牛蛙(Lithobates catesbeianus)原产自北美洲,19世纪50年代引入中国,是一种具有较高经济价值的两栖动物。因具有生长速度快、养殖成本低、肉味鲜美等特点,近年来,牛蛙养殖在我国中南部迅速发展,成为养殖产量最大的两栖动物[1-2]。
蛋白质是水生动物生长发育过程中的必需营养素。鱼粉是牛蛙饲料的重要蛋白质源,其蛋白质含量高,氨基酸和脂肪酸平衡,消化率高[2]。然而,随着水产养殖业的快速发展,对饲用鱼粉的需求不断扩大,造成鱼粉供不应求,价格不断上升。因此,开发可替代鱼粉的蛋白质源成为水产养殖业可持续发展的重要任务[3-5]。豆粕的蛋白质丰富、来源广泛,且价格稳定,成为水产饲料中普遍使用的植物蛋白质。但是,与鱼粉相比,豆粕中存在植酸、皂苷、大豆抗原蛋白和胰蛋白酶抑制剂等抗营养因子,对养殖对象的生长性能和健康造成负面影响[6]。Kikuchi等[7]研究表明,用豆粕替代饲料中30%的鱼粉后,红鳍东方鲀的增重率(weight gain rate,WGR)和蛋白质效率降低。此外,豆粕中存在的抗营养因子还会影响营养物质的消化和吸收,诱发肠道功能障碍。如饲料中豆粕替代50%鱼粉会诱发牛蛙肠炎,并降低其生长性能和饲料利用率[8]。因此,通过加工处理豆粕产品以提高饲料利用率对牛蛙饲料生产具有重要意义。
微生物发酵是一种经济有效的加工豆粕产品的技术。豆粕经过发酵后,不仅能降解抗营养因子,将大分子蛋白质分解为小肽,还能产生益生菌和益生元[9]。此外,通过发酵还能提高大豆生物活性肽的水平,对机体抗氧化、降低血脂和免疫调节具有促进作用[10]。目前,发酵豆粕已广泛应用于养殖动物饲料中,并获得很好的应用效果[11-13]。因此,本研究以牛蛙为试验对象,探究发酵豆粕替代鱼粉对牛蛙生长性能、肠道消化酶活性、血清生化指标及肠道组织结构的影响,为牛蛙配合饲料中发酵豆粕的利用提供参考。
1 材料与方法 1.1 试验饲料基础饲料中含有20%鱼粉,分别用发酵豆粕替代0、25%、50%、75%和100%的鱼粉制作成5种等氮等能的试验饲料(分别标记为FM、FSM25、FSM50、FSM75和FSM100)。试验饲料组成及营养水平见表 1。按照饲料配方,将各种原料粉碎,过60目筛网后逐级混合,使用小型饲料膨化机制成直径为2.5 mm的浮性颗粒饲料。
|
|
表 1 试验饲料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of experimental diets (DM basis) |
试验牛蛙购自厦门市同安区一家牛蛙养殖场,蛙苗规格为25~30 g/只。养殖试验在集美大学水产试验场内进行。试验开始前,将牛蛙放入暂养缸中暂养12 d。暂养期间,每天用基础饲料投喂2次(09:00和17:00),并换水。暂养结束后,停喂24 h,挑选出体质健康、规格均匀的牛蛙225只,试验牛蛙初始体重为(36.0±1.0) g,随机分为5组,每组3个重复(桶),每个重复15只牛蛙。5组分别饲喂用发酵豆粕替代0(FM组)、25%(FSM25组)、50%(FSM50组)、75%(FSM75组)和100%(FSM100组)鱼粉的试验饲料。
试验期间,每天饱食投喂2次(09:00、17:00),每次喂食0.5 h后观察摄食情况,记录残饵量,并换水。养殖期间水温25~29 ℃。试验期为56 d。
1.3 样品采集养殖56 d后,将牛蛙禁食24 h,对各养殖桶牛蛙计数和称重。每个养殖桶随机取6只牛蛙,用双毁髓法处理,然后用1 mL的注射器抽取血液注入1.5 mL离心管中,静置20 h,3 000 r/min离心10 min,分离取得血清,置于-80 ℃冰箱保存。将采完血的6只牛蛙解剖,取下后腿肌肉,置于-20 ℃冰箱保存,用于测定肌肉营养成分;取其中3只牛蛙的空肠分别放入装有波恩氏液的冻存管中,置于4 ℃冰箱保存,用于制作肠道组织切片;分别取出另外3只牛蛙的十二指肠、空肠和回肠,放入冻存管中,置于-80 ℃冰箱保存,用于测定肠道消化酶活性。再从每个缸中随机取出3只牛蛙装入自封袋,置于-20 ℃冰箱保存,用于测定全体营养成分。
1.4 指标测定 1.4.1 常规成分饲料原料、饲料、牛蛙全体和肌肉粗蛋白质、粗脂肪、粗灰分和水分含量测定参考AOAC(2005)的方法进行。其中,水分含量采用105 ℃烘箱干燥恒重法测定,粗蛋白质含量采用杜马斯燃烧定氮法(Rapid N Ⅲ氮/蛋白质分析仪)测定,粗脂肪含量采用索氏抽提法(乙醚为溶剂)测定,粗灰分含量采用马弗炉550 ℃高温灼烧法测定。
1.4.2 血清生化指标血清超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、谷草转氨酶(GOT)、谷丙转氨酶(GPT)活性和丙二醛(MDA)含量均使用从南京建成生物工程研究所购买的试剂盒测定。
1.4.3 肠道消化酶活性肠道淀粉酶活性使用南京建成生物工程研究所的试剂盒测定。肠道蛋白酶活性采用福林-酚试剂法测定[14],以标准L-酪氨酸浓度和吸光度值做标准曲线,用于计算蛋白酶活性。蛋白酶活性单位定义:每分钟内分解出1 μg酪氨酸的酶量为1个蛋白酶活性单位。
1.4.4 肠道组织结构从波恩氏液中剪取0.3~0.4 cm的肠道组织,先用乙醇脱水,再用石蜡包埋,用切片机切片,切片厚度约6 μm,之后进行染色封片。完成肠道组织切片后,在显微镜下观察并拍照。
1.5 计算公式

|
式中:Wt为终末牛蛙总重量(g);W0为初始牛蛙总重量(g);Nf为终末牛蛙数量;Ni为初始牛蛙数量;Wf为干物质总摄食量(g);t为饲喂天数(d);Pt为终末全体蛋白质含量(%);P0为初始全体蛋白质含量(%);WP为摄入饲料蛋白质总量(g)。
1.6 数据统计及方差分析所有数据采用SPSS 22.0统计软件进行单因素方差分析(one-way ANOVA),并进行Duncan氏法多重比较,差异显著水平为P < 0.05。所有数据用平均值±标准误表示。
