2. 天津农学院动物科学与动物医学学院, 天津市农业动物繁育与健康养殖重点实验室, 天津 300384
2. Tianjin Key Laboratory of Agricultural Animal Breeding and Healthy Husbandry, College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384, China
细胞和组织氧化应激反应的产生是由于其细胞内氧化和抗氧化系统失衡,导致细胞内活性氧(reactive oxygen species,ROS)过量。氧化应激可以破坏肠内稳态,是肠损伤发生的关键因素[1]。应激可诱导肠细胞产生大量ROS代谢物,影响细胞内的核酸、蛋白质和脂质稳定性,增加细胞凋亡,导致肠黏膜损伤[2]。有研究发现,断奶会破坏仔猪机体的氧化平衡,从而产生氧化应激[3],致使断奶仔猪小肠细胞周期阻滞和细胞凋亡[4-5]。氧化应激已被证实参与肠道屏障功能障碍和各种消化道疾病的发生,其对肠道形态的不良影响还伴随着紧密连接蛋白表达的下调[6]。猪霍乱沙门氏菌(Salmonella choleraesuis,SC)属于革兰氏阴性菌,作为一种人畜共患病原体可以侵入宿主并导致败血症的发生,并且耐药性沙门氏菌等在疾病中出现的概率增加[7]。当SC侵入肠道黏膜后,通过分泌免疫球蛋白A(IgA)、抗菌肽和消化酶等逃避宿主肠腔防御机制[8]。沙门氏菌黏附在上皮细胞的表面后,通过介导内吞作用侵入肠上皮的M细胞、上皮细胞和树突状细胞等非吞噬性肠细胞[9],并且在病原体繁殖时产生大量内毒素,内毒素作用于白细胞进而引起炎症、氧化应激,导致机体高烧和腹泻。有研究表明,沙门氏菌黏附到肠上皮细胞后,宿主防御机制被激活,这种早期促炎状态可以通过激活微生物特异性Toll样受体(TLRs)来启动,TLRs激活核转录因子-κB(NF-κB)、丝裂原活化蛋白激酶(MAPK)和含半胱氨酸的天冬氨酸蛋白水解酶(Caspase)依赖性信号通路[10]。
核因子E2相关因子(Nrf2)/抗氧化反应元件(ARE)信号通路是细胞内最重要的抗氧化应激通路之一,在氧化应激条件下,细胞核中Nrf2积聚,Nrf2与ARE结合后启动下游一系列保护性基因的表达,如谷胱甘肽过氧化物酶(GPX)、超氧化物歧化酶(SOD)等[11]。有研究表明,p38 MAPK可以将Nrf2中的3个丝氨酸残基(Ser215、Ser408和Ser577)磷酸化,这促进了Nrf2核积累的减少[12-13]。丁酸梭菌(Clostridium butyricum,CB)是一种革兰氏阳性菌,存在于人和动物胃肠道中[14]。据报道,CB能够改善肠道环境、促进宿主健康[14],其作用机制主要包括:1)在肠道内通过发酵产生短链脂肪酸、B族维生素、多种消化酶等益生物质,为肠道上皮细胞提供能量,促进肠道对营养物质的消化与吸收[15-16];2)通过与致病菌竞争营养物质及黏膜定植位点,阻止病原菌生长与定植[17];3)通过调节肠道微生物及其代谢产物(如丁酸、乙酸、丙酸),降低肠道pH,保护肠道免受致病菌感染[18-20];4)通过TLRs介导的免疫信号通路调控免疫细胞分泌促炎细胞因子(如白细胞介素-6)和抑炎细胞因子(如白细胞介素-10),提高机体免疫力[21-22]。此外,有研究表明,CB能促进小鼠机体中Nrf2的表达,缓解氧化应激[23]。但CB能否缓解SC对机体造成的影响尚不清晰,因此,本研究以猪小肠上皮细胞(IPEC-J2细胞)为模型,以p38 MAPK和Nrf2信号通路为切入点,探究CB缓解SC导致氧化损伤的分子机制。
1 材料与方法 1.1 试验菌株和细胞SC来自中国兽医微生物菌种保藏管理中心,保存于天津农学院动物科学与动物医学学院实验室。CB为健康仔猪粪便中分离菌株,已通过菌落形态、革兰氏染色和16S rDNA核苷酸序列确定其为CB。试验前,将活化的SC接种到营养肉汤,37 ℃静置培养24 h;将活化的CB接种到液体强化梭菌培养基(RCM),37 ℃静置培养48 h;以菌液:甘油(体积比1 ∶ 1)保存于-80 ℃冰箱中,用于后续试验。分别用营养琼脂和琼脂RCM对SC和CB进行活菌计数。
IPEC-J2细胞购买于上海冠导生物工程有限公司。在37 ℃、5%二氧化碳(CO2)的条件下,用含89% RPMI Medium 1640基础培养基、10%胎牛血清(BSA)和1%青链霉素的完全培养基进行培养。
