骨骼肌质量在动物机体中占比高达50%,是畜禽主要产肉器官,其生长发育对机体性能极为重要[1]。诸多研究发现骨骼肌生长过程中Wnt信号网络不可或缺,而其信号紊乱则会导致骨骼肌生长受阻和再生障碍[2-4]。随着Wnt信号网络的研究逐渐深入[5],对其进行营养精准调控以促进肌肉生长已成为动物营养和生命医学领域的研究热点。R-spondin1作为Wnt信号网络的激动因子,已有研究发现其具有缓解肝肺损伤、肠道损伤及促进毛囊发育等功能[6-9]。此外,还有研究表明,R-spondin1参与Wnt信号网络调控骨骼肌再生修复过程[10],但其作用机理仍不明确。因此,本文旨在综述R-spondin1启动Wnt信号网络调控骨骼肌生长的功能,期望通过梳理R-spondin1作用机制发掘新的研究靶点,为骨骼肌生长调控提供理论依据。
1 Wnt信号网络参与骨骼肌的生长发育骨骼肌生长过程极其复杂,涉及骨骼肌卫星细胞增殖分化和外源营养物质调控等多重因素。其中,卫星细胞作为位于肌膜和基底膜之间的具有成肌潜力的干细胞,对骨骼肌的生长发育及再生至关重要[11]。当肌肉受到损伤或其他外源刺激时,卫星细胞被激活进入高速扩增状态,逐步融入旧有肌纤维或相互融合形成新的肌纤维以修补肌肉受损部位[12]。此外,多项研究表明,特异性消融肌肉中卫星细胞会导致肌肉再生过程被阻断,提示卫星细胞在肌肉再生过程中的主导地位[13-14]。
据报道,Wnt信号网络在骨骼肌卫星细胞的增殖、迁移和分化等过程中均具有关键调控作用[15]。研究发现,肌肉再生过程中,Wnt信号由低水平表达转入高度活跃状态,而干扰或阻断Wnt信号则会导致肌肉再生延迟或受阻[16]。根据胞内转运组分不同,Wnt信号网络可被划分为经典的Wnt/β-连环蛋白(Wnt/β-catenin)信号通路、非经典的Wnt/细胞平面极性(Wnt/PCP)信号通路以及Wnt/钙离子(Wnt/Ca2+)信号通路[5, 15]。
Chen等[17]研究表明,饲粮中添加精氨酸可促进猪背最长肌肌内脂肪沉积,而该过程可能是通过抑制Wnt/β-catenin信号通路完成的。有意思的是骨骼肌卫星细胞也可能参与成脂过程[18],但是三者是否有必然联系还需进一步研究。同时,本课题组前期研究发现,赖氨酸缺乏会抑制Wnt/β-catenin信号通路,降低猪背最长肌质量及卫星细胞数量,补足赖氨酸则会重新激活Wnt/β-catenin信号通路[19]。此外,Yang等[20]通过在猪成肌细胞中添加GSK3β的抑制剂氯化锂,也证明Wnt/β-catenin信号通路是调节猪成肌细胞肌源性分化的重要途径。
Wnt/PCP信号通路的激活会促进卫星细胞的对称分裂,从而增加卫星细胞数量,促进骨骼肌/肌纤维损伤修复潜力[12]。同时,过表达Wnt/PCP信号通路会显著提高卫星细胞迁移及融合能力,最终导致肌纤维肥大[21-22]。此外, 卫星细胞进入分化状态后Wnt/β-catenin的表达水平会随之升高。与之相反,下调Wnt/β-catenin的表达水平则会延缓卫星细胞进入分化状态,阻碍骨骼肌损伤修复[23]。而Wnt/Ca2+信号通路主要调控卫星细胞的迁移及分化过程,该通路的激活会提高卫星细胞的分化程度和迁移能力[24]。
2 R-spondin家族蛋白质结构及功能R-spondin家族是含有血小板反应蛋白质1型重复序列(thrombospondin type 1 repeat,TSR-1)的超家族。其家族有4个成员,分别为R-spondin1~4;它们的氨基酸序列同源性为40%~60%,且均含有1个羧基尾巴和2个富含半胱氨酸的弗林蛋白质酶域(furin-like cysteine-rich domains,FLD)[25-26]。研究表明,FLD具有R-spondin家族蛋白质50%的活性,其主要负责R-spondin对dickkopf相关蛋白1(dickkopf-related protein 1,DKK1)功能的干扰[27]。
尽管R-spondin1~4结构相似,均对Wnt信号具有增强作用,但其表达规律及功能却不尽相同。其中,R-spondin1在早期研究中被发现与性别逆转有关[28],后续研究发现R-spondin1能够有效促进肠道生长发育,同时也是体外培养肠道隐窝干细胞的重要生长因子[29]。Nam等[30]研究发现,在卫星细胞分化过程中R-spondin1表达显著上调。另外,R-spondin1能够促进成年小鼠皮肤细胞毛囊的新生以及调节毛发周期进程[9, 31]。除此之外,它还具有诱导骨形成、血管生成和胰岛细胞分泌胰岛素等功能[32-34]。R-spondin2主要影响早期胚胎中的肢体和肺部的发育,其敲除会导致肢体及肺部发育不良[35-36]。Cadieu等[37]对80个品种的1 000多只家犬进行全基因组关联分析,发现R-spondin2的功能对于毛发的卷曲程度有重要的影响。而R-spondin3与胎盘生长发育相关,该基因定向敲除会抑制胎盘血管生成,进而导致胚胎死亡[38-39]。在甲癣(一种与指甲发育缺陷的疾病)发生个体中R-spondin4功能严重受损,表明R-spondin4可能与指甲发育和疾病的发生有关,但相关作用机制尚不清楚[40]。
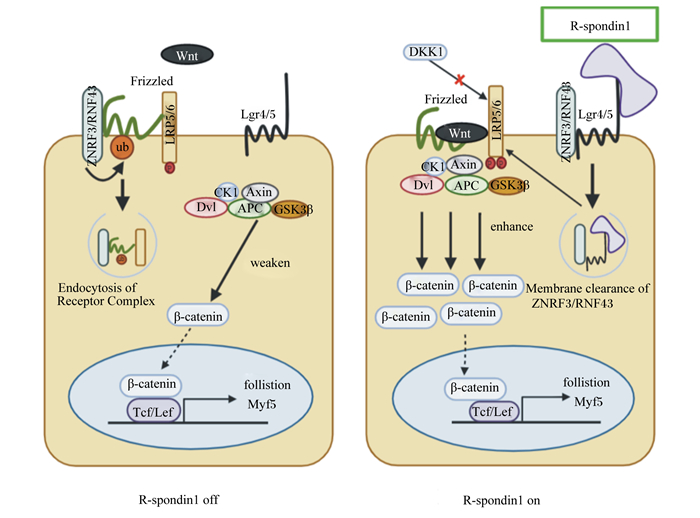
3 R-spondin1作用机制研究进展 3.1 R-spondin1作用机制概述研究表明,Wnt配体和受体卷曲蛋白质(frizzled,Fzd)结合能够激活Wnt信号;然后,低密度脂蛋白受体相关蛋白5/6(low density lipoprotein receptor related protein 5/6, LRP5/6)会被磷酸化,从而促进下游信号通路的激活[41]。然而,细胞膜上除Fzd和LRP5/6等有利于Wnt信号传导的受体外,E3泛素连接酶RNF43(ring finger protein 43)和ZNRF3(zinc and ring finger 3)与Fzd结合则会导致其泛素化降解,从而弱化Wnt信号的传导。R-spondin1作为Wnt信号网络的增强子,其FLD区域可通过与G蛋白质偶联受体4/5(G-protein-coupled receptors, Lgr4/5)结合,进而连接RNF43和ZNRF3的胞外区域,使得膜上RNF43和ZNRF3被清除,最终增强Wnt信号的传导[42-43](图 1)。