2 结果 2.1 发酵豆粕替代鱼粉对牛蛙生长性能的影响由表 2可知,各组牛蛙成活率均为100%。FM组牛蛙增重率显著高于FSM100组(P < 0.05),但与其他各组差异不显著(P>0.05)。FM组牛蛙摄食率、饲料效率和氮保留率与其他各组差异不显著(P>0.05)。
|
|
表 2 发酵豆粕替代鱼粉对牛蛙生长性能的影响 Table 2 Effects of fish meal replacement by fermented soybean meal on growth performance of bullfrog |
由表 3可知,各组之间牛蛙全体和肌肉的水分、粗蛋白质、粗脂肪含量没有显著差异(P>0.05)。FSM100组牛蛙肌肉粗灰分含量显著低于FSM75组(P < 0.05)。
|
|
表 3 发酵豆粕替代鱼粉对牛蛙全体和肌肉营养成分的影响 Table 3 Effects of fish meal replacement by fermented soybean meal on whole body and muscle nutritional composition of bullfrog |
由表 4可知,随着饲料中发酵豆粕替代鱼粉比例的增加,牛蛙十二指肠、空肠和回肠蛋白酶活性呈现逐渐降低的趋势,各替代组蛋白酶活性均显著低于FM组(]0.05)。各组之间牛蛙十二指肠淀粉酶活性无显著差异(P>0.05),FSM75组牛蛙空肠淀粉酶活性显著高于FM组(P < 0.05),FSM50、FSM75和FSM100组牛蛙回肠淀粉酶活性显著高于FM组(P < 0.05)。
|
|
表 4 发酵豆粕替代鱼粉对牛蛙肠道消化酶活性的影响 Table 4 Effects of fish meal replacement by fermented soybean meal on intestinal digestive enzyme activities of bullfrog |
由表 5可知,FSM75组血清CAT活性显著高于FM组(P < 0.05)。FSM100组血清MDA含量显著低于FM、FSM25和FSM50组(P < 0.05),与FSM75组差异不显著(P>0.05)。FSM25组血清SOD活性显著高于FM组(P < 0.05);从FSM25组到FSM100组,随着饲料中发酵豆粕替代鱼粉比例的增加,血清SOD活性呈下降趋势。各组之间血清GOT活性差异不显著(P>0.05)。从FSM25组到FSM100组,随着饲料中发酵豆粕替代鱼粉比例的增加,血清GPT活性呈上升趋势;FM组血清GPT活性显著高于FSM25组(P < 0.05), 与FSM50、FSM75和FSM100组差异不显著(P>0.05)。
|
|
表 5 发酵豆粕替代鱼粉对牛蛙血清生化指标的影响 Table 5 Effects of fish meal replacement by fermented soybean meal on serum biochemical parameters of bullfrog |
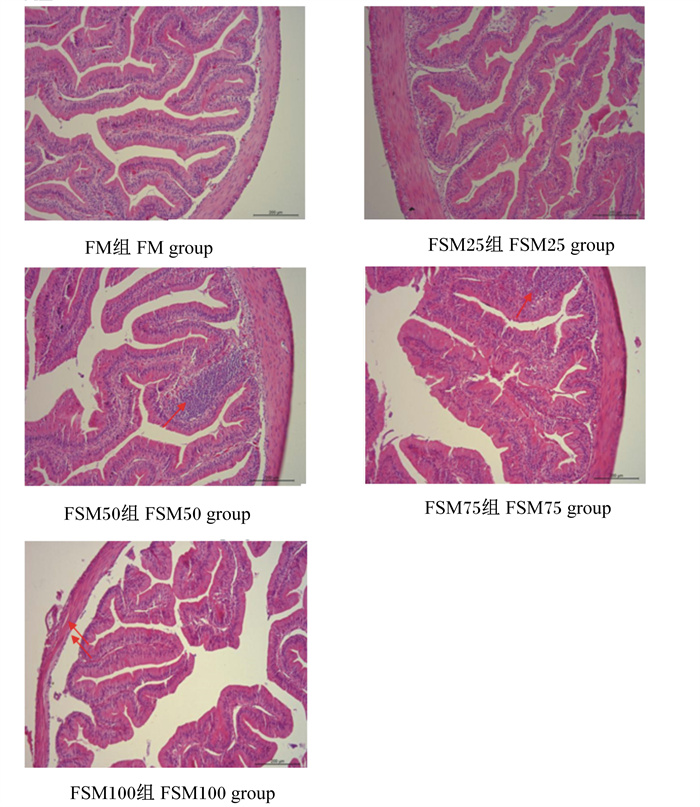
通过显微镜观察牛蛙空肠的组织切片,如图 1所示,当发酵豆粕替代50%鱼粉时(FSM50组),小肠绒毛固有层内局部出现大量的嗜碱性颗粒。当发酵豆粕替代75%鱼粉时(FSM75组),小肠绒毛固有层出现大面积嗜碱性颗粒聚集,且肠绒毛变短。当发酵豆粕完全替代鱼粉时(FSM100组),小肠肠壁变薄,杯状细胞数目减少,肠绒毛稀疏且较短,肠组织结构不完整。
|
FSM50和FSM75组图中箭头指向为小肠绒毛固有层局部大量嗜碱性颗粒聚集。FSM100组图中箭头指向为小肠肠壁变薄。 The arrow in the figure of FSM50 and FSM75 groups indicate a large number of basophilic particles gathered locally in the lamina propria of intestinal villus. The arrow in the figure of FSM100 group indicate thinning of the intestinal wall of small intestine. 图 1 发酵豆粕替代鱼粉对牛蛙空肠组织结构的影响 Fig. 1 Effects of fish meal replacement by fermented soybean meal on jejunum tissue structure of bullfrog |
已有研究表明,饲料中豆粕大量替代鱼粉会降低养殖鱼类的摄食率[15-16]。然而本试验中,发酵豆粕替代鱼粉对牛蛙的摄食率没有显著影响,这可能与牛蛙在自然状态下为杂食性有关,也说明牛蛙对发酵豆粕有较好的适应性[17]。有研究表明,发酵豆粕可部分替代饲料中鱼粉而不影响水生动物的生长。例如,发酵豆粕替代45%鱼粉对大菱鲆生长无不良影响[18],发酵豆粕替代20%鱼粉不影响许氏平鲉的生长性能[19]。罗智等[20]通过折线回归分析,得出饲料中发酵豆粕可替代10%鱼粉而不影响石斑鱼生长性能。Ding等[21]发现饲料中发酵豆粕100%替代鱼粉对日本沼虾的生长性能没有显著影响。Xu等[22]研究结果表明,2种发酵豆粕A和B分别可替代中华绒螯蟹饲料中10%和15%的鱼粉,并具有一定的促生长作用。此外,在花鲈[23]、大黄鱼[24]、异育银鲫[25]、圆斑星鲽[26]中也有类似的报道。本试验中,发酵豆粕替代100%鱼粉组牛蛙增重率低于其他各组,表明发酵豆粕完全替代鱼粉对牛蛙生长有一定抑制作用;而发酵豆粕替代75%鱼粉时,牛蛙增重率无显著下降,说明饲料中一定比例的鱼粉或动物蛋白质对牛蛙的生长有重要作用。研究表明,豆粕经发酵处理后,大分子的蛋白质分解为小肽、氨基酸,同时,抗营养因子含量大幅降低,更利于水产动物的消化吸收[20]。Xu等[22]研究发现,发酵豆粕可以提高豆粕酸溶性、水溶性和小分子蛋白质含量,从而提高豆粕的消化率和营养价值。此外,本试验中补充了赖氨酸、蛋氨酸,平衡了饲料中的必需氨基酸,从而满足发酵豆粕替代鱼粉后牛蛙对氨基酸的需求。上述结果说明,发酵豆粕是牛蛙饲料的优质蛋白质源。
