TGF-β/Smads通路,促进细胞外基质(ECM)合成,使ECM在基层大量沉积导致纤维化的发生[52-53]。而DHQ是一种抗纤维化物质,可以有效抑制心脏、肾脏、肝脏以及肺脏等器官的纤维化[54-56]。Guo等[56]通过建立小鼠主动脉缩窄(TAC)模型发现,饲料中添加DHQ后,心肌细胞内的ROS含量和心脏组织中TGF-β的mRNA和蛋白表达量均显著降低,激活的Smads信号通路被抑制,改善了TAC小鼠的心肌肥厚和心室纤维化。小鼠单侧输尿管梗阻(unilateral ureteral obstruction,UUO)的主要症状是肾小管萎缩、胶原沉积及肾脏纤维化,而饲料添加DHQ可改善这一现象[57],可能的机制是DHQ通过阻止ROS的产生减轻肾脏氧化损伤;另外,DHQ通过TGF-β信号因子抑制Smad3蛋白的磷酸化阻止成纤维蛋白的活化和肾脏的纤维化过程[57]。同时,Nrf2作为组织纤维化过程的负调控因子也发挥着重要作用[58]。另一项研究采用腹腔注射CCl4的方法构建小鼠肝脏纤维化模型,发现小鼠灌服DHQ对组织纤维化具有缓解作用,主要通过抑制ECM的产生及TGF-β/Smads和PI3K/AKT/mTOR通路发挥调控作用[59]。博莱霉素(bleomycin,BLM)是一种广泛使用的抗肿瘤的药物,但容易造成肺间质导致纤维化[60]。Impellizzeri等[55]建立BLM小鼠肺损伤模型,发现肺损伤与环加氧酶-2(COX-2)蛋白表达高度相关,DHQ处理后,显著下调了COX-2蛋白的表达量。COX-2是一种促炎酶,参与肺纤维化病理过程,其含量下调说明肺纤维化症状得到改善[61]。此外,血小板衍生因子(PDGF)与心脏[62]、肺脏[63]、皮肤[64]以及肾脏[65]等组织纤维化有关。对于黄酮类物质缓解肝脏纤维化的报道指出,杨梅素能显著抑制小鼠肝脏星状细胞的活化、增殖以及ECM积累,可能的作用机制是抑制ERK和AKT的磷酸化参与的PDGF通路[66]。然而,DHQ发挥抗纤维化作用是否通过调控PDGF通路还不清楚。另外,组织纤维化常引起动物疾病的发生,而补充DHQ后,动物的肝脏、肾脏、心脏以及肺脏等不同组织纤维化都取得了良好的改善效果,但对其他组织器官的纤维化影响目前还没有相关报道,有待更深层次的开展研究。
2.4 抗癌研究表明,黄酮类物质对癌症的治疗效果可能通过缓解氧化应激发挥作用[67]。近年来,DHQ也因其抗癌特性而受到广泛关注,其发挥抗癌作用可能涉及的机制包括:1)抑制上皮间质转化[68];2)降低干细胞调控因子的表达直接或间接参与癌症干细胞系的调节[69];3)调控相关信号通路,控制细胞周期G1、G2期,诱导细胞凋亡[70];4)通过表观遗传途径发挥作用[19];5)调节癌症相关酶的产生[71]。上皮间质转化(EMT)与癌症的发生密切相关。报道指出,DHQ可以通过抑制无翼型MMTV集成站点家族/β-连环蛋白信号(Wingless-type MMTV integration site family/β-catenin,Wnt/β-catenin)抑制EMT过程,对高侵袭性乳腺癌细胞发挥抗癌作用[68],这与Wang等[72]在肺癌细胞试验中的研究结果一致。在癌症的临床治疗中,放疗或化疗会消除大部分癌细胞,但干细胞对抗癌药物的耐药性容易导致癌症复发,因此,针对性的清除癌症干细胞可以延迟甚至是阻止癌症复发。目前已有研究证实,DHQ可通过降低干细胞调控因子胚胎干细胞关键蛋白2(SOX2)和干细胞多能性调节基因4(OCT4)的表达,抑制肺癌细胞A549细胞和H1975细胞的干细胞特性,这种效应可能与PI3K和Wnt的信号传导有关[72]。细胞周期阻滞是大多数化疗药物杀伤癌细胞作用的另一个重要机制。Chen等[73]通过流式细胞术分析发现,在U2OS和Saos-2骨肉瘤细胞系模型中,DHQ可能通过抑制AKT信号通路降低细胞周期调控相关蛋白(c-myc和SKP-2)的表达水平,使细胞生长停滞于G1期。另有研究发现,DHQ抑制mTOR/PI3K/AKT信号通路,使皮肤疤痕癌细胞在G2期生长受到抑制,诱导癌细胞凋亡[74]。同时,在大肠癌细胞系中,DHQ可控制Wnt/β-连环蛋白信号通路调节细胞周期、诱导细胞凋亡,且与时间和剂量存在一定的相关性[70]。另外,DHQ经CpG去甲基化和表观遗传上调12-O-十四烷酰佛波醋酸酯-13(12-O-tetradecanoylphorbol-13-acetate,TPA)诱导的小鼠皮肤表皮细胞中Nrf2及其下游靶基因HO-1和NQO1的表达,通过调控表观遗传修饰提高Nrf2的活性,有效预防皮肤癌[19]。除此之外,Ge等[71]研究表明,DHQ能够通过抑制大鼠和人的雄激素生物合成酶[滋养层细胞抗原抗体(HSD3B)和类固醇17α羟化酶(CYP17A1)]阻止睾丸间质细胞产生雄激素,这对治疗雄激素依赖性前列腺癌具有重要作用。由于大多数接受传统化疗或新型靶向抗癌药物治疗的死亡都与多药耐药性有关[75],联合使用黄酮类化合物及常规化疗药物可能是有益的[76]。报道称,DHQ作为调节剂可用于缓解化疗药物P-糖蛋白的耐药性[77]。这一发现为DHQ作为癌症临床药物的开发提供了新的依据和见解,DHQ与传统化疗药物相结合,可能开发出新的抗癌药物。另外,将DHQ用于动物养殖中可能具有预防癌症、提高生长性能和实现健康养殖的效果。