|
R-spondin1 off:R-spondin1缺失;R-spondin1 on:R-spondin1激活;ZNRF3:锌指环指蛋白3 zinc and ring finger 3;RNF43:环指蛋白43 ring finger protein 43;DKK1:Dickkopf相关蛋白1;Lgr4/5:G蛋白质偶联受体4/5 G-protein-coupled receptors 4/5;LRP5/6:低密度脂蛋白受体相关蛋白5/6 low density lipoprotein receptor related protein 5/6;Frizzled:受体卷曲蛋白质;ub:泛素化ubiquitination;Dvl:dishevelled protein散乱蛋白;Axin:轴蛋白axin scaffolding protein;APC:结肠腺瘤性息肉病蛋白adenomatous polyposis coli;GSK3β:糖原合成酶激酶3β glycogen synthase kinase 3β;CK1:酪蛋白质激酶1 casein kinase 1;β-catenin:β-连环蛋白质;Tcf/Lef:T细胞因子/淋巴增强因子T-cell factor/lymphoid enhancer factor;follistion:卵泡抑素;Myf5:生肌因子5 myogenic factor 5;Endocytosis of Receptor Complex: 受体复合体的内吞作用; weaken: 减弱;enhance:增强;Membrane clearance of ZNRF3/RNF43:清除膜上ZNRF3/RNF43。 图 1 R-spondin1与膜蛋白质结合启动Wnt/β-catenin信号通路的作用机制 Fig. 1 Mechanisms of R-spondin1 binding to membrane proteins to activate Wnt/β-catenin signaling pathway[25, 44] |
R-spondin1能够增强Wnt3a的活性。R-spondin1介导的β-catenin/T细胞因子(Tcf)信号通路依赖于内源性Wnt3a,相比于单独添加Wnt3a或R-spondin1,Wnt3a和R-spondin1联合处理能够更加显著增加LRP5/6磷酸化水平[45]。增强后的Wnt3a与Fzd和LRP5/6受体结合后,会抑制下游的轴蛋白质(Axin)-结肠腺瘤性息肉病蛋白(APC)-糖原合成酶激酶3β(GSK3β)复合物的形成,从而阻断β-catenin磷酸化,促进胞质内β-catenin转运入细胞核形成β-catenin/Tcf复合物以诱导靶基因转录[46-47]。而细胞表面LRP5/6的水平也能够限制细胞对经典Wnt配体的响应,同时减少c-Myc和β-catenin核内蛋白质水平[48]。另外,R-spondin1能通过与Kremen相互作用干扰Dickkopf相关蛋白1(DKK1)介导的LRP5/6磷酸化降解,导致细胞膜表面LRP5/6水平升高[49]。上述研究表明R-spondin1可通过LRP5/6磷酸化调控经典Wnt信号通路的表达[50-51]。
3.3 R-spondin1与Lgr4/5结合增强Wnt信号R-spondin1主要通过与Lgr4和Lgr5结合,诱导Wnt信号的负反馈调节因子ZNRF3和RNF43的清除,增加膜上可用的Wnt配体数量,从而增强Wnt信号诱导的LRP5/6的磷酸化,以此来介导Wnt/β-catenin信号的增强。ZNRF3能够抑制体内Wnt/β-catenin信号并破坏Wnt/PCP信号传导[43]。Koo等[42]发现在人胚胎肾HEK293T细胞和人结直肠癌HCT116细胞中,ZNRF3和RNF43都有效抑制了Wnt信号的活性。同样,Sun等[52]对肝细胞的研究表明,ZNRF3缺失会促进肝细胞增殖,而肝细胞的增殖也受到了RNF43上调的限制, 在ZNRF3突变的小鼠中同时缺失RNF43会诱导肝细胞的扩增,最终会促进肝脏肿瘤的发生。另外,Lgr4和Lgr5对动物的生长发育必不可少,研究表明,敲除Lgr5会导致新生仔鼠死亡,Lgr4被敲除则会导致小鼠存活率降低和生长迟缓[53]。而R-spondin1功能的发挥依赖Lgr4/5的存在。在HEK293T细胞中,R-spondin1增强了由Wnt3a启动的经典Wnt信号。Lgr4的去除尽管不影响Wnt3a的信号传递,但会抑制R-spondin1的功能增强,而导入重组的Lgr4/5则可以恢复R-spondin1的功能[54-55]。同时,在HEK293T细胞和爪蟾胚胎中,Lgr4和Lgr5可增强R-spondin1介导的Wnt/β-catenin和Wnt/PCP信号通路[56]。Lgr5在骨骼肌再生中是不可或缺的,其阳性细胞可在骨骼肌损伤后重建受损的肌肉纤维,并补充静止的卫星细胞池[57]。
R-spondin1和Lgr4通过与含GTP酶激活蛋白质1的IQ基序(IQ motif containing GTPase-activating protein 1,IQGAP1)支架蛋白质形成超复合物R-spondin1-Lgr-IQGAP1来增强Wnt信号。IQGAP1与Lgr4的7个跨膜结构域(7 transmembrane, 7TM)结合,然后通过丝裂原活化蛋白激酶激酶1/2(MEK1/2)介导的LRP6磷酸化增强β-catenin活化。R-spondin1也能够通过与Lgr4结合以清除膜表面的RNF43/ZNRF3增强Wnt信号活性[58]。因此,R-spondin1对Wnt信号的增强可能是一种双重调控机制,但具体过程还需进一步的研究。