3.2 发酵豆粕替代鱼粉对牛蛙血清生化指标的影响抗氧化系统包括酶促系统和非酶促系统,抗氧化酶包括SOD、CAT和谷胱甘肽过氧化物酶等,它们在组织中共同对抗自由基,形成第一道抗氧化防线[27]。SOD催化超氧阴离子经过歧化作用变为过氧化氢和氧,CAT则分解过氧化氢成为水和氧[28]。MDA是脂质过氧化的最终产物,是生物体氧化损伤的重要标志物[29]。本研究中,随着发酵豆粕替代水平的增加,牛蛙血清中MDA含量的总体变化趋势与SOD相似,都呈下降趋势,但血清CAT活性增加,说明发酵豆粕的添加可降低机体氧化水平,减少有害氧化产物的生成。分析其原因:一方面可能是鱼粉中较高的脂肪含量,易于被氧化;另一方面可能是豆粕经过发酵后增加了抗氧化产物水平。研究表明,发酵工艺能有效改善食品的营养价值和功能特性[30]。Lin等[31]研究发现,与商品豆粕相比,乳酸菌发酵豆粕减少了白虾的氧化损伤。米曲霉发酵提高了豆粕中抗氧化化合物的生物利用度,进而提高了鱼的抗氧化能力[32]。多酚类物质是豆类中广泛存在的天然抗氧化剂,研究表明,微生物发酵可提高一些豆科植物如鹰嘴豆[33]、菜豆[34]、兵豆[35]和大豆[36]等的抗氧化能力和总酚含量。GOT和GPT主要存在于肝细胞中,是机体氨基酸代谢过程中的2种关键酶,肝细胞受损时,GOT和GPT游离到血清中,因而血清中GOT和GPT活性可指示机体肝脏受损伤的程度[37]。本试验中,发酵豆粕替代25%鱼粉时牛蛙血清GOT和GPT活性均最低,可能说明饲料中添加部分发酵豆粕可以改善牛蛙的肝脏健康,这可能也是FSM25组中牛蛙生长性能提高的原因。
3.3 发酵豆粕替代鱼粉对牛蛙肠道消化酶活性的影响蛋白酶和淀粉酶是水产动物肠道的2种主要消化酶,影响它们分泌的因素有很多,如摄食习惯、饲料组成和抗营养因子等[38-39]。本试验中,发酵豆粕替代鱼粉提高了牛蛙空肠和回肠的淀粉酶活性,这与本实验室前期的研究结果[40]相似。这可能是饲料中碳水化合物含量的增加刺激了肠道淀粉酶活性的上升[41],相似的结论也出现在对大菱鲆的研究中[42]。此外,本试验中,随着饲料中发酵豆粕替代鱼粉比例的增加,牛蛙十二指肠、空肠和回肠蛋白酶活性呈现逐渐降低的趋势,这可能是由于发酵豆粕中还存在大豆抗原蛋白、蛋白酶抑制因子等抗营养因子,抑制了蛋白酶的活性,类似的结果也出现在罗非鱼[43]、大西洋鲑[44]、埃及胡子鲇[45]、花鲈[46]和黄金鲫[47]等的研究中。
3.4 发酵豆粕替代鱼粉对牛蛙空肠组织结构的影响肠道是消化和吸收的主要器官,其组织完整性关系到营养物质消化吸收和动物健康。本试验中,随着发酵豆粕替代比例的增加,牛蛙肠道绒毛出现断裂现象,当发酵豆粕100%替代鱼粉时,肠壁肌层厚度明显降低,肠道完整性遭到破坏,表明高比例的发酵豆粕替代鱼粉对牛蛙肠道结构造成影响,损伤肠黏膜,这与王健[40]的试验结果一致。此外,在花鲈[46]和黄金鲫[47]的研究中也发现类似的结果。研究表明,豆粕对肠道组织的负面影响归因于抗营养因子,如大豆抗原蛋白、皂苷、棉子糖、水苏糖和植物凝集素等[48-50]。Buttle等[51]报道,凝集素与肠道上皮细胞表面的多糖结合可破坏肠道微绒毛,导致营养物质的吸收和消化减少。Hu等[52]研究发现,大豆异黄酮和大豆皂苷破坏黄鳝肠道结构和肠道屏障,引起肠道炎症。Zhang等[53]研究发现,大豆球蛋白引起草鱼肠上皮细胞核移位和杯状细胞充血。本实验室前期研究发现,豆粕替代50%鱼粉后,牛蛙空肠绒毛高度、肌层厚度和柱状上皮高度显著降低[8]。本试验中,发酵豆粕替代鱼粉水平在50%以下时,没有对牛蛙肠道造成明显负面影响,说明发酵处理可能在一定程度上减少了豆粕中抗营养因子或产生了有益的微生物代谢产物,改善了牛蛙肠道健康。
4 结论本试验条件下,饲料中发酵豆粕替代75%鱼粉不会影响牛蛙生长性能、氮保留率和饲料效率,但对牛蛙肠道组织结构造成一定的负面影响。建议牛蛙饲料中发酵豆粕替代鱼粉的比例为50%~75%。
| [1] |
WANG L, WANG J, LU K L, et al. Total replacement of fish meal with soybean meal in diets for bullfrog (Lithobates catesbeianus): effects on growth performance and gut microbial composition[J]. Aquaculture, 2020, 524: 735236. DOI:10.1016/j.aquaculture.2020.735236 |
| [2] |
ZHANG C X, HUANG K K, WANG L, et al. Apparent digestibility coefficients and amino acid availability of common protein ingredients in the diets of bullfrog, Rana (Lithobates) catesbeiana[J]. Aquaculture, 2015, 437: 38-45. DOI:10.1016/j.aquaculture.2014.11.015 |
| [3] |
TACON A G J, METIAN M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects[J]. Aquaculture, 2008, 285(1/2/3/4): 146-158. |