2.5 抗病毒DHQ具有抗病毒的作用。研究表明,DHQ对柯萨奇病毒B4(Coxsackievirus B4,CVB4)具有明显的抵抗作用[78]。Galochkina等[44]发现,DHQ在75与150 g/mL浓度条件下能以剂量依赖性方式降低感染动物胰腺组织中的病毒滴度,发挥较强的抗CVB4活性,涉及到的作用机制有2种:一是直接抑制病毒的复制,发生于病毒复制早期;二是抑制病毒在靶器官中的繁殖和传播,阻止病毒对靶器官的破坏[44]。但DHQ抗CVB4的剂量范围、特异性靶点及其分子机制目前还不是完全清楚。抑制病毒多聚蛋白水解过程中所必需的蛋白酶也是治疗病毒感染的一种有力手段。Fischer等[79]通过计算机技术对6.06亿化合物进行筛选,认为DHQ可能是一种新型冠状病毒(SARS-CoV-2)蛋白酶的潜在抑制剂。Gogoi等[17]在对柑橘类水果的筛选中也有类似发现。另外,Raj等[80]发现,DHQ可能是优良的埃博拉病毒(Ebola virus,EBOV)蛋白靶点抑制剂,且对多个靶点有效,可以同时阻断多种信号通路发挥抗病毒作用。然而,目前还缺乏体内试验证实DHQ抗SARS-CoV-2和抗EBOV的应用效果。此外,碧萝芷的主要成分之一是DHQ,碧萝芷在50 μg/mL剂量时显示出抗丙型肝炎病毒作用且没有细胞毒性,与丙肝药物干扰素或利巴韦林具有协同作用[81]。但是,对DHQ的单体进行研究,发现其对丙肝病毒的抑制作用不明显[81]。因此,DHQ是否为碧萝芷的抗病毒功效成分还需要进行进一步探究。
禽流感、非洲猪瘟、口蹄疫等畜禽传染性疾病多年来一直威胁着畜牧业的健康、可持续发展,亟需采取有效措施,以期为临床上控制动物病毒性疾病提供理论基础。但由于目前DHQ的抗病毒作用多是通过计算机技术筛选得来,缺乏理论支撑和数据证明。因此,应当先建立啮齿动物模型,进行抗病毒试验,以确定DHQ的抗病毒功效及相关作用机制,为下一步DHQ在畜禽病毒性疾病中的应用提供支持。
2.6 其他功能DHQ作为一种天然的提取物,除以上功能外,还具有护肝[59]、抑制破骨细胞形成[82]以及预防心血管疾病[83]等作用。研究发现,小鼠腹腔注射CCl4引起丙氨酸氨基转移酶(ALT)和门冬氨酸氨基转移酶(AST)活性升高,导致严重的肝细胞坏死和肝组织病变[14, 36],而经DHQ处理后,这些变化被逆转,肝细胞凋亡被抑制[59]。同时,Salama等[36]发现,DHQ能够通过激活PI3K/AKT信号通路发挥促细胞增殖作用,激发肝细胞再生功能。另外,在提高肝功能方面,与相同剂量的其他酚类如儿茶素、槲皮素相比,DHQ效果最好,更适合用作肝保护剂[84]。由于DHQ具有良好的保肝活性,其作为水飞蓟素的有效成分,已被批准用于中毒性肝损伤、慢性炎症性肝病以及肝硬化的辅助性治疗药物[85-86]。破骨细胞与免疫细胞有很多共同的调控机制,炎症条件下产生的细胞因子会对破骨细胞的形成产生很大影响[87]。在小鼠颅骨骨溶解模型中,添加DHQ可有效抑制NF-κB信号通路,进而抑制破骨细胞形成,防止骨丢失[88]。同时,DHQ能够以剂量依赖性的方式直接或间接参与清除羟基自由基,保护骨髓间充质干细胞免受羟基诱导的损伤[88]。另外,Saito等[83]报道,DHQ有助于防止β-淀粉样蛋白低聚物的聚集,维持大脑淀粉样血管病(cerebral amyloid angiopathy,CAA)模型小鼠脑血管功能的完整性。此外,DHQ还可抵抗邻苯二甲酸诱导的线粒体功能障碍、维持机体正常的糖代谢并抑制心肌细胞凋亡[89-90]。综上所述,DHQ的有益功能较多,但对于其发挥各个功能的详细作用机制以及是否存在互作关系,仍需要进一步深入挖掘。
3 DHQ在畜禽生产中的应用目前,DHQ作为生物活性添加剂已被广泛应用于医药和食品领域[91]。采用动物试验模型,DHQ的多种生物学功能已被反复证实,但在畜牧业中的应用还较少。总结DHQ在畜禽生产中应用的研究进展,发现DHQ具有预防动物疾病[92]和提高畜禽生产性能[93]等作用。Cai等[89]发现,DHQ能够治疗邻苯二甲酸二乙酯(DEHP)诱导的鸡心肌肥大、糖代谢紊乱及线粒体功能障碍,这可能与ROS相关的JAK/STAT信号通路有关。类似的报道指出,DHQ补充可以降低钙离子浓度、调节细胞色素P450,减轻DEHP引起的家禽心肌细胞坏死及氧化应激损伤[90, 94]。维生素E可以促进肝脏稳态,抑制非酒精性脂肪肝(non-alcoholic fatty liver disease,NAFLD)的发展[95]。而DHQ能够提高肉鸡肝脏中维生素E的浓度,发挥抗NAFLD作用[29]。Bakalivanova等[96]探究DHQ对禽肉保存期的影响,发现DHQ显著缓解新鲜禽肉的脂质过氧化,延长了鲜肉的低温(-18 ℃)保质期,这为DHQ作为保鲜剂用于延长肉制品的保存提供了新的思路。在奶牛养殖业中,酮症是高产奶牛最易患的疾病之一,在围产期奶牛饲粮中添加DHQ有利于维护奶牛健康,预防奶牛酮病,改善肝脏功能并提高奶牛繁殖力[92]。DHQ是水飞蓟素的有效成分之一,Tedesco等[97]研究发现,口服水飞蓟素可增加奶牛的产奶量,对反刍动物的泌乳性能具有改善作用,另外还发现DHQ能够明显提高奶牛的平均产奶量[92],这表明水飞蓟素中起主导作用的活性物质可能是DHQ。在仔猪和育肥猪饲粮中添加DHQ,可以缓解热应激,提高猪只日增重[92]。然而,也有报道指出,标准饲养温度下,饲粮中补充DHQ对肉鸡的生长性能、能量及营养代谢影响不大[29]。究其原因,可能是由于高温状态下,机体氧化还原稳态的破坏对动物的生长性能产生负面影响,而DHQ在提高机体抗氧化能力的同时逆转了热应激对动物生长性能的影响。
DHQ作为饲料添加剂具有其独特的优势。首先,从来源上看,DHQ的主要来源落叶松树存活能力强,甚至在气候恶劣的环境中也能四季生长,尤其在我国吉林省长白山地区,另外,常见的柑橘类水果中也有DHQ的存在。从性质上看,DHQ是一种酚类物质,是目前发现的无毒、无副作用且具备较强抗氧化能力和清除体内自由基的化合物。因此,DHQ来源广、活性强及安全性高等优势使其在保护畜禽机体健康方面具有巨大的应用前景。然而,由于存在利用方式单一、纯化成本高等问题,限制了DHQ在畜牧业中的应用,迫切需要建立更多的DHQ利用途径及分离纯化技术,以发挥其最大的作用效果。此外,DHQ在畜禽生产中的研究较少,其在畜禽养殖中的应用是否能够达到与实验室模型相似的作用效果还有待证实。总之,DHQ作为潜在的畜禽饲料添加剂,对其发挥作用的分子机制、改善畜禽健康的作用途径还需进行更多研究。