1.2 试验试剂RPMI Medium 1640基础培养基、胎牛血清和胰蛋白酶乙二胺四乙酸(EDTA)消化液均购于美国Gibco公司。青链霉素和二甲基亚砜(DMSO)均购自北京索莱宝生物公司。Lipofectamine2000转染试剂盒和Opti-MEM购自美国Invitrogen公司。细胞增殖-毒性检测试剂盒(CCK-8)购自东仁化学科技有限公司。丙二醛(MDA)、GPX和SOD酶联免疫吸附检测(ELISA)试剂盒均购自南京建成生物工程研究所。反转录试剂盒和实时荧光定量PCR(RT-qPCR)试剂盒均购自美国Gene Copoeia公司。抗p38 MAPK抗体购买于美国Cell Signaling Technology公司。抗Nrf2抗体和抗甘油醛-3-磷酸脱氢酶(GAPDH)抗体均购自英国Abcam公司。
1.3 试验设计 1.3.1 试验1:SC适宜剂量选择将浓度为1×104个/mL的IPEC-J2细胞悬液接种于96孔板中,每孔100 μL,培养24 h后进行以下处理,即分为对照组(添加100 μL基础培养基)、SC1组(添加1×101 CFU/mL SC)、SC2组(添加1×102 CFU/mL SC)、SC3组(添加1×103 CFU/mL SC)和SC4组(添加1×104 CFU/mL SC),每组3个重复。分别培养3、6、12、18和24 h后,检测细胞活力及培养上清液中SOD、GPX活性和MDA含量。
1.3.2 试验2:CB对SC感染IPEC-J2细胞后p38 MAPK/Nrf2信号通路活化的影响将浓度为1×105个/mL的IPEC-J2细胞接种至6孔细胞培养板,做不同处理。CB选用感染复数(MOI)为50、孵育时间为3 h进行后续试验[24]。试验分为3组,即对照组(添加基础培养基)、SC组(添加1×103 CFU/mL SC)和CB+SC组(CB孵育3 h和添加1×103 CFU/mL SC),每组3个重复。待SC分别处理3、6、12和24 h后收集细胞并裂解,检测细胞中Nrf2、SOD1、SOD2、GPX1、GPX2和GAPDH mRNA相对表达量,并在SC处理12 h后检测细胞中Nrf2和p38 MAPK蛋白相对表达量。
1.3.3 试验3:Nrf2信号通路在CB影响SC感染IPEC-J2细胞氧化损伤中的作用猪Nrf2的小干扰RNA(siRNA)序列:正向5′-GCCCAUGAUGUCUCUGUTT-3′,反向5′-AUCAGAGAUGAUGGCTT-3′,引物由上海生工生物工程有限公司合成。在进行试验之前,以Nrf2非特异性siRNA作为阴性对照(NC),检测了Nrf2特异性siRNA的转染效率,发现Nrf2非特异性siRNA对Nrf2的表达没有影响,而Nrf2特异性siRNA能够显著下调Nrf2的表达,因此,可以采用Nrf2特异性siRNA进行后续细胞转染试验。将浓度为1×105个/mL的IPEC-J2细胞接种至6孔细胞培养板,试验分为7组,即对照组(添加基础培养基)、NC组(Nrf2非特异性siRNA单独处理细胞6 h)、siRNA组(siRNA单独处理细胞6 h)、NC+SC组(先进行Nrf2非特异性siRNA处理细胞6 h,再进行1×103 CFU/mL SC处理细胞12 h)、siRNA+SC组(先进行siRNA处理细胞6 h,再进行1×103 CFU/mL SC处理细胞12 h)、NC+CB+SC组(先进行Nrf2非特异性siRNA处理细胞6 h,再进行CB孵育3 h,最后进行1×103 CFU/mL SC处理细胞12 h)和siRNA+CB+SC组(先进行siRNA处理细胞6 h,再进行CB孵育3 h,最后进行1×103 CFU/mL SC处理细胞12 h),每组3个重复。待SC处理12 h后,收集细胞及其培养上清液,检测上清液中SOD、GPX活性以及细胞中SOD1、SOD2、GPX1、GPX2和Nrf2 mRNA相对表达量。
1.3.4 试验4:p38 MAPK信号通路在CB影响SC感染IPEC-J2细胞氧化损伤中的作用用DMSO溶解SB-203580(p38 MAPK抑制剂),制备终浓度为50 μmol/L的p38 MAPK抑制剂。将IPEC-J2细胞接种至6孔细胞培养板中,待细胞贴壁后,试验分为7组,即对照组(添加基础培养基)、DMSO组(50 μmol/L DMSO处理细胞1 h)、p38组(p38 MAPK抑制剂处理细胞1 h)、DMSO+SC组(50 μmol/L DMSO处理细胞1 h后再用SC感染细胞12 h)、p38+SC组(p38 MAPK抑制剂处理细胞1 h后再用SC感染细胞12 h)、DMSO+CB+SC组(50 μmol/L DMSO处理细胞1 h后,CB孵育细胞3 h,SC感染细胞12 h)和p38+CB+SC组(p38 MAPK抑制剂处理细胞1 h后,CB孵育细胞3 h,SC感染细胞12 h),每组3个重复。待SC处理12 h后,收集细胞及培养上清液,检测上清液中SOD、GPX活性以及细胞中p38 MAPK、SOD1、SOD2、GPX1、GPX2和Nrf2的mRNA相对表达量。