现有报道关于R-spondin1作用机制的解析主要集中于Wnt/β-catenin信号通路。大量数据表明R-spondin1可显著增强Wnt/β-catenin信号通路活性[25-27]。此外,Wnt信号介导的其他支路,如Wnt/Ca2+信号通路是否也受到R-spondin1的调控作用尚不可知。
4 R-spondin1在骨骼肌生长发育中的作用 4.1 R-spondin1启动经典Wnt/β-catenin信号通路调控骨骼肌生长有关R-spondin1通过经典Wnt/β-catenin信号通路调控骨骼肌生长及再生的研究相对较多。近期研究表明,R-spondin1缺失小鼠在急性损伤后的肌肉再生阶段会因Wnt/β-catenin靶基因激活的减少而表现出短暂的延迟生长现象[59]。此外,骨骼肌的再生主要依赖卫星细胞的增殖和分化[13-14]。据报道,R-spondin1基因过表达可以增强C2C12成肌细胞和小鼠原代卫星细胞中碱性螺旋-环-螺旋(basic helix-loop-helix,bHLH)类成肌调节因子生肌因子5(Myf5)的mRNA丰度和蛋白质表达水平[10]。而Myf5与卫星细胞的增殖过程密切相关,但卫星细胞是否参与R-spondin1调控的骨骼肌生长尚不可知[60]。Han等[10]研究表明,在外源R-spondin1基因表达的C2C12细胞(C2C12/R-spondin1HA)中,与对照C2C12细胞的细胞质基质中的β-catenin水平相比,前者的β-catenin水平显著高于后者,表明R-spondin1能够通过激活经典Wnt信号通路提高β-catenin水平,进而激活Myf5调控成肌细胞的增殖。有趣的是,在胫骨前肌中,R-spondin1表达缺失的小鼠肌纤维的数量、大小和横截面积没有改变,同时每条肌纤维的肌核数和整个肌肉的横截面积也没有表现出任何差异[59],这可能是因为肌肉的生成过程是由多因素调控的,其他因素弥补了R-spondin1缺失带来的影响。在卫星细胞的肌源性分化过程中,R-spondin1和R-spondin3表达均增加,而R-spondin2表达保持不变[10]。R-spondin3可能通过补偿效应代替R-spondin1发挥相应的作用,这也可以解释为什么在R-spondin1缺失时机体也会启动卫星细胞的增殖、分化,产生含有更多肌核肌管,而R-spondin家族的另一个成员R-spondin2不仅能够激活Myf5,还能促进C2C12细胞的肌源性分化并促进肌管肥大。研究表明,Myf5的缺失能够导致小鼠原代成肌细胞的早熟分化,而当C2C12细胞中Myf5基因被抑制时,也会抑制成肌细胞融合[10]。上述研究表明,R-spondin1可能通过激活Myf5参与成肌细胞的分化和成肌融合。然而,在过表达Myf5的C2C12细胞中并没有发现肌管融合指数的增加,其具体原因尚待明确。
4.2 R-spondin1或可通过Wnt/PCP信号通路调控骨骼肌生长在肌肉再生过程中,Wnt7a过表达能够显著增强生肌作用,促进肌纤维肥大[15]。同时,重组Wnt7a蛋白也可以被用于治疗杜氏肌营养不良症[18]。对其作用机制进行解析发现Wnt7a是通过激活Wnt/PCP信号通路,诱导卫星细胞的对称分裂,增加卫星细胞数量,更新卫星细胞池,从而提升骨骼肌损伤修复潜力[17]。与之相反,而R-spondin1缺失则会上调Fzd7和RAC1(rac family small GTPase 1)表达,激活Wnt/PCP信号通路以促进成肌细胞的融合[59]。造成两者差异的原因或许与细胞类型或状态有关。另外,当非经典Wnt/PCP信号通路被激活,其关键组分RAC1和RHOA(ras homolog family member A)表达上调, 但并未检测到R-spondin1的功能发挥[10]。这可能是由于R-spondin1是Wnt/PCP信号通路的上游,但该观点还需更多的研究支持。
4.3 R-spondin1通过Wnt信号网络参与骨骼肌生长R-spondin1能够平衡经典和非经典Wnt信号通路之间的关系,参与调控骨骼肌生长发育。据报道,Wnt/β-catenin信号通路能够通过刺激成肌细胞中的卵泡抑素和肌生成素的表达诱导成肌分化和肌细胞融合[61]。Wnt7a能够介导Wnt/PCP信号通路驱动卫星细胞的对称分裂,也能激活蛋白激酶B(Akt)/哺乳动物雷帕霉素靶蛋白(mTOR)信号通路诱导肌纤维肥大[17, 62]。Lacour等[59]以R-spondin1敲除的小鼠为试验模型,证明R-spondin1调控肌细胞融合以促进骨骼肌再生。与野生型细胞相比,R-spondin1缺失小鼠的肌肉祖细胞分化效率降低,但在成肌细胞体外和体内均具有更好的融合效率;此外,R-spondin1缺失将导致Wnt/β-catenin信号下调,抑制成肌细胞分化;研究还发现,非经典Wnt7a/Fzd7/RAC1信号却会上调,并表现为成肌细胞融合比例的增加[59]。造成该结果的原因可能是经典和非经典Wnt信号通路表达时序性不同,也有可能是非经典Wnt信号通路对于经典Wnt信号通路具有补偿作用,当分化被抑制后,机体启动非经典Wnt信号通路,加速已完成成肌分化的细胞参与细胞融合。因此,R-spondin1或可通过调节Wnt信号网络来参与骨骼肌生长或再生的调控,但其具体的作用机制仍需进一步探索。
5 小结与展望目前,有关R-spondin1在骨骼肌生长发育过程中的作用机制仍存在诸多未知,其功能和作用机理研究仍有待挖掘。本课题组已经完成猪R-spondin1基因克隆和表达纯化,并对重组猪R-spondin1蛋白质的活性和功能进行了初步验证,为后续研究奠定了基础[63]。作为Wnt信号网络中重要激活因子,有研究提出R-spondin1在体外培养肌肉组织方面可能会成为一个重要突破方向。此外,R-spondin1与Wnt信号及其他调控因子的联系,将是科研工作者今后考虑的方向。今后,我们将进一步探索R-spondin1调控骨骼肌生长的机制,以期通过介导R-spondin1的表达水平或外源添加R-spondin1调控Wnt信号网络及发现调控骨骼肌生长和修复的新的研究靶点,进而提高肌肉生长潜力,为畜牧业生产提供理论指导。