4 小结DHQ因具有抗氧化、抗炎、抗纤维化、抗癌和抗病毒等多种生物学功能,成为天然植物提取物研究领域的热点之一。DHQ作为饲用抗生素的替代品,在畜禽生产中具有极大应用前景。未来对DHQ的研究可从以下方面展开:1)低剂量DHQ对动物无显著积极影响,且DHQ安全范围内的极限剂量还不明确,因此,DHQ在畜禽生产中的适宜添加量还有待研究;2)DHQ对畜禽肠道健康的影响鲜有报道,探究DHQ是否通过改变肠道微生物菌群,提高肠道免疫功能也是我们未来的研究内容之一;3)DHQ的作用机理尚不完全清楚,如何提高DHQ的生物活性和靶向性需要进一步的验证。综上所述,系统深入研究DHQ的生物学功能和特性,对畜牧业生产中可能的新型饲料添加剂的开发具有重要意义。
| [1] |
BIANCHIN M, PEREIRA D, ALMEIDA J F, et al. Antioxidant properties of lyophilized rosemary and sage extracts and its effect to prevent lipid oxidation in poultry Pátê[J]. Molecules, 2020, 25(21): 5160. DOI:10.3390/molecules25215160 |
| [2] |
XIE Q F, CHENG J J, CHEN J F, et al. Comparation of anti-inflammatory and antioxidantactivities of curcumin, tetrahydrocurcumin and octahydrocurcuminin LPS-stimulated RAW264.7 macrophages[J]. Evidence-based Complementary and Alternative Medicine, 2020, 2020: 8856135. |
| [3] |
ZHAO F, YAN S, TIAN M. Blueberry polyphenol extracts enhance the intestinal antioxidant capacity in weaned rats by modulating the Nrf2-Keap1 signal pathway[J]. Frontiers in Physiology, 2021, 12: 640737. DOI:10.3389/fphys.2021.640737 |
| [4] |
PANCHE A N, DIWAN A D, CHANDRA S R. Flavonoids: an overview[J]. Journal of Nutritional Science, 2016, 5: e47. DOI:10.1017/jns.2016.41 |
| [5] |
PAL H C, ATHAR M, ELMETS C A, et al. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NF-κB signaling pathways in SKH-1 hairless mice[J]. Photochemistry and Photobiology, 2015, 91(1): 225-234. DOI:10.1111/php.12337 |
| [6] |
PEW J C. A flavonone from Douglas-fir heartwood[J]. Journal of the American Chemical Society, 1948, 70(9): 3031-3034. DOI:10.1021/ja01189a059 |
| [7] |
LEI L M, CHAI Y F, LIN H M, et al. Dihydroquercetin activates AMPK/Nrf2/HO-1 signaling in macrophages and attenuates inflammation in LPS-Induced endotoxemic mice[J]. Frontiers in Pharmacology, 2020, 11: 662. DOI:10.3389/fphar.2020.00662 |
| [8] |
FUKUI Y, NAKADOME K, ARIYOSHI H. Studies on the monomer flavonoides of the plants of coniferae.Ⅱ.Isolation of a new taxifolin glucoside from the leaves of Chamaecyparis obtusa Endlicher[J]. Yakugaku Zasshi, 1966, 86(3): 184-187. DOI:10.1248/yakushi1947.86.3_184 |
| [9] |
SEKER M E, CELIK A, DOST K, et al. Corrigendum to: investigation of pycnogenol content in five different pine barks species grown in Turkey by HPLC-UV and LC-MS[J/OL]. Journal of Chromatographic Science, (2021-03-25)[2021-05-14]. https://academic.oup.com/chromsci/advance-article/doi/10.1093/chromsci/bmab039/6187090.