1.4 细胞活力分析细胞在培养特定时间后,用磷酸盐缓冲液(PBS)清洗,每孔加入90 μL RPMI 1640基础培养基和10 μL CCK-8孵育2 h,用酶标仪检测波长为450 nm的吸光度并计算细胞活力。
1.5 细胞培养上清中GPX、SOD活性和MDA含量检测严格按照制造商说明书要求分别检测培养上清液中SOD、GPX活性和MDA含量。
1.6 RT-qPCR将收集的细胞裂解,严格按照说明书使用TRIzol试剂提取细胞中的RNA。运用NanoDrop 1000超微量紫外分光光度计测定RNA的含量和纯度。根据反转录试剂盒说明书要求将RNA逆转录成cDNA后进行qPCR。引物序列信息见表 1,猪GPX2特异性引物通过在NCBI获取其基因的全序列,使用Primer 5.0设计。采用Light Cycler® 480 Real-Time PCR检测目的基因mRNA的Ct值并用2-ΔΔCt法计算p38 MAPK、Nrf2、SOD1、SOD2、GPX1、GPX2和GAPDH的mRNA相对表达量。
|
|
表 1 引物序列信息 Table 1 Primer sequence information |
将细胞裂解后,与上样缓冲液充分混匀,煮沸10 min,获得蛋白样品,采用蛋白定量法测定蛋白总量。运用12%变性十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离蛋白,将目的蛋白转印至聚偏氟乙烯(PVDF)膜上,参照Qiao等[31]的方法进行Western blotting试验。运用Image软件来检测相关蛋白的灰度值(相对灰度值=相关蛋白的灰度值/内参GAPDH灰度值),检测Nrf2、p38 MAPK和GAPDH蛋白相对表达量。
1.8 数据处理与分析试验结果使用Excel 2016软件对数据进行初步处理,采用SPSS 20.0进行单因素方差分析(one-way ANOVA),并通过Graphpad Prism 7.0软件制作柱形图,P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果与分析 2.1 SC适宜剂量选择由图 1可知,同一处理时间,与对照组相比,其他各组细胞活力均显著或极显著下降(P < 0.05或P < 0.01),且呈剂量依赖性。在SC处理3 h后,与SC2组相比,SC1组和SC3组细胞活力没有显著差异(P>0.05);SC4组细胞活力显著低于SC1组和SC2组(P < 0.05)。在SC处理12 h后,SC2组和SC3组细胞活力没有显著差异(P>0.05),且与SC1组和SC4组存在极显著差异(P < 0.01)。在SC处理6、18和24 h后,SC1组、SC2组、SC3组和SC4组之间细胞活力均存在显著或极显著差异(P < 0.05或P < 0.01)。相同浓度的SC,随处理时间的延长,细胞活力呈现下降趋势。细胞培养上清液检测结果表明,不同浓度的SC在同一处理时间内,与对照组相比,随着SC浓度的增加,MDA含量(3 h的SC1组除外)均呈显著或极显著上升(P < 0.05,P < 0.01),抗氧化酶SOD、GPX活性呈极显著下降(P < 0.01),并且每个时间点SC4组MDA含量最多,SOD、GPX活性最低。相同浓度的SC在不同处理时间内,MDA含量增加,SOD、GPX活性降低,且均呈时间依赖性。试验结果表明,SC3组细胞活力显著降低,且在此浓度长时间处理细胞后对细胞损伤较小。因此,选择1×103 CFU/mL作为SC的适宜剂量进行后续试验。
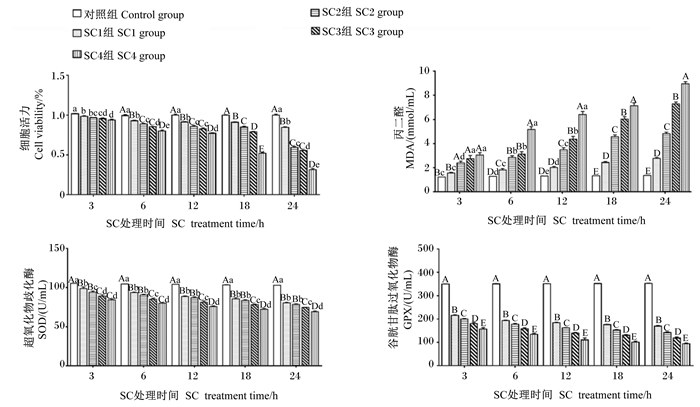
|
数据柱形标注不同小写字母表示差异显著(P < 0.05),不同大写字母表示差异极显著(P < 0.01),相同小写字母或无字母表示差异不显著(P>0.05)。下图同。 Value columns with different small letters mean significant difference (P < 0.05), with different capital letters mean extremely significant difference (P < 0.01), while with the same small letter or no letter superscripts mean no significant difference (P>0.05). The same as below. 图 1 SC适宜剂量选择 Fig. 1 SC Appropriate dose selection |