| [4] |
GATLIN D M, BARROWS F T, BROWN P, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review[J]. Aquaculture Research, 2007, 38(6): 551-579. DOI:10.1111/j.1365-2109.2007.01704.x |
| [5] |
HARDY R W. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal[J]. Aquaculture Research, 2010, 41(5): 770-776. DOI:10.1111/j.1365-2109.2009.02349.x |
| [6] |
FRANCIS G, MAKKAR H P S, BECKER K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish[J]. Aquaculture, 2001, 199(3/4): 197-227. |
| [7] |
KIKUCHI K, FURUTA T. Inclusion of blue mussel extract in diets based on fish and soybean meals for tiger puffer Takifugu rubripes[J]. Fisheries Science, 2009, 75: 183-189. DOI:10.1007/s12562-008-0012-x |
| [8] |
丁李, 王玲, 鲁康乐, 等. 豆粕替代鱼粉对牛蛙生长性能、消化酶活性和肠道健康的影响[J]. 淡水渔业, 2019, 49(4): 62-68. DING L, WANG L, LU K L, et al. Effects of replacement of fish meal with soybean meal on growth performance, digestive enzyme activity and intestinal health of bullfrog Rana catesbeiana[J]. Freshwater Fisheries, 2019, 49(4): 62-68 (in Chinese). DOI:10.3969/j.issn.1000-6907.2019.04.010 |
| [9] |
HONG K J, LEE C H, KIM S W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals[J]. Journal of Medicinal Food, 2004, 7(4): 430-435. DOI:10.1089/jmf.2004.7.430 |
| [10] |
SINGH B P, VIJ S, HATI S. Functional significance of bioactive peptides derived from soybean[J]. Peptides, 2014, 54: 171-179. DOI:10.1016/j.peptides.2014.01.022 |
| [11] |
WU P, GOLLY M K, GUO Y T, et al. Effect of partial replacement of soybean meal with high-temperature fermented soybean meal in antibiotic-growth-promoter-free diets on growth performance, organ weights, serum indexes, intestinal flora and histomorphology of broiler chickens[J]. Animal Feed Science and Technology, 2020, 269: 114616. DOI:10.1016/j.anifeedsci.2020.114616 |
| [12] |
刘凡, 张天勇, 李艳芳, 等. 发酵豆粕特性及在畜禽养殖中的应用研究[J]. 饲料研究, 2020, 43(6): 147-149. LIU F, ZHANG T Y, LI Y F, et al. Study on the characteristics of fermented soybean meal and the application in livestock and poultry breeding[J]. Feed Research, 2020, 43(6): 147-149 (in Chinese). |
| [13] |
何明, 喻一峰, 李小勤, 等. 大口黑鲈饲料中发酵豆粕营养价值的评定[J]. 动物营养学报, 2020, 32(10): 4943-4955. HE M, YU Y F, LI X Q, et al. Evaluation of nutrient value of fermented soybean meal in diet for largemouth bass (Micropterus salmoides)[J]. Chinese Journal of Animal Nutrition, 2020, 32(10): 4943-4955 (in Chinese). |
| [14] |
