2. 青海省高原放牧家畜营养与饲料科学重点实验室, 西宁 810016;
3. 青海省牦牛工程技术研究中心, 西宁 810016
2. Key Laboratory of Plateau Grazing Animal Nutrition and Feed Science of Qinghai Province, Xi'ning 810016, China;
3. Yak Engineering Technology Research Center of Qinghai Province, Xi'ning 810016, China
牦牛主要栖息在青藏高原及周围地区,是青藏高原的优势畜种,也是当地牧民重要的生产生活资料[1]。由于青藏高原独特的地理环境,一年只有“冷季”与“暖季”之分,冷季长达7~9个月,牧草产量与营养水平不能满足牦牛营养需要量,导致牦牛掉膘严重,养殖效益低下[2-3]。目前,已有大量研究表明,冷季补饲能够提高放牧牦牛的生长性能,但是鲜有涉及补饲对其瘤胃发酵及菌群结构影响的研究[4-5],且已有研究表明瘤胃微生物与反刍动物的生长发育有密切的联系[6-7]。瘤胃是反刍动物消化饲粮的重要场所,其含有大量微生物,包括细菌、真菌、原虫和古生菌等[8]。影响反刍动物瘤胃菌群结构组成的因素除了动物品种、年龄、生理阶段等内部因素外,最主要的外部因素就是饲粮营养水平[9]。周力等[10]研究发现,补饲降低了藏羔羊瘤胃细菌多样性与丰富度,但提高了相关淀粉降解菌的相对丰度。Mao等[11]研究表明,随着饲粮精粗比的提高,奶牛瘤胃液总挥发性脂肪酸(TVFA)浓度和淀粉降解菌的相对丰度呈上升趋势,而瘤胃液pH和纤维降解菌相对丰度呈下降趋势。由此可见,通过调控饲粮营养水平,能够改善瘤胃发酵,提高营养物质的利用率,进而提高生产性能。因此,本试验拟通过16S rDNA高通量测序技术,从微生物角度阐述补饲精料对冷季放牧牦牛生长性能、瘤胃发酵和菌群结构的影响,以期为青藏高原冷季放牧牦牛精准补饲提供理论依据。
1 材料与方法 1.1 试验地点试验于2019年11月至2020年2月在青海省贵南县老扎西牧场的冬季草场进行。该草场属于高寒草甸草地,优势种有小嵩草、高山嵩草、矮嵩草、线叶嵩草等。
1.2 试验动物与试验设计选取3周岁左右、体重[(204.90±10.65) kg]相近、体况良好的公牦牛24头,随机分为2组(每组12头):对照组仅放牧,不补饲;试验组在放牧的基础上每头每天精料补饲量为体重的1.2%。试验期为100 d,其中预试期10 d,正试期90 d。
1.3 饲养管理与试验饲粮所有试验牦牛在同一草场放牧,设置2个面积大小一样的围栏小区,每个围栏小区的面积大小约为300亩(1亩≈667 m2),08:00出牧,18:00收牧,试验组在出牧前和收牧后分2次进行补饲,所有试验牦牛自由饮水。补饲精料参照我国《肉牛饲养标准》(NY/T 815—2004),结合放牧牦牛采食量[12]配制。牧草营养水平、精料组成及营养水平见表 1。
|
|
表 1 牧草营养水平、精料组成及营养水平(干物质基础) Table 1 Pasture nutrient levels, composition and nutrient levels of the concentrate (DM basis) |
在试验中期,观察试验耗牛所吃的牧草,然后驱离试验牦牛采集该区域牧草样品,采集方法采用模拟采摘法。在同一草场随机选取10个1 m×1 m样方,齐地面刈割取样,65 ℃烘干后计算地上生物量,经计算牧草地上生物量为71.46 g/m2。
1.4.2 瘤胃液样品在正试期第90天,参考Geishauser[13]的方法,晨饲前采用胃管式采样器采集瘤胃液150 mL,4层纱布过滤后立即测定pH,剩余的瘤胃液样品分装至15 mL离心管中,置于-80 ℃冻存备用。
1.5 指标测定与方法 1.5.1 牧草及精料营养成分的测定牧草、精料中的干物质(DM)含量采用GB/T 6435—2014中的方法测定,粗蛋白质(CP)含量采用GB/T 6432—2018中的方法测定,钙(Ca)含量采用GB/T 6436—2002中的方法测定,磷(P)含量采用按照GB/T 6437—2002中的方法测定,中性洗涤纤维(NDF)、酸性洗涤纤维(ADF)含量采用Van Soest等[14]的方法测定,所用仪器为ANKOM 200i半自动纤维分析仪。
1.5.2 生长性能的测定正试期开始前和结束时于晨饲前连续2 d空腹称重,计算总增重(TWG)、平均日増重(ADG)。
1.5.3 瘤胃发酵参数的测定使用HANNA HI221型台式酸度计测定瘤胃液pH,测定前对该酸度计使用相应标准液进行校正;参照冯宗慈等[15]改进的比色法测定瘤胃液氨态氮(NH3-N)浓度,仪器为紫外可见分光光度计(TU-1810),预热30 min,在波长625 nm处测定溶液吸光度(OD)值,利用标准曲线确定样品的氨态氮浓度;瘤胃液微生物蛋白(MCP)浓度采用考马斯亮蓝法[16]在595 nm波长处比色测定(试剂盒购于南京建成生物工程研究所);瘤胃液挥发性脂肪酸(VFA)浓度测定参考文献[17-18],使用日本岛津GC-2014型气相色谱仪测定,其中挥发性脂肪酸浓度测定条件为:氢火焰离子化(FID)检测器,色谱柱为毛细管柱(FFAP,30.00 m×0.32 mm×0.50 μm);升温条件为初始60 ℃,以10 ℃/min升温至120 ℃,保留2 min,以15 ℃/min升温至180 ℃,保留5 min;汽化室温度250 ℃;FID温度250 ℃;进样量1 μL,载气为高纯氮气(99.99%),压力0.7 MPa;氢气压力0.4 MPa,空气压力0.4 MPa,毛细管柱压力0.6~0.8 MPa,分流比40 ∶ 1。