|
| [10] |
WALLACE S N, CARRIER D J, CLAUSEN E C. Batch solvent extraction of flavanolignans from milk thistle (Silybum marianum L.Gaertner)[J]. Phytochemical Analysis, 2005, 16(1): 7-16. DOI:10.1002/pca.803 |
| [11] |
TOPAL F, NAR M, GOCER H, et al. Antioxidant activity of taxifolin: an activity-structure relationship[J]. Journal of Enzyme Inhibition and Medicinal Chemistry, 2016, 31(4): 674-683. DOI:10.3109/14756366.2015.1057723 |
| [12] |
王宇, 王遂. RP-HPLC法测定落叶松中二氢槲皮素的含量[J]. 化学工程师, 2009, 23(2): 22-24. WANG Y, WANG S. Determination of dihydroquercetin in larch by RP-HPLC[J]. Chemical Engineer, 2009, 23(2): 22-24 (in Chinese). DOI:10.3969/j.issn.1002-1124.2009.02.009 |
| [13] |
ROGOZHIN V V, PERETOLCHIN D V. Kinetic regulations of dihydroquercetin oxidation with horseradish peroxide[J]. Bioorganicheskaia Khimiia, 2009, 35(5): 640-645. |
| [14] |
HU C, YE J W, ZHAO L C, et al. 5, 7, 3', 4'-flavan-on-ol (taxifolin) protects against acetaminophen-induced liver injury by regulating the glutathione pathway[J]. Life Sciences, 2019, 236: 116939. DOI:10.1016/j.lfs.2019.116939 |
| [15] |
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), TURCK D, CASTENMILLER J, et al. Safety of hot water extract of fruits and peduncles of Hovenia dulcis as a novel food pursuant to regulation 1(EU) 2015/2283[J]. EFSA Journal, 2020, 18(8): e06196. |
| [16] |
RAJNOCHOVÁ S A, RYŠAVÁ A, PSOTOVÁ M, et al. The phototoxic potential of the flavonoids, taxifolin and quercetin[J]. Photochemistry and Photobiology, 2017, 93(5): 1240-1247. DOI:10.1111/php.12755 |
| [17] |
GOGOI N, CHOWDHURY P, GOSWAMI A K, et al. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease[J/OL]. Molecular Diversity, (2020-11-25)[2021-05-14]. https://pubmed.ncbi.nlm.nih.gov/33236176/.