| [1] |
李伯江, 李平华, 吴望军, 等. 骨骼肌肌纤维形成机制的研究进展[J]. 中国农业科学, 2014(6): 1200-1207. LI B J, LI P H, WU W J, et al. Progresses in research of the mechanisms of skeletal muscle fiber formation[J]. Scientia Agricultura Sinica, 2014(6): 1200-1207 (in Chinese). DOI:10.3864/j.issn.0578-1752.2014.06.017 |
| [2] |
POLESSKAYA A, SEALE P, RUDNICKI M A. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration[J]. Cell, 2003, 113(7): 841-852. DOI:10.1016/S0092-8674(03)00437-9 |
| [3] |
RUDOLF A, SCHIRWIS E, GIORDANI L, et al. β-catenin activation in muscle progenitor cells regulates tissue repair[J]. Cell Reports, 2016, 15(6): 1277-1290. DOI:10.1016/j.celrep.2016.04.022 |
| [4] |
PARISI A, LACOUR F, GIORDANI L, et al. APC is required for muscle stem cell proliferation and skeletal muscle tissue repair[J]. The Journal of Cell Biology, 2015, 210(5): 717-726. DOI:10.1083/jcb.201501053 |
| [5] |
GIRARDI F, LE GRAND F. Chapter five-Wnt signaling in skeletal muscle development and regeneration[J]. Progress in Molecular Biology and Translational Science, 2018, 153: 157-179. |
| [6] |
XU C F, LIU Y J, WANG Y, et al. Downregulation of R-spondin1 contributes to mechanical stretch-induced lung injury[J]. Critical Care Medicine, 2019, 47(7): e587-e596. DOI:10.1097/CCM.0000000000003767 |
| [7] |
LIN Y, FANG Z P, LIU H J, et al. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5+ liver stem cells[J]. Nature Communications, 2017, 8: 1175. DOI:10.1038/s41467-017-01341-6 |
| [8] |
CHEN W, JU S W, LU T, et al. Directional delivery of RSPO1 by mesenchymal stem cells ameliorates radiation-induced intestinal injury[J]. Cytokine, 2017, 95: 27-34. DOI:10.1016/j.cyto.2017.02.004 |
| [9] |
WEBER E L, LAI Y C, LEI M X, et al. Human fetal scalp dermal papilla enriched genes and the role of R-spondin-1 in the restoration of hair neogenesis in adult mouse cells[J]. Frontiers in Cell and Developmental Biology, 2020, 8: 583434. DOI:10.3389/fcell.2020.583434 |
| [10] |
HAN X H, JIN Y R, SETO M, et al. A Wnt/β-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis[J]. Journal of Biological Chemistry, 2011, 286(12): 10649-10659. DOI:10.1074/jbc.M110.169391 |
| [11] |
WANG Y X, RUDNICKI M A. Satellite cells, the engines of muscle repair[J]. Nature Reviews Molecular Cell Biology, 2012, 13(2): 127-133. DOI:10.1038/nrm3265 |
| [12] |