1.5.4 瘤胃微生物DNA提取、测序每组随机选择4个瘤胃液样品置于冰上解冻并充分混合,使用CTAB方法提取瘤胃液样品基因组DNA,用1.0%琼脂糖凝胶电泳检测DNA的纯度和浓度,取适量的样本于离心管中,用无菌水稀释样本至1 ng/μL。根据细菌16S rDNA基因的V3~V4区,合成带有Barcode的特异引物。通用引物序列:515F,5′-GTGCCAGCMGCCGCGG-3′;806R,5′-GGACTACHVGGGTWTCTAAT-3′。PCR采用25 μL扩增体系;DNA模板5 ng,5 μmol/L的上、下游引物各1 μL,2 ng/μL的牛血清白蛋白(BSA)3 μL,2×Taq Plus Master Mix 12.5 μL,双蒸水(ddH2O)补加至25 μL;PCR扩增条件:94 ℃预变性5 min;94 ℃变性30 s,50 ℃退火30 s,72 ℃延伸60 s,共30个循环;72 ℃延伸7 min。PCR扩增产物用1.0%琼脂糖凝胶电泳检测,用QIAquickPCR纯化试剂盒(德国Qiagen公司)纯化回收,符合要求的DNA样品送至北京奥维森基因科技有限公司测序,测序平台为Mixed PE 300。
1.5.5 数据分析测序平台得到的原始数据使用软件Trimmomatic(Version 0.36)软件去除低质量数据,然后通过vsearch软件和物种数据库去除嵌合体,得到有效序列。筛选相似性在97%以上的序列归类为操作分类单元(OTU),然后对OTU聚类、物种分类学、多样性指数、群落结构进行统计分析。
1.6 数据统计与分析试验数据用Excel 2016初步整理后,采用SPSS 24.0软件进行单因素方差分析,并用Duncan氏法进行组间的多重比较,P < 0.05表示差异显著,P < 0.01表示差异极显著,结果均以平均值和均值标准误(SEM)表示。
2 结果与分析 2.1 补饲精料对冷季放牧牦牛生长性能的影响由表 2可知,试验开始前对照组和试验组牦牛体重无显著差异(P=0.872),经过90 d的补饲后,冷季传统放牧条件下,牦牛掉膘严重,补饲精料极显著提高了牦牛的平均日增重(P < 0.01)。
|
|
表 2 补饲精料对冷季放牧牦牛生长性能的影响 Table 2 Effects of concentrate supplementation on growth performance of grazing yaks in cold season |
由表 3可知,与对照组相比,试验组瘤胃液氨态氮、总挥发性脂肪酸、丙酸、丁酸、异丁酸、戊酸和异戊酸浓度极显著提高(P < 0.01),微生物蛋白和乙酸浓度显著提高(P < 0.05);pH和乙酸/丙酸值极显著降低(P < 0.01)。上述结果表明,补饲精料能够促进冷季放牧牦牛瘤胃发酵。
|
|
表 3 补饲精料对冷季放牧牦牛瘤胃发酵参数的影响 Table 3 Effects of concentrate supplementation on rumen fermentation parameters of grazing yaks in cold season |
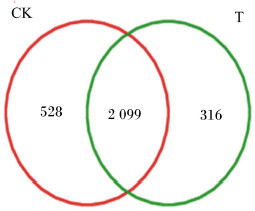
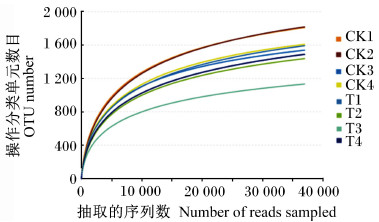
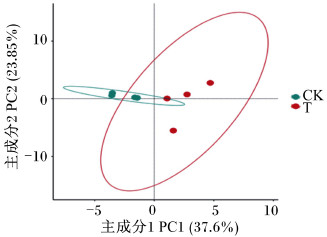
由图 1可知,2组共产生2 945个OTU,其中对照组和试验组分别检测到2 627、2 415个OTU,相对应的独有OTU数目分别为528和316个,共有OTU数目为2 099个,占总OTU数目的79.90%。由图 2可知,随着测序深度的增加,2组瘤胃细菌物种稀释曲线趋于平缓,说明测序程度已基本覆盖到样品中所有的细菌物种。通过alpha多样性分析(表 4)可知,试验组Shannon指数和谱系多样性显著低于对照组(P < 0.05)。通过主坐标分析(PCoA,图 3)可知,2组放牧牦牛瘤胃菌群结构具有明显差异。以上结果表明,补饲精料降低了冷季放牧牦牛瘤胃菌群的多样性与丰富度。
|
CK表示对照组,T表示试验组。下图同。 CK represented control group, and T represented test group. The same as below. 图 1 OTU维恩图 Fig. 1 OTU Venn diagram |
|
图 2 稀释曲线 Fig. 2 Rarefaction curve |
|
|
表 4 补饲精料对冷季放牧牦牛瘤胃菌群alpha多样性的影响 Table 4 Effects of concentrate supplementation on alpha diversity of rumen microbial community of grazing yaks in cold season |
|
图 3 主坐标分析 Fig. 3 PCoA |