王学标, 何云侠, 刘强. 福林酚试剂法与缩二脲试剂法测定饲用蛋白酶活力的比较分析[J]. 畜牧与兽医, 2019, 51(3): 23-28. WANG X B, HE Y X, LIU Q. Comparison of the folinphenol reagent method and the Biuret reagent method for determination of feed protease activity[J]. Animal Husbandry & Veterinary Medicine, 2019, 51(3): 23-28 (in Chinese). |
| [15] |
RAHIMNEJAD S, ZHANG J J, WANG L, et al. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed[J]. Aquaculture, 2021, 531: 735975. DOI:10.1016/j.aquaculture.2020.735975 |
| [16] |
高荣兵, 庄平, 章龙珍, 等. 豆粕替代鱼粉对点篮子鱼生长性能的影响[J]. 水产学报, 2010, 34(10): 1534-1540. GAO R B, ZHUANG P, ZHANG L Z, et al. Effects of replacement of fish meal by soybean meal on growth characters of siganidae (Siganus guttatus)[J]. Journal of Fisheries of China, 2010, 34(10): 1534-1540 (in Chinese). |
| [17] |
方卫东, 鲁康乐, 张春晓, 等. 豆粕替代鱼粉对牛蛙生长、体组成、消化酶活力及肝脏生化指标的影响[J]. 水产学报, 2016, 40(11): 1742-1752. FANG W D, LU K L, ZHANG C X, et al. Effects of fish meal replacement by soybean meal on growth, body composition, digestive enzyme activities and hepatic biochemical indices of Rana (Lithobates) catesbeiana[J]. Journal of Fisheries of China, 2016, 40(11): 1742-1752 (in Chinese). |
| [18] |
LI C Q, ZHANG B L, ZHOU H H, et al. Beneficial influences of dietary Aspergillus awamori fermented soybean meal on oxidative homoeostasis and inflammatory response in turbot (Scophthalmus maximus L.)[J]. Fish & Shellfish Immunology, 2019, 93: 8-16. |
| [19] |
LEE S M, AZARM H M, CHANG K H. Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli)[J]. Aquaculture, 2016, 459: 110-116. DOI:10.1016/j.aquaculture.2016.03.036 |
| [20] |
罗智, 刘永坚, 麦康森, 等. 石斑鱼配合饲料中发酵豆粕和豆粕部分替代白鱼粉的研究[J]. 水产学报, 2004, 28(2): 175-181. LUO Z, LIU Y J, MAI K S, et al. Partial replacement of fish meal by soybean protein in diets for grouper Epinephelus coioides juveniles[J]. Journal of Fisheries of China, 2004, 28(2): 175-181 (in Chinese). |
| [21] |
DING Z L, ZHANG Y X, YE J Y, et al. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: growth, nonspecific immunity, and resistance to Aeromonas hydrophila[J]. Fish & Shellfish Immunology, 2015, 44(1): 295-301. |
| [22] |
XU C Y, LIU W B, ZHANG D D, et al. Effects of partial fish meal replacement with two fermented soybean meals on the growth of and protein metabolism in the Chinese mitten crab (Eriocheir sinensis)[J]. Aquaculture Reports, 2020, 17: 100328. DOI:10.1016/j.aqrep.2020.100328 |
| [23] |
LIANG X F, HU L, DONG Y C, et al. Substitution of fish meal by fermented soybean meal affects the growth performance and flesh quality of Japanese seabass (Lateolabrax japonicus)[J]. Animal Feed Science and Technology, 2017, 229: 1-12. DOI:10.1016/j.anifeedsci.2017.03.006 |