由图 2可知,SC处理细胞3 h后,与对照组相比,SC组Nrf2和GPX1的mRNA相对表达量极显著降低(P < 0.01),GPX2的mRNA相对表达量极显著升高(P < 0.01),然而SOD1和SOD2的mRNA相对表达量没有显著变化(P>0.05);与SC组相比,CB+SC组的SOD1和SOD2的mRNA相对表达量没有显著变化(P>0.05),Nrf2和GPX1的mRNA相对表达量极显著升高(P < 0.01),GPX2的mRNA相对表达量极显著降低(P < 0.01)。SC处理细胞6 h后,与对照组相比,SC组除SOD1的mRNA相对表达量没有显著变化(P>0.05),其他基因的相对表达量均极显著降低(P < 0.01);与SC组相比,CB+SC组Nrf2、SOD2和GPX2的mRNA相对表达量均极显著升高(P < 0.01),SOD1和GPX1的mRNA相对表达量没有显著变化(P>0.05)。SC处理细胞12和24 h后,与对照组相比,SC组Nrf2、SOD1、SOD2、GPX1和GPX2的mRNA相对表达量均显著或极显著降低(P < 0.05或P < 0.01);与SC组相比,CB+SC组Nrf2、SOD1、SOD2、GPX1和GPX2的mRNA相对表达量均极显著升高(P < 0.01)。此外,Western blotting结果显示,SC处理细胞12 h后,与对照组相比,SC组Nrf2和p38 MAPK蛋白相对表达量极显著降低(P < 0.01);与SC组相比,CB+SC组Nrf2和p38 MAPK蛋白相对表达量极显著升高(P < 0.01)。试验结果表明,与SC组相比,CB+SC组Nrf2及其下游基因在12 h后的mRNA相对表达量均极显著升高,CB预处理能缓解SC诱导的细胞内抗氧化酶mRNA相对表达量的降低。因此,选用12 h作为SC处理细胞的时间点,进行后续试验。
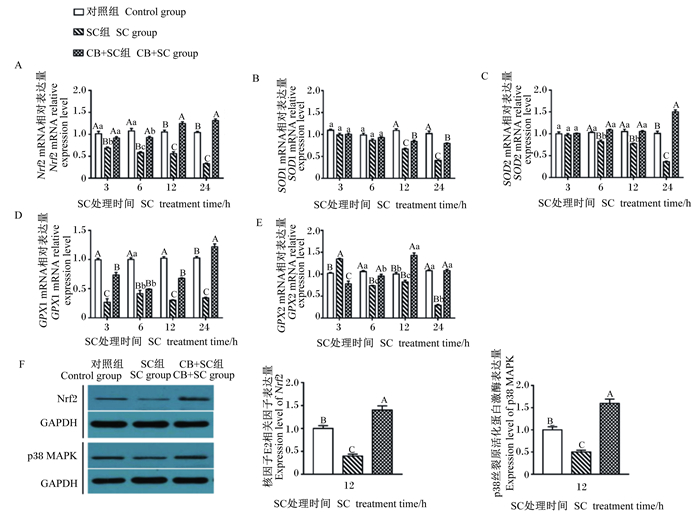
|
Nrf2:核因子E2相关因子NF-E2-related factor 2;GAPDH:甘油醛-3-磷酸脱氢酶glyceraldehyde-3-phosphate dehydrogenase;p38 MAPK:p38丝裂原活化蛋白激酶p38 mitogen activated protein kinase。 图 2 CB对SC感染IPEC-J2细胞内p38 MAPK/Nrf2信号通路相关基因表达的影响 Fig. 2 Effects of CB on expression of genes related to p38 MAPK/Nrf2 signaling pathway in SC-infected IPEC-J2 cells |
用siRNA沉默IPEC-J2细胞中Nrf2的表达,采用ELISA方法检测细胞培养上清液中SOD和GPX活性,RT-qPCR方法检测细胞中Nrf2及其下游基因的mRNA相对表达量,以此确定CB调控致病菌感染引起的IPEC-J2细胞氧化损伤是否与Nrf2信号通路的活化有关。由表 2和图 3可知,与对照组相比,NC组培养上清液中SOD和GPX活性以及细胞中Nrf2、SOD1、SOD2和GPX1的mRNA相对表达量均无显著变化(P>0.05),NC+SC组和siRNA+SC组培养上清液中SOD和GPX活性以及细胞中Nrf2相关基因的mRNA相对表达量均显著或极显著降低(P < 0.05或P < 0.01)。与NC+SC组相比,siRNA+SC组培养上清液中GPX活性以及细胞中Nrf2、SOD1、GPX1和GPX2的mRNA相对表达量显著降低(P < 0.05);NC+CB+SC组培养上清液中SOD活性极显著降低(P < 0.01),GPX活性极显著升高(P < 0.01),细胞中Nrf2相关基因的mRNA相对表达量均显著升高(P < 0.05);siRNA+CB+SC组仅GPX2的mRNA相对表达量显著升高(P < 0.05)。与siRNA+SC组相比,NC+CB+SC和siRNA+CB+SC组培养上清液中GPX活性以及细胞中Nrf2相关基因的mRNA相对表达量均显著升高(P < 0.05),并且与siRNA+CB+SC组相比,NC+CB+SC组培养上清液中GPX活性以及细胞中Nrf2、SOD1、GPX1和GPX2均显著或极显著升高(P < 0.05或P < 0.01)。试验结果表明,Nrf2信号通路的下调在一定程度上加重了SC诱导的IPEC-J2细胞氧化损伤,CB可通过上调Nrf2信号通路缓解这种损伤,且用siRNA沉默Nrf2表达后CB的益生作用会有所降低。
|
|
表 2 Nrf2信号通路在CB缓解SC诱导的IPEC-J2细胞氧化损伤中的积极作用 Table 2 Positive role of Nrf2 signaling pathway in CB alleviating SC-induced oxidative damage in IPEC-J2 cells |
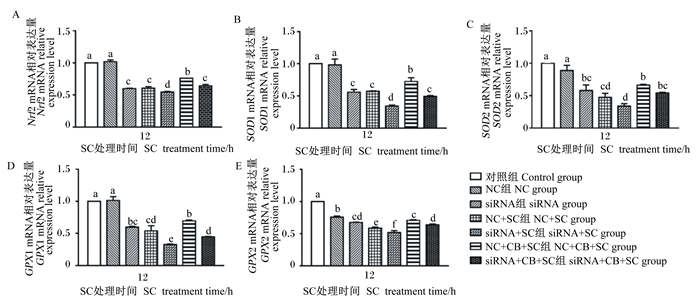
|