|
| [18] |
SCHAUSS A G, TSELYICO S S, KUZNETSOVA V A, et al. Toxicological and genotoxicity assessment of a dihydroquercetin-rich dahurian larch tree (Larix gmelinii Rupr) extract (lavitol)[J]. International Journal of Toxicology, 2015, 34(2): 162-181. DOI:10.1177/1091581815576975 |
| [19] |
KUANG H, TANG Z, ZHANG C, et al. Taxifolin activates the Nrf2 anti-oxidative stress pathway in mouse skin epidermal JB6 P+ cells through epigenetic modifications[J]. International Journal of Molecular Sciences, 2017, 18(7): 1546. DOI:10.3390/ijms18071546 |
| [20] |
JOMOVÁ K, HUDECOVA L, LAURO P, et al. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3', 4'-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(Ⅱ) ions: a spectroscopic, absorption titration and DNA damage study[J]. Molecules, 2019, 24(23): 4335. DOI:10.3390/molecules24234335 |
| [21] |
NOWAK M, TRYNISZEWSKI W, SARNIAK A, et al. Effect of physiological concentrations of vitamin C on the inhibitation of hydroxyl radical induced light emission from Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 systems in vitro[J]. Molecules, 2021, 26(7): 1993. DOI:10.3390/molecules26071993 |
| [22] |
MIYANISHI K, TANAKA S, SAKAMOTO H, et al. The role of iron in hepatic inflammation and hepatocellular carcinoma[J]. Free Radical Biology & Medicine, 2019, 133: 200-205. |
| [23] |
BABENKOVA I V, OSIPOV A N, TESELKIN Y O. The effect of dihydroquercetin on catalytic activity of iron (Ⅱ) ions in the fenton reaction[J]. Bulletin of Experimental Biology and Medicine, 2018, 165(3): 347-350. DOI:10.1007/s10517-018-4167-x |
| [24] |
DING T, WANG S F, ZHANG X Y, et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome[J]. Phytomedicine, 2018, 41: 45-53. DOI:10.1016/j.phymed.2018.01.026 |
| [25] |
XIE X B, FENG J, KANG Z F, et al. Taxifolin protects RPE cells against oxidative stress-induced apoptosis[J]. Molecular Vision, 2017, 23: 520-528. |
| [26] |
CHEN X F, HUANG J, HU Z H, et al. Protective effects of dihydroquercetin on an APAP-induced acute liver injury mouse model[J]. International Journal of Clinical and Experimental Pathology, 2017, 10(10): 10223-10232. |
| [27] |
NI H M, MCGILL M R, CHAO X J, et al. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice[J]. Journal of Hepatology, 2016, 65(2): 354-362. DOI:10.1016/j.jhep.2016.04.025 |
| [28] |
SHU Z P, YANG Y N, YANG L, et al. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway[J]. Food & Function, 2019, 10(1): 203-215. |
| [29] |
PIRGOZLIEV V R, MANSBRIDGE S C, WESTBROOK C A, et al. Feeding dihydroquercetin and vitamin E to broiler chickens reared at standard and high ambient temperatures[J]. Archives of Animal Nutrition, 2020, 74(6): 496-511. DOI:10.1080/1745039X.2020.1820807 |
| [30] |
TUROVSKAYA M V, GAIDIN S G, MAL'TSEVA V N, et al. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons[J]. Molecular and Cellular Neurosciences, 2019, 96: 10-24. DOI:10.1016/j.mcn.2019.01.005 |
| [31] |
MOHAN V, DAS S, RAO S B. Hydroxytyrosol, a dietary phenolic compound forestalls the toxic effects of methylmercury-induced toxicity in IMR-32 human neuroblastoma cells[J]. Environmental Toxicology, 2016, 31(10): 1264-1275. DOI:10.1002/tox.22134 |
| [32] |
CHEN M J, XI Y M, CHEN K L, et al. Upregulation sestrin2 protects against hydrogen peroxide-induced oxidative damage bovine mammary epithelial cells via a Keap1-Nrf2/ARE pathway[J]. Journal of Cellular Physiology, 2021, 236(1): 392-404. DOI:10.1002/jcp.29867 |
| [33] |
YANG Q, ZHAO Z Z, XIE J, et al. Senkyunolide Ⅰ attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative, anti-inflammatory and anti-apoptotic pathways[J]. International Immunopharmacology, 2021, 97: 107717. DOI:10.1016/j.intimp.2021.107717 |
| [34] |
ZHAO M Y, CHEN J J, ZHU P, et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells[J]. International Immunopharmacology, 2015, 28(2): 938-944. DOI:10.1016/j.intimp.2015.04.032 |
| [35] |
LI G T, WU X W, SUN P, et al. Dithiolation indolizine exerts viability suppression effects on A549 cells via triggering intrinsic apoptotic pathways and inducing G2/M phase arrest[J]. Biomedicine & Pharmacotherapy, 2021, 133: 110961. |
| [36] |
SALAMA S A, KABEL A M. Taxifolin ameliorates iron overload-induced hepatocellular injury: modulating PI3K/AKT and p38 MAPK signaling, inflammatory response, and hepatocellular regeneration[J]. Chemico-Biological Interactions, 2020, 330: 109230. DOI:10.1016/j.cbi.2020.109230 |
| [37] |
ZAI W J, CHEN W, LUAN J Y, et al. Dihydroquercetin ameliorated acetaminophen-induced hepatic cytotoxicity via activating JAK2/STAT3 pathway and autophagy[J]. Applied Microbiology and Biotechnology, 2018, 102(3): 1443-1453. DOI:10.1007/s00253-017-8686-6 |
| [38] |