| [24] |
何娇娇, 王萍, 冯建, 等. 发酵豆粕对大黄鱼生长、肠道结构及肠道微生物菌群的研究[J]. 水生生物学报, 2018, 42(5): 919-928. HE J J, WANG P, FENG J, et al. Effects of fermented soybean meal on the growth and intestinal histology and microbiota of juvenile large yellow croaker Larimichthys crocea[J]. Acta Hydrobiologica Sinica, 2018, 42(5): 919-928 (in Chinese). |
| [25] |
CAO S P, MO P, XIAO Y B, et al. Dietary supplementation with fermented plant meal enhances growth, antioxidant capacity and expression of TOR signaling pathway genes in gibel carp (Carassius auratus gibelio var. CASV)[J]. Aquaculture Reports, 2021, 19: 100559. DOI:10.1016/j.aqrep.2020.100559 |
| [26] |
吕云云, 常青, 陈四清, 等. 发酵豆粕对圆斑星鲽生长及消化能力的影响[J]. 水生生物学报, 2016, 40(1): 10-18. LYU Y Y, CHANG Q, CHEN S Q, et al. The effects of dietary fermented soybean meal on the growth and digestive ability of spotted halibut, Verasper variegatus[J]. Acta Hydrobiologica Sinica, 2016, 40(1): 10-18 (in Chinese). |
| [27] |
孔纯, 华雪铭, 杨璐, 等. 暗纹东方鲀饲料中豆粕替代鱼粉的营养生理效应及其与大豆抗原蛋白的相关性[J]. 水产学报, 2017, 41(5): 734-745. KONG C, HUA X M, YANG L, et al. Nutritional physiological effects of soybean meal substituting for fish meal in the feed of obscure puffer (Takifugu fasciatus) and its relationship with soybean antigenic proteins[J]. Journal of Fisheries of China, 2017, 41(5): 734-745 (in Chinese). |
| [28] |
TOVAR-RAMÍREZ D, MAZURAIS D, GATESOUPE J F, et al. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae[J]. Aquaculture, 2010, 300(1/4): 142-147. |
| [29] |
DRAPER H H, HADLEY M. Malondialdehyde determination as index of lipid Peroxidation[J]. Methods in Enzymology, 1990, 186: 421-431. |
| [30] |
SONG Y S, FRIAS J, MARTINEZ-VILLALUENGA C, et al. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products[J]. Food Chemistry, 2008, 108(2): 571-581. DOI:10.1016/j.foodchem.2007.11.013 |
| [31] |
LIN Y H, MUI J J. Comparison of dietary inclusion of commercial and fermented soybean meal on oxidative status and non-specific immune responses in white shrimp, Litopenaeus vannamei[J]. Fish & Shellfish Immunology, 2017, 63: 208-212. |
| [32] |
KIM S S, GALAZ G B, PHAM M A. Effects of dietary supplementation of a meju, fermented soybean meal, and Aspergillus oryzae for juvenile parrot fish (Oplegnathus fasciatus)[J]. Asian-Australasian Journal of Animal Sciences, 2009, 22(6): 849-856. DOI:10.5713/ajas.2009.80648 |
| [33] |
XIAO Y, XING G L, RUI X, et al. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18[J]. Journal of Functional Foods, 2014, 10: 210-222. DOI:10.1016/j.jff.2014.06.008 |