图 3 Nrf2信号通路在CB缓解SC诱导的IPEC-J2细胞氧化损伤中的积极作用 Fig. 3 Positive role of Nrf2 signaling pathway in CB alleviating SC-induced oxidative damage in IPEC-J2 cells |
由图 4可知,与对照组相比,DMSO组p38 MAPK的mRNA相对表达量显著上升(P < 0.05);p38组p38 MAPK的mRNA相对表达量显著下降(P < 0.05),DMSO+SC组和p38+SC组p38 MAPK的mRNA相对表达量亦显著下降(P < 0.05)。与DMSO+SC和p38+SC组分别相比,DMSO+CB+SC组和p38+CB+SC组p38 MAPK的mRNA相对表达量均显著升高(P < 0.05)。试验结果表明,CB能上调SC造成的p38 MAPK mRNA相对表达量的下降,并对Nrf2信号通路起到激活作用。
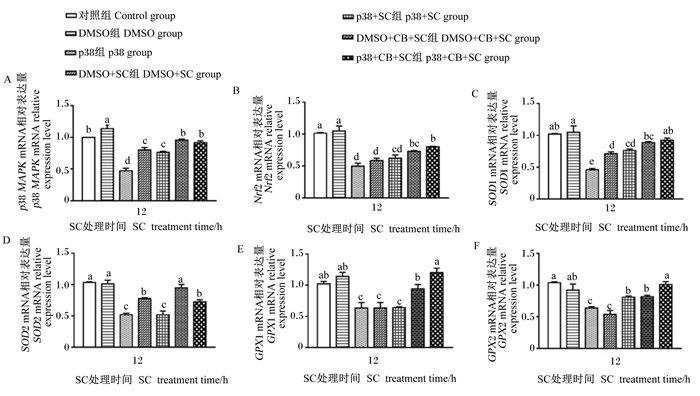
|
图 4 p38 MAPK信号通路在CB缓解SC诱导的IPEC-J2细胞氧化损伤中的积极作用 Fig. 4 Positive role of p38 MAPK signaling pathway in CB alleviating SC-induced oxidative damage in IPEC-J2 cells |
用抑制剂抑制IPEC-J2细胞中p38 MAPK的活化后,采用ELISA方法检测细胞培养上清液中SOD和GPX活性,RT-qPCR方法检测细胞中Nrf2及其下游抗氧化酶基因的相对表达量来检测p38 MAPK信号通路对Nrf2信号通路的积极作用。由表 3和图 4可知,与对照组相比,DMSO组培养上清液中SOD和GPX活性以及细胞中Nrf2相关基因的mRNA相对表达量无显著变化(P>0.05);p38组、DMSO+SC组和p38+SC组培养上清液中SOD和GPX活性以及细胞中Nrf2相关基因的mRNA相对表达量均显著或极显著下降(P < 0.05或P < 0.01)。与DMSO+SC组相比,DMSO+CB+SC组和p38+CB+SC组培养上清液中GPX活性以及细胞中SOD1、GPX1、GPX2和Nrf2的mRNA相对表达量均显著或极显著升高(P < 0.05或P < 0.01),仅DMSO+CB+SC组细胞中SOD2的mRNA相对表达量显著升高(P < 0.05)。与p38+SC组相比,DMSO+CB+SC组培养上清液中GPX活性以及细胞中SOD2和GPX1的mRNA相对表达量显著或极显著升高(P < 0.05或P < 0.01);p38+CB+SC组培养上清液中GPX活性以及细胞中Nrf2相关基因的mRNA相对表达量均显著或极显著升高(P < 0.05或P < 0.01)。试验结果表明,p38 MAPK信号通路的下调会造成Nrf2及其下游基因的相对表达量降低,CB可通过上调p38 MAPK通路缓减SC诱导的IPEC-J2细胞氧化损伤。
|
|
表 3 p38 MAPK信号通路在CB缓解SC诱导的IPEC-J2细胞氧化损伤中的积极作用 Table 3 Positive role of p38 MAPK signaling pathway in CB alleviating SC-induced oxidative damage in IPEC-J2 cells |
活性氧(ROS)包括超氧阴离子(O2-)、羟基自由基(·OH)和过氧化氢(H2O2),这些分子主要来源于在线粒体、过氧化物酶体和内质网(ER)中发生的各种代谢反应中消耗的氧气[32]。细胞内ROS水平的调节对细胞内稳态至关重要,过量的ROS会损害细胞成分,如导致DNA损伤、脂类过氧化水平升高、蛋白质损伤等[33],因此这些损伤的生物大分子可作为氧化应激的评估指标,例如MDA就是一种脂质过氧化产物,是反映氧化损伤的典型参数[34]。
CB对动物具有多种有益作用,包括缓解氧化应激[35]、平衡肠道菌群[36]、调节脂质代谢[37]、改善神经功能缺损[38]等。有研究表明,浓度为5×108 CFU/mL的CB预处理可降低不同胃溃疡模型小鼠血清中MDA含量,提高胃组织中SOD和过氧化氢酶(CAT)活性[39]。四氯化碳诱导小鼠急性肝损伤后,小鼠肝脏中MDA含量增加,CB预处理后增加了肝脏中SOD和CAT活性,并降低了MDA含量,另外还增加了Nrf2含量[23, 35]。本试验通过检测p38 MAPK、Nrf2、SOD1、SOD2、GPX1、GPX2的mRNA相对表达量来说明CB对IPEC-J2细胞的保护作用,研究发现,在SC处理12 h后,CB预处理能够显著提高IPEC-J2细胞p38 MAPK、Nrf2、SOD1、SOD2、GPX1和GPX2的mRNA相对表达量。