由表 5可知,在门水平上,试验组瘤胃中拟杆菌门和放线菌门丰度显著高于对照组(P < 0.05),疣微菌门和螺旋体门丰度极显著高于对照组(P < 0.01);对照组瘤胃中厚壁菌门丰度显著高于试验组(P < 0.05),黏胶球形菌门和纤维杆菌门丰度极显著高于试验组(P < 0.01)。由表 6可知,在属水平上,试验组瘤胃中普雷沃菌属_1和瘤胃球菌科_NK4A214丰度极显著高于对照组(P < 0.01),克里斯滕森菌科_R-7和解琥珀酸菌属丰度显著高于对照组(P < 0.05)。以上结果表明,补饲精料对冷季放牧牦牛瘤胃菌群结构有一定的影响。
|
|
表 5 补饲精料对冷季放牧牦牛瘤胃菌群组成的影响(门水平) Table 5 Effects of concentrate supplementation on rumen microbial community composition of grazing yaks in cold season (at phylum level) |
|
|
表 6 补饲精料对冷季放牧牦牛瘤胃菌群组成的影响(属水平) Table 6 Effects of concentrate supplementation on rumen microbial community composition of grazing yaks in cold season (at genus level) |
由于青藏高原独特的地理环境,一年“无四季之分,只有冷暖之别”,冷季长达7~8个月,冷季牧草产量与营养营养水平极度匮乏,导致牦牛的生长一直处于“夏饱、秋肥、冬瘦、春死亡”的恶性循环中,生长效率低下,经济效益差[2-3]。本试验结果表明,传统放牧条件下,冷季放牧牦牛体重严重下降,3个月体重损失率高达10.35%,补饲精料的试验组牦牛的ADG为453.32 g/d,与对照组相比显著促进了牦牛的生长,这与徐田伟等[19]、张建勋[20]和王威等[21]的研究结果相一致。其原因主要是:一方面,补饲精料直接为放牧牦牛提高了营养物质的摄入量,营养物质的增加可提高反刍动物抵御冷应激的能力[22];另一方面,补饲精料一定程度上满足了放牧牦牛对矿物元素的需要,提高了瘤胃的消化能力,从而提高了牦牛对牧草的消化率[23-24],有利于促进放牧牦牛的生长发育。
3.2 补饲精料对冷季放牧牦牛瘤胃发酵参数的影响瘤胃液中的挥发性脂肪酸主要来自饲粮中淀粉、纤维等物质的发酵,其主要包括乙酸、丙酸和丁酸等短链脂肪酸,是反刍动物的主要能量来源[25]。蔡晶晶等[26]研究发现,随着饲粮非纤维性碳水化合物(NFC)/中性洗涤纤维(NDF)的提高,奶牛瘤胃液pH显著降低。本研究结果表明,试验组牦牛瘤胃液pH极显著低于对照组,与蔡晶晶等[26]的研究结果相一致。这可能是由于补饲精料后牦牛摄入的NFC的比例增加,在瘤胃中迅速发酵产生大量的挥发性脂肪酸主和乳酸等物质,导致瘤胃液pH下降。瘤胃液氨态氮是瘤胃微生物利用饲料中的蛋白质合成的,也是瘤胃微生物蛋白合成的前体,其浓度反映了瘤胃微生物分解饲粮中含氮物质产生氨态氮的速度及对其摄取利用情况。本试验条件下,试验组瘤胃液氨态氮和微生物蛋白浓度显著或极显著高于对照组,这与董利锋等[27]和朱昊鹏等[28]的研究结果基本一致,原因可能是补饲精料中可溶性蛋白质、易消化碳水化合物和非蛋白含氮物含量高,且降解速率缓慢,有利于氨态氮和微生物蛋白的合成。Giger-Reverdin等[29]研究发现,随着饲粮中精料比例的提高,奶山羊瘤胃液丙酸、丁酸和总挥发性脂肪酸浓度显著提高,而乙酸浓度和乙酸/丙酸值显著降低。Murphy等[30]研究也发现,降低饲粮中精料比例降低了奶牛瘤胃液中丙酸、丁酸、戊酸和总挥发性脂肪酸浓度,而提高了乙酸浓度。本试验中,试验组牦牛瘤胃液总挥发性脂肪酸、乙酸、丙酸、丁酸、异丁酸、戊酸和异戊酸浓度显著或极显著高于对照组,而乙酸/丙酸值显著低于对照组,与以上研究结果不一致。其可能原因是冷季牧草产量与营养水平极度降低,不能满足放牧牦牛正常的采食量需要,无法为瘤胃微生物提供足够的发酵底物,从而导致瘤胃液中挥发性脂肪酸浓度降低。
3.3 补饲精料对冷季放牧牦牛瘤胃菌群丰富度与多样性的影响Chao1指数是衡量菌群丰富度的指标,Shannon指数是衡量菌群多样性的指标。Chao1指数越大,表明菌群丰富度越高;Shannon指数越大,表明菌群多样性越高。周力等[10]研究发现,补饲精料降低了藏羔羊瘤胃菌群多样性与丰富度;Liu等[31]研究也发现,提高饲粮中精料的比例,显著降低了牦牛瘤胃菌群Chao1和Shannon指数,这可能是增加饲粮中精料比例导致瘤胃液pH下降,而瘤胃液pH降低则会抑制瘤胃液中不耐酸的纤维降解菌的生长与繁殖[32]。本试验中,试验组牦牛瘤胃菌群Shannon指数较对照组显著降低,Chao1指数呈下降的趋势,与以上研究结果基本一致。