| [34] |
LIMÓN R I, PEÑAS E, TORINO M I, et al. Fermentation enhances the content of bioactive compounds in kidney bean extracts[J]. Food Chemistry, 2015, 172: 343-352. DOI:10.1016/j.foodchem.2014.09.084 |
| [35] |
TORINO M I, LIMÓN R I, MARTÍNEZ-VILLALUENGA C, et al. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils[J]. Food Chemistry, 2013, 136(2): 1030-1037. DOI:10.1016/j.foodchem.2012.09.015 |
| [36] |
CHENG K C, WU J Y, LIN J T, et al. Enhancements of isoflavone aglycones, total phenolic content, and antioxidant activity of black soybean by solid-state fermentation with Rhizopus spp[J]. European Food Research and Technology, 2013, 236: 1107-1113. DOI:10.1007/s00217-013-1936-7 |
| [37] |
YAN Q, XIE S, ZHU X, et al. Dietary methionine requirement for juvenile rockfish, Sebastes schlegeli[J]. Aquaculture Nutrition, 2007, 13(3): 163-169. DOI:10.1111/j.1365-2095.2007.00461.x |
| [38] |
PAVASOVIC A, ANDERSON A J, MATHER P B, et al. Influence of dietary protein on digestive enzyme activity, growth and tail muscle composition in redclaw crayfish, Cherax quadricarinatus (von Martens)[J]. Aquaculture Research, 2007, 38(6): 644-652. DOI:10.1111/j.1365-2109.2007.01708.x |
| [39] |
HIDALGO M C, UREA E, SANZ A. Comparative study of digestive enzymes in fish with different nutritional habits.Proteolytic and amylase activities[J]. Aquaculture, 1999, 170(3): 267-283. |
| [40] |
王健. 蛙源酵母菌ZR1发酵豆粕对牛蛙生长、消化和肠道健康的影响[D]. 硕士学位论文. 厦门: 集美大学, 2018. WANG J. Effects of autochthonous yeast ZR1(Pseudozyma aphidis strain) fermented soybean meal on growth, digestion and intestinal health in bullfrog (Rana (Lithobates) catesbeiana)[D]. Master's Thesis. Xiamen: Jimei University, 2018. (in Chinese) |
| [41] |
ZHOU C P, GE X P, NIU J, et al. Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus[J]. Aquaculture, 2015, 437: 390-397. DOI:10.1016/j.aquaculture.2014.12.016 |
| [42] |
王海英, 孙谧, 薛长湖, 等. 大菱鲆配合饲料中植物蛋白替代鱼粉的可行性研究[J]. 海洋科学, 2008, 32(6): 9-12, 34. WANG H Y, SUN M, XUE C H, et al. The feasibility of partial or total replacement of fish meal by soybean meal in diet for turbot[J]. Marine Sciences, 2008, 32(6): 9-12, 34 (in Chinese). |
| [43] |
LIN S M, LUO L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus×O. aureus[J]. Animal Feed Science and Technology, 2011, 168(1/2): 80-87. |
| [44] |
KROGDAHL A. BAKKE-MCKELLEP A M, BAEVERFJORD G.Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.)[J]. Aquaculture Nutrition, 2003, 9(6): 361-371. DOI:10.1046/j.1365-2095.2003.00264.x |