AHISKALI I, PINAR C L, KIKI M, et al. Effect of taxifolin on methanol-induced oxidative and inflammatory optic nerve damage in rats[J]. Cutaneous and Ocular Toxicology, 2019, 38(4): 384-389. DOI:10.1080/15569527.2019.1637348 |
| [39] |
PAN S L, ZHAO X X, JI N, et al. Inhibitory effect of taxifolin on mast cell activation and mast cell-mediated allergic inflammatory response[J]. International Immunopharmacology, 2019, 71: 205-214. DOI:10.1016/j.intimp.2019.03.038 |
| [40] |
FUSCO R, CORDARO M, SIRACUSA R, et al. Effects of hydroxytyrosol against lipopolysaccharide-induced inflammation and oxidative stress in bovine mammary epithelial cells: a natural therapeutic tool for bovine mastitis[J]. Antioxidants (Basel, Switzerland), 2020, 9(8): 693. |
| [41] |
ZHANG L Q, ZHANG J L, JIANG X L, et al. Hydroxytyrosol inhibits LPS-induced neuroinflammatory responses via suppression of TLR-4-mediated NF-κB P65 activation and ERK signaling pathway[J]. Neuroscience, 2020, 426: 189-200. DOI:10.1016/j.neuroscience.2019.12.005 |
| [42] |
LIU J H, CAO L, ZHANG C H, et al. Dihydroquercetin attenuates lipopolysaccharide-induced acute lung injury through modulating FOXO3-mediated NF-κB signaling via miR-132-3p[J]. Pulmonary Pharmacology & Therapeutics, 2020, 64: 101934. |
| [43] |
CHEN J, SUN X, XIA T, et al. Pretreatment with dihydroquercetin, a dietary flavonoid, protected against concanavalin A-induced immunological hepatic injury in mice and TNF-α/ActD-induced apoptosis in HepG2 cells[J]. Food & Function, 2018, 9(4): 2341-2352. |
| [44] |
GALOCHKINA A V, ANIKIN V B, BABKIN V A, et al. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against Coxsackie virus B4 in a model of viral pancreatitis[J]. Archives of Virology, 2016, 161(4): 929-938. DOI:10.1007/s00705-016-2749-3 |
| [45] |
叶艳琼, 王晓莉, 蔡骞, 等. 花旗松素对过氧化氢诱导H9C2细胞焦亡的保护作用[J]. 中南大学学报(医学版), 2017, 42(12): 1367-1374. YE Y Q, WANG X L, CAI Q, et al. Protective effect of taxifolin on H2O2-induced H9C2 cell pyroptosis[J]. Journal of Central South University (Medical Science), 2017, 42(12): 1367-1374 (in Chinese). DOI:10.11817/j.issn.1672-7347.2017.12.003 |
| [46] |
CAO X Y, BI R C, HAO J L, et al. A study on the protective effects of taxifolin on human umbilical vein endothelial cells and THP-1 cells damaged by hexavalent chromium: a probable mechanism for preventing cardiovascular disease induced by heavy metals[J]. Food & Function, 2020, 11(5): 3851-3859. |
| [47] |
CAI C, LIU C Y, ZHAO L M, et al. Effects of taxifolin on osteoclastogenesis in vitro and in vivo[J]. Frontiers in Pharmacology, 2018, 9: 1286. DOI:10.3389/fphar.2018.01286 |
| [48] |
SU X, LIU K, XIE Y, et al. Protective effect of a polyphenols-rich extract from Inonotus Sanghuang on bleomycin-induced acute lung injury in mice[J]. Life Sciences, 2019, 230: 208-217. DOI:10.1016/j.lfs.2019.05.074 |
| [49] |
XIE X L, CONG L, LIU S J, et al. Genistein alleviates chronic vascular inflammatory response via the miR-21/NF-κB p65 axis in lipopolysaccharide-treated mice[J]. Molecular Medicine Reports, 2021, 23(3): 192. DOI:10.3892/mmr.2021.11831 |
| [50] |
OTTERBEIN L E, BACH F H, ALAM J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway[J]. Nature Medicine, 2000, 6(4): 422-428. DOI:10.1038/74680 |
| [51] |
HOU J X, HU M Y, ZHANG L, et al. Dietary taxifolin protects against dextran sulfate sodium-induced colitis via NF-κB signaling, enhancing intestinal barrier and modulating gut microbiota[J]. Frontiers in Immunology, 2020, 11: 631809. |
| [52] |
HAO B W, SUN R T, GUO X T, et al. NOX4-Derived ROS promotes collagen Ⅰ deposition in bronchial smooth muscle cells by activating noncanonical p38MAPK/Akt-mediated TGF-β signaling[J]. Oxidative Medicine and Cellular Longevity, 2021, 6668971. |
| [53] |
CHEN J W, HU Y B, MOU X, et al. Amygdalin alleviates renal injury by suppressing inflammation, oxidative stress and fibrosis in streptozotocin-induced diabetic rats[J]. Life Sciences, 2021, 265: 118835. DOI:10.1016/j.lfs.2020.118835 |
| [54] |
REN L, GUO H N, YANG J, et al. Dissecting efficacy and metabolic characteristic mechanism of Taxifolin on renal fibrosis by multivariate approach and ultra-performance liquid chromatography coupled with mass spectrometry-based metabolomics strategy[J]. Frontiers in Pharmacology, 2020, 11: 608511. |
| [55] |
IMPELLIZZERI D, TALERO E, SIRACUSA R, et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice[J]. The British Journal of Nutrition, 2015, 114(6): 853-865. DOI:10.1017/S0007114515002597 |
| [56] |
GUO H P, ZHANG X, CUI Y Q, et al. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload[J]. Toxicology and Applied Pharmacology, 2015, 287(2): 168-177. DOI:10.1016/j.taap.2015.06.002 |
| [57] |