3.4 补饲精料对冷季放牧牦牛瘤胃菌群组成的影响饲粮结构是影响反刍动物瘤胃微生物区系组成的首要因数,营养组成不同会直接影响瘤胃微生物的种类与数目[33]。大量研究表明,厚壁菌门和拟杆菌门是反刍动物瘤胃中的优势菌门,拟杆菌门是降解碳水化合物的主要细菌,而厚壁菌门主要参与纤维物质的分解,两者在瘤胃发酵过程中起着至关重要的作用[34-35]。本研究结果表明,试验组和对照组牦牛瘤胃液中拟杆菌门和厚壁菌门丰度和占整个细菌门的85%,与以上研究结果相一致。Kim等[36]研究表明,粗料组肉牛瘤胃中厚壁菌门丰度显著低于高谷物组。李蒋伟等[37]研究也发现,降低饲粮精料比例有提高藏羔羊瘤胃厚壁菌门丰度的趋势。本试验中,试验组牦牛瘤胃拟杆菌门丰度显著高于对照组,而厚壁菌门丰度显著低于对照组,这与以上研究结果相一致。放线菌门是一类革兰氏阳性菌,具有分支的纤维和孢子,其大部分是腐生菌,也有寄生菌,可致病[38]。本试验中,补饲显著提高了牦牛瘤胃放线菌门的丰度,表明生产中牦牛精料补饲量不宜过高。疣微菌门在哺乳动物胃肠道免疫中具有重要的作用,其丰度的上升与机体免疫力增强有密切的联系[39]。本试验中,试验组牦牛瘤胃疣微菌门丰度极显著高于对照组,说明了补饲精料一定程度提高了放牧牦牛的免疫力。研究表明,螺旋体门可以有效地降解果胶和磷酸酯,利用可发酵的碳水化合物合成挥发性脂肪酸,为反刍动物机体提供能量[40]。徐晓锋等[41]研究发现,高精料饲粮提高了奶牛瘤胃螺旋体门丰度,这与本研究结果相一致。黏胶球形菌门和纤维杆菌门与纤维二糖的降解密切相关,能够促进瘤胃对纤维物质的降解[42]。本研究结果表明,试验组牦牛瘤胃黏胶球形菌门和纤维杆菌门丰度较对照组极显著降低,原因可能是试验组牦牛瘤胃发酵的纤维物质比例低于对照组。
在属水平上,普雷沃菌属是瘤胃中的优势菌属,参与瘤胃内的多种代谢活动,主要降解饲粮中的半纤维成分,并且普雷沃菌属能够产生大量的复合酶,促进非纤维性多糖和蛋白质的降解[43]。霍俊宏等[44]研究发现,随着饲粮精粗比的提高,努比亚山羊瘤胃普雷沃菌属丰度呈上升的趋势。陈芸等[45]研究也表明,增加饲粮中精料的比例能显著提高了山羊瘤胃普雷沃菌属丰度。本试验中,试验组牦牛瘤胃普雷沃菌属_1丰度极显著高于对照组,这与以上研究结果相一致;试验组牦牛瘤胃克里斯滕森菌科_R-7丰度显著高于对照组,与李岚捷[46]研究结果相一致。克里斯滕森菌科_R-7与乙酸产量有关,本研究中试验组牦牛瘤胃液乙酸浓度显著高于对照组也验证这一点。瘤胃球菌科_NK4A214属于瘤胃球菌属,归属于厚壁菌门,主要降解纤维物质。Fernando等[47]研究发现,高精料组奶牛瘤胃中瘤胃球菌属丰度显著低于粗料组,与本研究结果不一致。本试验中,试验组牦牛瘤胃中瘤胃球菌科_NK4A2丰度极显著高于对照组,原因可能是冷季牧草产量低,牦牛采食的纤维物质量低,补饲精料一定程度上促进了牦牛对纤维物质的摄入量,导致瘤胃球菌科_NK4A2的丰度有所上升。有研究报道,解琥珀酸菌属丰度与饲粮纤维成分有密切联系,可以降解纤维或纤维二糖产生琥珀酸和乙酸等物质[48]。本试验中,补饲精料显著提高了牦牛瘤胃解琥珀酸菌属丰度,试验组瘤胃液乙酸浓度较对照组上升也验证这一点。
4 结论在本试验条件下,补饲精料量为体重的1.2%时,能够显著提高冷季放牧牦牛的平均日增重,促进瘤胃中微生物蛋白和挥发性脂肪酸的合成,降低瘤胃菌群丰富度与多样性,提高瘤胃拟杆菌门、放线菌门、疣微菌门和螺旋体门丰度,降低瘤胃厚壁菌门、黏胶球形菌门和纤维杆菌门丰度,提高瘤胃普雷沃菌属_1、瘤胃球菌科_NK4A214、克里斯滕森菌科_R-7和解琥珀酸菌属丰度。
| [1] |
LONG R J, DING L M, SHANG Z H. The yak grazing system on the Qinghai-Tibetan plateau and its status[J]. Rangeland Journal, 2008, 30(2): 241-246. DOI:10.1071/RJ08012 |
| [2] |
DING X Z, GUO X, YAN P, et al. Seasonal and nutrients intake regulation of lipoprotein lipase (LPL) activity in grazing yak (Bos grunniens) in the alpine regions around Qinghai lake[J]. Livestock Science, 2012, 143(1): 29-34. DOI:10.1016/j.livsci.2011.08.004 |
| [3] |
XUE B, ZHAO X Q, ZHANG Y S. Seasonal changes in weight and body composition of yak grazing on alpine-meadow grassland in the Qinghai-Tibetan plateau of China[J]. Journal of Animal Science, 2005, 83(8): 1908-1913. DOI:10.2527/2005.8381908x |
| [4] |
LONG R J, DONG S K, WEI X H, et al. The effect of supplementary feeds on the bodyweight of yaks in cold season[J]. Livestock Production Science, 2005, 93(3): 197-204. DOI:10.1016/j.livprodsci.2004.08.016 |
| [5] |