研究发现,Nrf2基因敲除的小鼠更容易受到氧化应激导致肿瘤发生以及加重肥胖带来的有害影响[40-41]。目前,有关沉默Nrf2的表达对IPEC-J2细胞影响的研究尚未见报道。本研究用siRNA干扰细胞中Nrf2的表达后发现,培养上清液中GPX活性以及细胞中Nrf2、SOD1、SOD2、GPX1和GPX2的mRNA相对表达量均显著降低,加入SC处理细胞后,上清液中抗氧化酶活性和细胞中各基因的mRNA相对表达量均显著降低,而添加CB后能显著增加细胞中Nrf2、SOD1、SOD2、GPX1和GPX2的mRNA相对表达量,表明CB可通过Nrf2信号通路减轻致病菌造成的IPEC-J2细胞损伤。
MAPK在细胞分化、有丝分裂和凋亡等多种细胞活动中发挥一定作用而受到了广泛关注[42]。MAPK信号通路还参与很多生物学过程,如调节氧化应激、炎症、凋亡和细胞增殖等,与肠上皮屏障功能密切相关[43]。MAPK信号通路由三级激酶组成,包括丝裂原活化蛋白激酶激酶激酶(MAPKKK)、丝裂原活化蛋白激酶激酶(MAPKK)和MAPK,依次被激活,实现信号从细胞表面向细胞核传递,诱导下游的信号转导,调控细胞的生长、分化、增殖和炎症反应[44-45]。MAPK通路由细胞外调节蛋白激酶(ERK)1和ERK2,p38 MAPK,C-Jun氨基末端激酶(JNK)1、JNK2和JNK3,以及ERK5共4种分支组成[45-46]。其中,p38 MAPK信号通路与炎症和免疫反应密切相关,是促炎性细胞因子合成的关键调控路径[47]。ERK1和ERK2、p38 MAPKs以及JNK1、JNK2和JNK3是研究最广泛的MAPK[47]。在MAPK中,p38 MAPK参与了一系列的信号通路,这些通路刺激了多种不同的生物功能[48]。p38 MAPK对炎症细胞因子和ROS等应激刺激有反应。有研究发现磷酸化p38 MAPK蛋白水平在致病菌诱导的断奶仔猪腹泻中显著升高,表明p38 MAPK对致病菌诱导的仔猪腹泻有重要贡献[49]。Nrf2启动抗氧化基因转录的活性也受到p38 MAPK的正调控[50]。本研究发现,CB能上调SC造成的p38 MAPK的mRNA相对表达量的下降,并对p38 MAPK信号通路起到激活作用。另外,本研究通过抑制p38 MAPK的表达后,检测p38 MAPK信号通路对Nrf2信号通路的积极作用。试验结果表明,p38 MAPK抑制剂单独处理、DMSO与SC处理和p38 MAPK抑制剂与SC处理均会降低上清液中抗氧化酶的活性以及细胞中Nrf2及其下游基因的mRNA相对表达量,而CB预处理后上调致病菌造成的抗氧化基因的mRNA相对表达量降低。这表明p38 MAPK信号通路下调会造成Nrf2及其下游基因的相对表达量降低,加重SC造成的IPEC-J2细胞氧化损伤,CB可通过上调p38 MAPK通路缓解这种损伤。
微生物在消化道中定植离不开其细胞壁成分与肠道黏膜之间的相互作用[51]。据报道,金黄色葡萄球菌细胞壁成分(脂磷壁酸)能够诱导HT-29细胞炎症反应和凋亡,而CB的脂磷壁酸可以缓解这一现象[52]。Zhao等[53]研究证实了CB可以通过其细胞壁成分和丁酸等代谢物降低Caco-2细胞脂肪的生成。此外,有研究发现CB能够通过激活G蛋白偶联受体(GPRs)抑制肠道肿瘤细胞增殖[54]。值得注意的是,丁酸可以通过与其受体(如GPR41、GPR43、GPR109A)结合来影响肠道内多种信号通路[55]。例如,丁酸能够激活GPR109A,抑制NF-κB信号通路,诱导肿瘤细胞凋亡[56],并且丁酸在进入细胞后会分解产生氢离子[57],激活钠离子(Na+)/氢离子(H+)交换系统,维持细胞内外pH稳定,抑制病原体增殖,维持肠道微生物平衡[58];同时H+可以通过与自由基的结合,增强了机体抗氧化酶的活性,减轻氧化应激,提高机体抗氧化能力[59]。以上研究表明,CB发挥益生作用可能依赖于其细胞壁成分及代谢产物。然而,具体的某种细胞壁成分或代谢产物所具有的益生作用及其机制仍然需要进行更深入的研究。
4 结论综上所述,CB能够通过上调p38 MAPK的表达,激活Nrf2信号通路,进而介导下游抗氧化相关基因的表达,缓解SC诱导的IPEC-J2细胞氧化损伤。
| [1] |
BHATTACHARYYA A, CHATTOPADHYAY R, MITRA S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases[J]. Physiological Reviews, 2014, 94(2): 329-354. DOI:10.1152/physrev.00040.2012 |
| [2] |