WANG W, MA B L, XU C G, et al. Dihydroquercetin protects against renal fibrosis by activating the Nrf2 pathway[J]. Phytomedicine, 2020, 69: 153185. DOI:10.1016/j.phymed.2020.153185 |
| [58] |
SONG M K, LEE J H, RYOO I G, et al. Bardoxolone ameliorates TGF-β1-associated renal fibrosis through Nrf2/Smad7 elevation[J]. Free Radical Biology & Medicine, 2019, 138: 33-42. |
| [59] |
LIU X L, LIU W C, DING C B, et al. Taxifolin, extracted from waste Larix olgensis roots, attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/smads signaling pathways[J]. Drug Design, Development and Therapy, 2021, 15: 871-887. DOI:10.2147/DDDT.S281369 |
| [60] |
QIAN W B, CAI X R, QIAN Q H, et al. Astragaloside Ⅳ modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis[J]. Journal of Cellular and Molecular Medicine, 2018, 22(9): 4354-4365. DOI:10.1111/jcmm.13725 |
| [61] |
ZANNIKOU M, BARBAYIANNI I, FANIDIS D, et al. MAP3K8 regulates Cox-2-mediated prostaglandin E2 production in the lung and suppresses pulmonary inflammation and fibrosis[J]. Journal of Immunology, 2021, 206(3): 607-620. DOI:10.4049/jimmunol.2000862 |
| [62] |
AULA H, SKYTTÄ T, TUOHINEN S, et al. Decreases in TGF-β1 and PDGF levels are associated with echocardiographic changes during adjuvant radiotherapy for breast cancer[J]. Radiation Oncology, 2018, 13(1): 201. DOI:10.1186/s13014-018-1150-7 |
| [63] |
DADRICH M, NICOLAY N H, FLECHSIG P, et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis[J]. Oncoimmunology, 2016, 5(5): e1123366. DOI:10.1080/2162402X.2015.1123366 |
| [64] |
BUCUR M, DINCA O, VLADAN C, et al. Variation in expression of inflammation-related signaling molecules with profibrotic and antifibrotic effects in cutaneous and oral mucosa scars[J]. Journal of Immunology Research, 2018, 2018: 5196023. |
| [65] |
LI Q, MING Y, JIA H, et al. Poricoic acid A suppresses TGF-β1-induced renal fibrosis and proliferation via the PDGF-C, Smad3 and MAPK pathways[J]. Experimental and Therapeutic Medicine, 2021, 21(4): 289. DOI:10.3892/etm.2021.9720 |
| [66] |
GENG Y, SUN Q, LI W, et al. The common dietary flavonoid myricetin attenuates liver fibrosis in carbon tetrachloride treated mice[J]. Molecular Nutrition & Food Research, 2017, 61(4): 1600392. |
| [67] |
ZHANG Z Y, PAN Y, ZHAO Y, et al. Delphinidin modulates JAK/STAT3 and MAPKinase signaling to induce apoptosis in HCT116 cells[J]. Environmental Toxicology, 2021, 36(8): 1557-1566. DOI:10.1002/tox.23152 |
| [68] |
LI J, HU L, ZHOU T, et al. Taxifolin inhibits breast cancer cells proliferation, migration and invasion by promoting mesenchymal to epithelial transition via β-catenin signaling[J]. Life Sciences, 2019, 232: 116617. DOI:10.1016/j.lfs.2019.116617 |
| [69] |
YUAN X H, LI N, ZHANG M M, et al. Taxifolin attenuates IMQ-induced murine psoriasis-like dermatitis by regulating T helper cell responses via Notch1 and JAK2/STAT3 signal pathways[J]. Biomedicine & Pharmacotherapy, 2020, 123: 109747. |
| [70] |
RAZAK S, AFSAR T, ULLAH A, et al. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway[J]. BMC Cancer, 2018, 18(1): 1043. DOI:10.1186/s12885-018-4959-4 |
| [71] |
GE F, TIAN E, WANG L, et al. Taxifolin suppresses rat and human testicular androgen biosynthetic enzymes[J]. Fitoterapia, 2018, 125: 258-265. DOI:10.1016/j.fitote.2018.01.017 |
| [72] |
WANG R H, ZHU X J, WANG Q, et al. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice[J]. Annals of Translational Medicine, 2020, 8(9): 590. DOI:10.21037/atm-20-3329 |
| [73] |
CHEN X, GU N, XUE C, et al. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells[J]. Molecular Medicine Reports, 2018, 17(2): 3239-3245. |
| [74] |
ZHOU W, LIU Z M, WANG M, et al. Taxifolin inhibits the scar cell carcinoma growth by inducing apoptosis, cell cycle arrest and suppression of PI3K/AKT/mTOR pathway[J]. Journal of B.U.ON: Official Journal of the Balkan Union of Oncology, 2019, 24(2): 853-858. |
| [75] |
SILVA V L, SAXENA J, NICOLINI F, et al. Chloroxine overrides DNA damage tolerance to restore platinum sensitivity in high-grade serous ovarian cancer[J]. Cell Death & Disease, 2021, 12(4): 395. |
| [76] |
KIKUCHI H, YUAN B, HU X M, et al. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents[J]. American Journal of Cancer Research, 2019, 9(8): 1517-1535. |
| [77] |
CHEN H J, CHUNG Y L, LI C Y, et al. Taxifolin resensitizes multidrug resistance cancer cells via uncompetitive inhibition of P-glycoprotein function[J]. Molecules, 2018, 23(12): 3055. DOI:10.3390/molecules23123055 |
| [78] |
GALOCHKINA A V, ZARUBAEV V V, KISELEV O I, et al. Antiviral activity of the dihydroquercetin during the coxsackievirus B4 replication in virto[J]. Voprosy virusologii, 2016, 61(1): 27-31. |
| [79] |