| [45] |
吴莉芳, 秦贵信, 孙泽威, 等. 饲料中去皮豆粕替代鱼粉对埃及胡子鲇消化酶活力和肠道组织的影响[J]. 中山大学学报(自然科学版), 2010, 49(4): 99-105. WU L F, QIN G X, SUN Z W, et al. Effect of dietary dehulled soyabean meal replacing fish meal on the activity of digestive enzyme and the intestinal tissue of Clarias lazera[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni (Natural Science Edition), 2010, 49(4): 99-105 (in Chinese). |
| [46] |
ZHANG C X, RAHIMNEJAD S, WANG Y R, et al. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes[J]. Aquaculture, 2018, 483: 173-182. DOI:10.1016/j.aquaculture.2017.10.029 |
| [47] |
林佳洁. 发酵豆粕替代鱼粉对黄金鲫生长、免疫及肠道组织的影响[D]. 硕士学位论文. 长春: 吉林农业大学, 2016. LIN J J. Effects of replacement of fish meal by fermented soybean meal in diets on growth, immunity and intestinal tissue of Carassius auratus[D]. Master's Thesis. Changchun: Jilin Agricultural University, 2016. (in Chinese) |
| [48] |
VENOU B, ALEXIS M N, FOUNTOULAKI E, et al. Effects of extrusion and inclusion level of soybean meal on diet digestibility, performance and nutrient utilization of gilthead sea bream (Sparus aurata)[J]. Aquaculture, 2006, 261(1): 343-356. DOI:10.1016/j.aquaculture.2006.07.030 |
| [49] |
CHEN W, AI Q H, MAI K S, et al. Effects of dietary soybean saponins on feed intake, growth performance, digestibility and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus)[J]. Aquaculture, 2011, 318(1/2): 95-100. |
| [50] |
PENG M, XU W, AI Q H, et al. Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybean meal (Scophthalmus maximus L. )[J]. Aquaculture, 2013, 392/395: 51-58. DOI:10.1016/j.aquaculture.2013.02.002 |
| [51] |
BUTTLE L G, BURRELLS A C, GOOD J E, et al. The binding of soybean agglutinin (SBA) to the intestinal epithelium of Atlantic salmon, Salmo salar and rainbow trout, Oncorhynchus mykiss, fed high levels of soybean meal[J]. Veterinary Immunology and Immunopathology, 2001, 80(3/4): 237-244. |
| [52] |
HU Y J, ZHANG J Z, XUE J J, et al. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity and gut microbiota community on rice field eel (Monopterus albus)[J]. Aquaculture, 2021, 537: 736506. DOI:10.1016/j.aquaculture.2021.736506 |
| [53] |
ZHANG Y L, DUAN X D, JIANG W D, et al. Soybean glycinin decreased growth performance, impaired intestinal health, and amino acid absorption capacity of juvenile grass carp (Ctenopharyngodon idella)[J]. Fish Physiology and Biochemistry, 2019, 45(5): 1589-1602. DOI:10.1007/s10695-019-00648-z |