LE GRAND F, JONES A E, SEALE V, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells[J]. Cell Stem Cell, 2009, 4(6): 535-547. DOI:10.1016/j.stem.2009.03.013 |
| [13] |
SCHMIDT M, SCHVLER S C, HVTTNER S S, et al. Adult stem cells at work: regenerating skeletal muscle[J]. Cellular and Molecular Life Sciences, 2019, 76(13): 2559-2570. DOI:10.1007/s00018-019-03093-6 |
| [14] |
LEPPER C, PARTRIDGE T A, FAN C M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration[J]. Development, 2011, 138(17): 3639-3646. DOI:10.1242/dev.067595 |
| [15] |
叶茂, 宋志文, 金成龙, 等. 骨骼肌生长发育及再生过程中Wnt信号网络的作用机制[J]. 动物营养学报, 2020, 32(8): 3560-3567. YE M, SONG Z W, JIN C L, et al. Mechanism of Wnt signaling network in skeletal muscle growth, development and regeneration[J]. Chinese Journal of Animal Nutrition, 2020, 32(8): 3560-3567 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.08.014 |
| [16] |
张宗明, 金成龙, 严会超, 等. Wnt信号调控骨骼肌生长、发育与再生的机制[J]. 动物营养学报, 2019, 31(2): 560-566. ZHANG Z M, JIN C L, YAN H C, et al. Regulatory mechanism of Wnt signaling in skeletal muscle growth, development and regeneration[J]. Chinese Journal of Animal Nutrition, 2019, 31(2): 560-566 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.02.010 |
| [17] |
CHEN X L, LUO Y L, JIA G, et al. The effect of arginine on the Wnt/β-catenin signaling pathway during porcine intramuscular preadipocyte differentiation[J]. Food & Function, 2017, 8(1): 381-386. |
| [18] |
闫研, 张军芳, 孙斌, 等. 添加环格列酮对延边黄牛骨骼肌卫星细胞成脂转分化的影响[J]. 中国畜牧兽医, 2020, 47(3): 714-721. YAN Y, ZHANG J F, SUN B, et al. Effect of ciglitazone on adipogenic transdifferentiation of skeletal muscle satellite cells in Yanbian yellow cattle[J]. China Animal Husbandry & Veterinary Medicine, 2020, 47(3): 714-721 (in Chinese). |
| [19] |
ZHANG Z M, GAO C Q, YAN H C, et al. Wnt/β-catenin pathway is required for porcine satellite cell proliferation and differentiation to promote skeletal muscle growth[J]. Journal of Animal Science, 2019, 97(S3): 97-97. |
| [20] |
YANG Y J, YANG J Z, LIU R X, et al. Accumulation of β-catenin by lithium chloride in porcine myoblast cultures accelerates cell differentiation[J]. Molecular Biology Reports, 2011, 38(3): 2043-2049. DOI:10.1007/s11033-010-0328-3 |
| [21] |
BENTZINGER C F, VON MALTZAHN J, DUMONT N A, et al. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength[J]. The Journal of Cell Biology, 2014, 205(1): 97-111. DOI:10.1083/jcb.201310035 |
| [22] |
VON MALTZAHN J, RENAUD J M, PARISE G, et al. Wnt7a treatment ameliorates muscular dystrophy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(50): 20614-20619. DOI:10.1073/pnas.1215765109 |