YANG C, HOU F, SUN Y, et al. Oats hay supplementation to yak grazing alpine meadow improves carbon return to the soil of grassland ecosystem on the Qinghai-Tibet plateau, China[J]. Global Ecology and Conservation, 2020, 23: e1158. |
| [6] |
ROSS E M, PETROVSKI S, MOATE P J, et al. Metagenomics of rumen bacteriophage from thirteen lactating dairy cattle[J]. BMC Microbiology, 2013, 13: 242. DOI:10.1186/1471-2180-13-242 |
| [7] |
BICKHART D M, WEIMER P J. Symposium review: host-rumen microbe interactions may be leveraged to improve the productivity of dairy cows[J]. Journal of Dairy Science, 2018, 101(8): 7680-7689. DOI:10.3168/jds.2017-13328 |
| [8] |
SMITH P E, ENRIQUEZ-HIDALGO D, HENNESSY D, et al. Sward type alters the relative abundance of members of the rumen microbial ecosystem in dairy cows[J]. Scientific Reports, 2020, 10(1): 9317. DOI:10.1038/s41598-020-66028-3 |
| [9] |
YÁÑEZ-RUIZ D R, ABECIA L, NEWBOLD C J. Manipulating rumen microbiome and fermentation through interventions during early life: a review[J]. Frontiers in Microbiology, 2015, 6: 1133. |
| [10] |
周力, 马博妍, 高占红, 等. 精料补充料补饲对藏羔羊生长发育及瘤胃菌群组成的影响[J]. 四川农业大学学报, 2021, 39(1): 101-107. ZHOU L, MA B Y, GAO Z H, et al. Effects of concentrate supplement on growth performance and rumen bacteria composition of Tibetan lambs[J]. Journal of Sichuan Agricultural University, 2021, 39(1): 101-107 (in Chinese). |
| [11] |
MAO S Y, ZHANG R Y, WANG D S, et al. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing[J]. Anaerobe, 2013, 24: 12-19. DOI:10.1016/j.anaerobe.2013.08.003 |
| [12] |
崔占鸿, 刘书杰, 柴沙驼, 等. 三江源区高寒草甸草场放牧牦牛采食量的测定[J]. 中国草食动物, 2007, 27(6): 20-22. CUI Z H, LIU S J, CHAI S T, et al. The determination of feed intake for yak grazing on alpine meadow in the region of the source of Yangtze, yellow and lantsang rivers[J]. China Herbivores, 2007, 27(6): 20-22 (in Chinese). DOI:10.3969/j.issn.2095-3887.2007.06.007 |
| [13] |
GEISHAUSER T. An instrument for the collection and transfer of ruminal fluid and for the administration of water soluble drugs in adult cattle[J]. Bovine Pract, 1993, 27: 38-42. |
| [14] |
VAN SOEST P J, ROBERTSON J B, LEWIS B A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition[J]. Journal of Dairy Science, 1991, 74(10): 3583-3597. DOI:10.3168/jds.S0022-0302(91)78551-2 |
| [15] |