CHEN Z G, YUAN Q L, XU G R, et al. Effects of quercetin on proliferation and H2O2-induced apoptosis of intestinal porcine enterocyte cells[J]. Molecules, 2018, 23(8): 2012. DOI:10.3390/molecules23082012 |
| [3] |
CAMPBELL J M, CRENSHAW J D, POLO J. The biological stress of early weaned piglets[J]. Journal of Animal Science and Biotechnology, 2013, 4(1): 19. DOI:10.1186/2049-1891-4-19 |
| [4] |
ZHU L H, ZHAO K L, CHEN X L, et al. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs[J]. Journal of Animal Science, 2012, 90(8): 2581-2589. DOI:10.2527/jas.2011-4444 |
| [5] |
ZHU L H, CAI X, GUO Q, et al. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways' response to oxidative stress in weaned piglets[J]. The British Journal of Nutrition, 2013, 110(11): 1938-1947. DOI:10.1017/S0007114513001608 |
| [6] |
ZHUANG Y, WU H R, WANG X X, et al. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway[J]. Oxidative Medicine and Cellular Longevity, 2019, 7591840. |
| [7] |
PAN Y L, FANG Y, FENG Y Q, et al. Discovery of mcr-3.1 gene carried by a prophage located in a conjugative IncA/C2 plasmid from a Salmonella Choleraesuis clinical isolate[J]. The Journal of Infection, 2021, 82(3): 414-451. |
| [8] |
HOEKE L, SHARBATI J, PAWAR K, et al. Intestinal Salmonella typhimurium infection leads to miR-29a induced caveolin 2 regulation[J]. PLoS One, 2013, 8(6): e67300. DOI:10.1371/journal.pone.0067300 |
| [9] |
MCGHIE E J, BRAWN L C, HUME P J, et al. Salmonella takes control: effector-driven manipulation of the host[J]. Current Opinion in Microbiology, 2009, 12(1): 117-124. DOI:10.1016/j.mib.2008.12.001 |
| [10] |
HULST M, SMITS M, VASTENHOUW S, et al. Transcription networks responsible for early regulation of Salmonella-induced inflammation in the jejunum of pigs[J]. Journal of Inflammation, 2013, 10(1): 18. DOI:10.1186/1476-9255-10-18 |
| [11] |
SHAW P, CHATTOPADHYAY A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms[J]. Journal of Cellular Physiology, 2020, 235(4): 3119-3130. DOI:10.1002/jcp.29219 |
| [12] |