| [23] |
BRACK A S, CONBOY I M, CONBOY M J, et al. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis[J]. Cell Stem Cell, 2008, 2(1): 50-59. DOI:10.1016/j.stem.2007.10.006 |
| [24] |
PENG S J, SONG C C, LI H, et al. Circular RNA SNX29 sponges miR-744 to regulate proliferation and differentiation of myoblasts by activating the Wnt5a/Ca2+ signaling pathway[J]. Molecular Therapy: Nucleic Acids, 2019, 16: 481-493. DOI:10.1016/j.omtn.2019.03.009 |
| [25] |
DE LAU W B M, SNEL B, CLEVERS H C. The R-spondin protein family[J]. Genome Biology, 2012, 13(3): 242. DOI:10.1186/gb-2012-13-3-242 |
| [26] |
KIM K A, ZHAO J S, ANDARMANI S, et al. R-spondin proteins: a novel link to β-catenin activation[J]. Cell Cycle, 2006, 5(1): 23-26. DOI:10.4161/cc.5.1.2305 |
| [27] |
KIM K A, WAGLE M, TRAN K, et al. R-spondin family members regulate the Wnt pathway by a common mechanism[J]. Molecular Biology of the Cell, 2008, 19(6): 2588-2596. DOI:10.1091/mbc.e08-02-0187 |
| [28] |
PARMA P, RADI O, VIDAL V, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy[J]. Nature Genetics, 2006, 38(11): 1304-1309. DOI:10.1038/ng1907 |
| [29] |
YAN K S, JANDA C Y, CHANG J L, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal[J]. Nature, 2017, 545(7653): 238-242. DOI:10.1038/nature22313 |
| [30] |
NAM J S, TURCOTTE T J, YOON J K. Dynamic expression of R-spondin family genes in mouse development[J]. Gene Expression Patterns, 2007, 7(3): 306-312. DOI:10.1016/j.modgep.2006.08.006 |
| [31] |
LI N, LIU S, ZHANG H S, et al. Exogenous R-spondin1 induces precocious telogen-to-anagen transition in mouse hair follicles[J]. International Journal of Molecular Sciences, 2016, 17(4): 582. DOI:10.3390/ijms17040582 |
| [32] |
SHI G X, ZHENG X F, ZHU C, et al. Evidence of the role of R-spondin 1 and its receptor Lgr4 in the transmission of mechanical stimuli to biological signals for bone formation[J]. International Journal of Molecular Sciences, 2017, 18(3): 564. DOI:10.3390/ijms18030564 |
| [33] |
GORE A V, SWIFT M R, CHA Y R, et al. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3[J]. Development, 2011, 138(22): 4875-4886. DOI:10.1242/dev.068460 |
| [34] |
CHAHAL J K, WONG V S C, CHABOISSIER M C, et al. R-spondin1 deficiency enhances β-cell neogenesis in a murine model of diabetes[J]. Pancreas, 2014, 43(1): 93-102. DOI:10.1097/MPA.0b013e3182a70bfb |
| [35] |
NAM J S, PARK E, TURCOTTE T J, et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb[J]. Developmental Biology, 2007, 311(1): 124-135. DOI:10.1016/j.ydbio.2007.08.023 |
| [36] |