冯宗慈, 高民. 通过比色测定瘤胃液氨氮含量方法的改进[J]. 畜牧与饲料科学, 2010, 31(6/7): 37. FENG Z C, GAO M. Improvement of the method of measuring ammonia nitrogen concentration of rumen liquid by colorimetric determination[J]. Animal Husbandry and Feed Science, 2010, 31(6/7): 37 (in Chinese). |
| [16] |
高雨飞. 高精料日粮条件下烟酸对牛瘤胃微生物区系的影响[D]. 硕士学位论文. 南昌: 江西农业大学, 2016. GAO Y F. Effects of niacin on microorganism system in the rumen of cattle under high-concentrate diet[D]. Master's Thesis. Nanchang: Jiangxi Agricultural University, 2016. (in Chinese) |
| [17] |
曹庆云, 周武艺, 朱贵钊, 等. 气相色谱测定羊瘤胃液中挥发性脂肪酸方法研究[J]. 中国饲料, 2006(24): 26-28. CAO Q Y, ZHOU W Y, ZHU G Z, et al. Study on the methods of determination of volatile fatty acid in the rumen liquid of lambs by gas chromatography[J]. China Feed, 2006(24): 26-28 (in Chinese). DOI:10.3969/j.issn.1004-3314.2006.24.011 |
| [18] |
王加启. 反刍动物营养学研究方法[M]. 北京: 现代教育出版社, 2011: 139-141. WANG J Q. Methods in ruminant nutrition research[M]. Beijing: Modern Education Press, 2011: 139-141 (in Chinese). |
| [19] |
徐田伟, 吉汉忠, 刘宏金, 等. 牧归后补饲精料对冷季藏系绵羊生长性能的影响[J]. 西北农业学报, 2016, 25(8): 1132-1136. XU T W, JI H Z, LIU H J, et al. Effect of concentrate supplementing after grazing on growth performance of tibetan sheep in alpine pastoral area during cold season[J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2016, 25(8): 1132-1136 (in Chinese). |
| [20] |
张建勋. 不同季节牦牛补饲效果及其机理研究[D]. 硕士学位论文. 雅安: 四川农业大学, 2013. ZHANG J X. Mechanism of the effects of seasonal supplementation on yak performance[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2013. (in Chinese) |
| [21] |
王威, 张建勋, 康坤, 等. 冷季补饲精料对牦牛繁殖性能和生长性能的影响[J]. 中国畜牧杂志, 2013, 49(7): 78-80. WANG W, ZHANG J X, KANG K, et al. Effects of concentrate supplementation on reproductive performance and growth performance of yak in cold season[J]. Chinese Journal of Animal Science, 2013, 49(7): 78-80 (in Chinese). DOI:10.3969/j.issn.0258-7033.2013.07.021 |
| [22] |
马培东. 冷应激条件下玉米源性日粮能量构成对不同阶段母牛生产性能的影响[D]. 硕士学位论文. 长春: 吉林农业大学, 2019. MA P D. Effects of different energy sources of corn-based diets on production performance of beefs at different stages under cold stress[D]. Master's Thesis. Changchun: Jilin Agricultural University, 2019. (in Chinese) |
| [23] |
GORDEN L J, PRINS H H T. The ecology of browsing and grazing[M]. Verlag Berlin Heidelberg: Springer, 2008.