FISCHER A, SELLNER M, NERANJAN S, et al. Potential inhibitors for novel coronavirus protease identified by virtual screening of 606 million compounds[J]. International Journal of Molecular Sciences, 2020, 21(10): 3626. DOI:10.3390/ijms21103626 |
| [80] |
RAJ U, VARADWAJ P K. Flavonoids as multi-target inhibitors for proteins associated with Ebola virus: in silico discovery using virtual screening and molecular docking studies[J]. Interdisciplinary Sciences, Computational Life Sciences, 2016, 8(2): 132-141. DOI:10.1007/s12539-015-0109-8 |
| [81] |
EZZIKOURI S, NISHIMURA T, KOHARA M, et al. Inhibitory effects of pycnogenol on hepatitis C virus replication[J]. Antiviral Research, 2015, 113: 93-102. DOI:10.1016/j.antiviral.2014.10.017 |
| [82] |
ZHANG H Q, WANG Y J, YANG G T, et al. Taxifolin inhibits receptor activator of NF-κB ligand-induced osteoclastogenesis of human bone marrow-derived macrophages in vitro and prevents lipopolysaccharide-induced bone loss in vivo[J]. Pharmacology, 2019, 103(1/2): 101-109. |
| [83] |
SAITO S, YAMAMOTO Y, MAKI T, et al. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy[J]. Acta Neuropathologica Communications, 2017, 5(1): 26. DOI:10.1186/s40478-017-0429-5 |
| [84] |
AKINMOLADUN A C, OLADEJO C O, JOSIAH S S, et al. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: relevance for therapy in pesticide-induced liver toxicity?[J]. Pathophysiology, 2018, 25(4): 365-371. DOI:10.1016/j.pathophys.2018.07.002 |
| [85] |
YANG J Z, LIANG J C, SHAO L, et al. Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis[J]. Metabolic Engineering, 2020, 59: 44-52. DOI:10.1016/j.ymben.2020.01.007 |
| [86] |
MOEZIAN G S A, JAVADINIA S A, SALES S S, et al. Oral silymarin formulation efficacy in management of AC-T protocol induced hepatotoxicity in breast cancer patients: a randomized, triple blind, placebo-controlled clinical trial[J/OL]. Journal of Oncology Pharmacy Practice, (2021-04-16)[2021-05-14]. https://pubmed.ncbi.nlm.nih.gov/33861657/.
|
| [87] |
SAKUNRANGSIT N, METHEEPAKORNCHAI P, KUMPUNYA S, et al. Etanercept prevents TNF-α mediated mandibular bone loss in FcγRⅡb-/- lupus model[J]. PLoS One, 2021, 16(4): e0250215. DOI:10.1371/journal.pone.0250215 |
| [88] |
LI X C, XIE H, JIANG Q, et al. The mechanism of (+) taxifolin's protective antioxidant effect for·OH-treated bone marrow-derived mesenchymal stem cells[J]. Cellular & Molecular Biology Letters, 2017, 22: 31. |
| [89] |
CAI J Z, SHI G L, ZHANG Y, et al. Taxifolin ameliorates DEHP-induced cardiomyocyte hypertrophy via attenuating mitochondrial dysfunction and glycometabolism disorder in chicken[J]. Environmental Pollution, 2019, 255(Pt.1): 113155. |
| [90] |
ZHANG Y, SHI G L, CAI J Z, et al. Taxifolin alleviates apoptotic injury induced by DEHP exposure through cytochrome P450 homeostasis in chicken cardiomyocytes[J]. Ecotoxicology and Environmental Safety, 2019, 183: 109582. DOI:10.1016/j.ecoenv.2019.109582 |
| [91] |
ZHANG X, LI D Q, DONG C Y, et al. Molybdenum sulfide-based electrochemical platform for high sensitive detection of taxifolin in Chinese medicine[J]. Analytica Chimica Acta, 2020, 1099: 85-93. DOI:10.1016/j.aca.2019.11.057 |
| [92] |
FOMICHEV Y, NIKANOVA L, LASHIN A. The effectiveness of using dihydroquercetin (taxifolin) in animal husbandry, poultry and apiculture for prevention of metabolic disorders, higher antioxidative capacity, better resistance and realisation of a productive potential of organism[J]. Agriculture & Food, 2016, 4: 140-159. |
| [93] |
SEMENOVA A A, NASONOVA V V, KUZNETSOVA T G, et al. PSⅢ-17 program chair poster pick: a study on the effect of dihydroquercetin added into a diet of growing pigs on meat quality[J]. Journal of Animal Science, 2020, 98(Suppl.4): 364. |
| [94] |
ZHENG Y Y, SHI G L, CAI J Z, et al. Di-(2-ethyl hexyl) phthalate induces necroptosis in chicken cardiomyocytes by triggering calcium overload[J]. Journal of Hazardous Materials, 2020, 387: 121696. DOI:10.1016/j.jhazmat.2019.121696 |
| [95] |
KEDARISETTY C K, BHARDWAJ A, KUMAR G, et al. Efficacy of combining pentoxiphylline and vitamin E versus vitamin E alone in non-alcoholic steatohepatitis-A randomized pilot study[J]. Indian Journal of Gastroenterology, 2021, 40(1): 41-49. DOI:10.1007/s12664-020-01131-x |
| [96] |
BAKALIVANOVA T, KALOYANOV N. Effect of taxifolin, rosemary and synthetic antioxidants treatment on the mechanically separated poultry meat lipid peroxidation[J]. Oxidation Communications, 2014, 37(1): 254-261. |
| [97] |
TEDESCO D, TAVA A, GALLETTI S, et al. Effects of silymarin, a natural hepatoprotector, in periparturient dairy cows[J]. Journal of Dairy Science, 2004, 87(7): 2239-2247. DOI:10.3168/jds.S0022-0302(04)70044-2 |