BELL S M, SCHREINER C M, WERT S E, et al. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis[J]. Development, 2008, 135(6): 1049-1058. DOI:10.1242/dev.013359 |
| [37] |
CADIEU E, NEFF M W, QUIGNON P, et al. Coat variation in the domestic dog is governed by variants in three genes[J]. Science, 2009, 326(5949): 150-153. DOI:10.1126/science.1177808 |
| [38] |
AOKI M, MIEDA M, IKEDA T, et al. R-spondin3 is required for mouse placental development[J]. Developmental Biology, 2007, 301(1): 218-226. DOI:10.1016/j.ydbio.2006.08.018 |
| [39] |
KAZANSKAYA O, OHKAWARA B, HEROULT M, et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development[J]. Development, 2008, 135(22): 3655-3664. DOI:10.1242/dev.027284 |
| [40] |
BERGMANN C, SENDEREK J, ANHUF D, et al. Mutations in the gene encoding the Wnt-signaling component R-spondin 4(RSPO4) cause autosomal recessive anonychia[J]. American Journal of Human Genetics, 2006, 79(6): 1105-1109. DOI:10.1086/509789 |
| [41] |
CROCE J C, MCCLAY D R. Evolution of the Wnt pathways[J]. Methods in Molecular Biology, 2008, 469: 3-18. |
| [42] |
KOO B K, SPIT M, JORDENS I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors[J]. Nature, 2012, 488(7413): 665-669. DOI:10.1038/nature11308 |
| [43] |
HAO H X, XIE Y, ZHANG Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner[J]. Nature, 2012, 485(7397): 195-200. DOI:10.1038/nature11019 |
| [44] |
NAGANO K. R-spondin signaling as a pivotal regulator of tissue development and homeostasis[J]. The Japanese Dental Science Review, 2019, 55(1): 80-87. DOI:10.1016/j.jdsr.2019.03.001 |
| [45] |
CARMON K S, LIN Q S, GONG X, et al. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/β-catenin signaling[J]. Molecular and Cellular Biology, 2012, 32(11): 2054-2064. DOI:10.1128/MCB.00272-12 |
| [46] |
BEJSOVEC A. Wnt pathway activation: new relations and locations[J]. Cell, 2005, 120(1): 11-14. |
| [47] |
GORDON M D, NUSSE R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors[J]. Journal of Biological Chemistry, 2006, 281(32): 22429-22433. DOI:10.1074/jbc.R600015200 |
| [48] |
CHEN C, XIANG H, PENG Y L, et al. Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/β-catenin signaling pathway by targeting LRP6[J]. Cellular Signalling, 2014, 26(6): 1155-1165. DOI:10.1016/j.cellsig.2014.02.003 |
| [49] |
MAO B Y, WU W, DAVIDSON G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling[J]. Nature, 2002, 417(6889): 664-667. DOI:10.1038/nature756 |
| [50] |