|
| [24] |
LEMAIRE G, HODGSON J, CHABBI A. Grassland productivity and ecosystem services[M]. Wallingford UK and Cambridge MA: CABI, 2011.
|
| [25] |
CHAUCHEYRAS-DURAND F, OSSA F. Review: the rumen microbiome: composition, abundance, diversity, and new investigative tools[J]. The Professional Animal Scientist, 2014, 30(1): 1-12. DOI:10.15232/S1080-7446(15)30076-0 |
| [26] |
蔡晶晶, 王洪荣, 付聪, 等. 不同NFC/NDF饲粮和硫胺素对奶牛瘤胃代谢的影响[J]. 动物营养学报, 2013, 25(9): 2012-2020. CAI J J, WANG H R, FU C, et al. Effects of different dietary NFC/NDF and thiamine on rumen metabolism in dairy cows[J]. Chinese Journal of Animal Nutrition, 2013, 25(9): 2012-2020 (in Chinese). |
| [27] |
董利锋, 杨修竹, 高彦华, 等. 日粮不同NDF/NFC水平对周岁后荷斯坦奶牛生产性能、营养物质消化率、瘤胃发酵特征和甲烷排放的影响[J]. 草业学报, 2021, 30(2): 156-165. DONG L F, YANG X Z, GAO Y H, et al. Effects of dietary NDF: NFC ratio on growth performance, nutritive digestibility, ruminal fermentation characteristics and methane emissions of Holstein heifers[J]. Acta Prataculturae Sinica, 2021, 30(2): 156-165 (in Chinese). |
| [28] |
朱昊鹏, 郑月, 颜培实. 精料补饲水平对锦江黄牛生长性能和瘤胃液理化指标的影响[J]. 畜牧与兽医, 2017, 49(3): 24-29. ZHU H P, ZHENG Y, YAN P S. Effects of different concentrate supplemental levels on production performance, rumen fluid physicochemical index of Jinjiang cattle[J]. Animal Husbandry & Veterinary Medicine, 2017, 49(3): 24-29 (in Chinese). |
| [29] |
GIGER-REVERDIN S, RIGALMA K, DESNOYERS M, et al. Effect of concentrate level on feeding behavior and rumen and blood parameters in dairy goats: relationships between behavioral and physiological parameters and effect of between-animal variability[J]. Journal of Dairy Science, 2014, 97(7): 4367-4378. DOI:10.3168/jds.2013-7383 |
| [30] |
MURPHY M, AKERLIND M, HOLTENIUS K. Rumen fermentation in lactating cows selected for milk fat content fed two forage to concentrate ratios with hay or silage[J]. Journal of Dairy Science, 2000, 83(4): 756-764. DOI:10.3168/jds.S0022-0302(00)74938-1 |
| [31] |
LIU C, WU H, LIU S J, et al. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type[J]. Frontiers in Microbiology, 2019, 10: 1116. DOI:10.3389/fmicb.2019.01116 |
| [32] |
张瑞阳. 组学技术研究亚急性瘤胃酸中毒对奶牛瘤胃微生物、代谢和上皮功能的影响[D]. 博士学位论文. 南京: 南京农业大学, 2015. ZHANG R Y. Omics-based approaches to assess the effects of subacute ruminal acidosis on rumen microbiota, metabolism and epithelial function in dairy cows[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2015. (in Chinese) |
| [33] |
CHEN Y, PENNER G B, LI M J, et al. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet[J]. Applied and Environmental Microbiology, 2011, 77(16): 5770-5781. DOI:10.1128/AEM.00375-11 |
| [34] |
SPENCE C, WELLS W G, SMITH C J. Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: regulation by carbon source and oxygen[J]. Journal of Bacteriology, 2006, 188(13): 4663-4672. DOI:10.1128/JB.00125-06 |
| [35] |
DE OLIVEIRA M N V, JEMELL K A, FREITAS F S, et al. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer[J]. Veterinary Microbiology, 2013, 164(3/4): 307-314. |
| [36] |
KIM M, KIM J, KUEHN L A, et al. Investigation of bacterial diversity in the feces of cattle fed different diets[J]. Journal of Animal Science, 2014, 92(2): 683-694. DOI:10.2527/jas.2013-6841 |
| [37] |
李蒋伟, 王志有, 侯生珍, 等. 日粮精粗比对育肥藏羊瘤胃组织形态及微生物菌群的影响[J]. 草业学报, 2021, 30(3): 100-109. LI J W, WANG Z Y, HOU S Z, et al. Effects of dietary concentrate: roughage ratio on rumen morphology and microbial flora in fattening Tibetan sheep[J]. Acta Prataculturae Sinica, 2021, 30(3): 100-109 (in Chinese). |
| [38] |
SERVIN J A, HERBOLD C W, SKOPHAMMER R G, et al. Evidence excluding the root of the tree of Life from the actinobacteria[J]. Molecular Biology and Evolution, 2008, 25(1): 1-4. |
| [39] |
DERRIEN M, VAN BAARLEN P, HOOIVELD G, et al. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila[J]. Frontiers in Microbiology, 2011, 2: 166. |
| [40] |
HESS M, SCZYRBA A, EGAN R, et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen[J]. Science, 2011, 331(6016): 463-467. DOI:10.1126/science.1200387 |
| [41] |
徐晓锋, 胡丹丹, 郭婷婷, 等. 不同精粗比饲粮条件下奶牛瘤胃细菌菌群结构变化的研究[J]. 动物营养学报, 2019, 31(12): 5541-5550. XU X F, HU D D, GUO T T, et al. Structure changes of rumen bacterial flora of dairy cows under different concentrate to roughage ratio diets[J]. Chinese Journal of Animal Nutrition, 2019, 31(12): 5541-5550 (in Chinese). |
| [42] |
LIMAM R D, BOUCHEZ T, CHOUARI R, et al. Detection of WWE2-related Lentisphaerae by 16S rRNA gene sequencing and fluorescence in situ hybridization in landfill leachate[J]. Canadian Journal of Microbiology, 2010, 56(10): 846-852. DOI:10.1139/W10-065 |
| [43] |
LIU H J, HU L Y, HAN X P, et al. Tibetan sheep adapt to plant phenology in alpine meadows by changing rumen microbial community structure and function[J]. Frontiers in Microbiology, 2020, 11: 587558. DOI:10.3389/fmicb.2020.587558 |
| [44] |
霍俊宏, 方绍培, 吴平山, 等. 不同精粗比日粮对努比亚山羊瘤胃菌群结构的影响[J]. 草业科学, 2020, 37(12): 2558-2566. HUO J H, FANG S P, WU P S, et al. Effects of diets with different concentration-roughage ratios on the microbial community structure of Nubian goat rumen[J]. Pratacultural Science, 2020, 37(12): 2558-2566 (in Chinese). |
| [45] |
陈芸, 刘旗, 邓俊良, 等. 复合抗菌肽对山羊瘤胃菌群结构的影响[J]. 浙江农业学报, 2017, 29(11): 1800-1808. CHEN Y, LIU Q, DENG J L, et al. Effects of composite antimicrobial peptide on rumen bacteria community structure of goat[J]. Acta Agriculturae Zhejiangensis, 2017, 29(11): 1800-1808 (in Chinese). DOI:10.3969/j.issn.1004-1524.2017.11.05 |
| [46] |
李岚捷. 不同NFC/NDF水平日粮对公犊牛生产性能、瘤胃发育及微生物区系的影响[D]. 硕士学位论文. 兰州: 甘肃农业大学, 2017. LI L J. Effects of diets with different NFC/NDF levels on the production performance, rumen development and microbial flora of male calves[D]. Master's Thesis. Lanzhou: Gansu Agricultural University, 2017. (in Chinese) |
| [47] |
FERNANDO S C, PURVIS H T, NAJAR F Z, et al. Rumen microbial population dynamics during adaptation to a high-grain diet[J]. Applied and Environmental Microbiology, 2010, 76(22): 7482-7490. DOI:10.1128/AEM.00388-10 |
| [48] |
KOIKE S, KOBAYASHI Y. Fibrolytic rumen bacteria: their ecology and functions[J]. Asian-Australasian Journal of Animal Sciences, 2009, 22(1): 131-138. DOI:10.5713/ajas.2009.r.01 |