BINNERTS M E, KIM K A, BRIGHT J M, et al. R-spondin1 regulates Wnt signaling by inhibiting internalization of LRP6[J]. Proceedings of the National Academy of Sciences, 2007, 104(37): 14700-14705. |
| [51] |
WEI Q O, YOKOTA C, SEMENOV M V, et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling[J]. Journal of Biological Chemistry, 2007, 282(21): 15903-15911. |
| [52] |
SUN T L, ANNUNZIATO S, BERGLING S, et al. ZNRF3 and RNF43 cooperate to safeguard metabolic liver zonation and hepatocyte proliferation[J/OL]. Cell Stem Cell, 2021: S1934-5909(21)00250. (2021-06-11). https://pubmed.ncbi.nlm.nih.gov/34129813.DOI: 10.1016/j.stem.2021.05.013.
|
| [53] |
CARMON K S, GONG X, LIN Q S, et al. R-spondins function as ligands of the orphan receptors Lgr4 and Lgr5 to regulate Wnt/beta-catenin signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(28): 11452-11457. |
| [54] |
DE LAU W, BARKER N, LOW T Y, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling[J]. Nature, 2011, 476(7360): 293-297. |
| [55] |
PARK S, CUI J, YU W S, et al. Differential activities and mechanisms of the four R-spondins in potentiating Wnt/β-catenin signaling[J]. Journal of Biological Chemistry, 2018, 293(25): 9759-9769. |
| [56] |
GLINKA A, DOLDE C, KIRSCH N, et al. Lgr4 and Lgr5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling[J]. EMBO Reports, 2011, 12(10): 1055-1061. |
| [57] |
LEUNG C, MURAD K B A, TAN A L T, et al. Lgr5 marks adult progenitor cells contributing to skeletal muscle regeneration and sarcoma formation[J]. Cell Reports, 2020, 33(12): 108535. |
| [58] |
CARMON K S, GONG X, YI J, et al. RSPO-Lgr4 functions via IQGAP1 to potentiate Wnt signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(13): E1221-E1229. |
| [59] |
LACOUR F, VEZIN E, BENTZINGER C F, et al. R-spondin1 controls muscle cell fusion through dual regulation of antagonistic Wnt signaling pathways[J]. Cell Reports, 2017, 18(10): 2320-2330. |
| [60] |
TAJBAKHSH S, BORELLO U, VIVARELLI E, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5[J]. Development, 1998, 125(21): 4155-4162. |
| [61] |
JONES A E, PRICE F D, LE GRAND F, et al. Wnt/β-catenin controls follistatin signalling to regulate satellite cell myogenic potential[J]. Skeletal Muscle, 2015, 5: 14. |
| [62] |
VON MALTZAHN J, BENTZINGER C F, RUDNICKI M A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle[J]. Nature Cell Biology, 2012, 14(2): 186-191. |
| [63] |
朱秋杰. 猪RSPO1基因克隆表达及功能验证[D]. 硕士学位论文. 广州: 华南农业大学, 2020. ZHU Q J. Cloning expression and function verification of RSPO1 gene for pigs[D]. Master's Thesis. Guangzhou: South China Agricultural University, 2020. |