KEUM Y S, YU S W, CHANG P P J, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells[J]. Cancer Research, 2006, 66(17): 8804-8813. DOI:10.1158/0008-5472.CAN-05-3513 |
| [13] |
SUN Z, HUANG Z P, ZHANG D D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response[J]. PLoS One, 2009, 4(8): e6588. DOI:10.1371/journal.pone.0006588 |
| [14] |
CHEN L, LI S, ZHENG J, et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide[J]. Journal of Animal Science and Biotechnology, 2018, 9: 62. DOI:10.1186/s40104-018-0275-8 |
| [15] |
LIU L, ZENG D, YANG M Y, et al. Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning Rex rabbits[J]. Probiotics and Antimicrobial Proteins, 2019, 11(4): 1278-1292. DOI:10.1007/s12602-018-9476-x |
| [16] |
KONG Q, HE G Q, JIA J L, et al. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice[J]. Current Microbiology, 2011, 62(2): 512-517. DOI:10.1007/s00284-010-9737-8 |
| [17] |
GAO Q X, QI L L, WU T X, et al. Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells[J]. Veterinary Microbiology, 2012, 160(3/4): 395-402. |
| [18] |
ZHANG J, CHEN X Y, LIU P, et al. Dietary Clostridium butyricum induces a phased shift in fecal microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model[J]. Journal of Agricultural and Food Chemistry, 2018, 66(20): 5157-5166. DOI:10.1021/acs.jafc.8b01253 |
| [19] |
SUN J, XU J X, YANG B, et al. Effect of Clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer's disease via regulating gut microbiota and metabolites butyrate[J]. Molecular Nutrition & Food Research, 2020, 64(2): e1900636. |
| [20] |
KANAI T, MIKAMI Y, HAYASHI A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease[J]. Journal of Gastroenterology, 2015, 50(9): 928-939. DOI:10.1007/s00535-015-1084-x |
| [21] |
HAYASHI A, SATO T, KAMADA N, et al. A single strain of Clostridium butyricum induces intestinal IL-10 producing macrophages to suppress acute experimental colitis in mice[J]. Cell Host & Microbe, 2013, 13(6): 711-722. |
| [22] |
KASHIWAGI I, MORITA R, SCHICHITA T, et al. Smad2 and Smad3 inversely regulate TGF-β autoinduction in Clostridium butyricum-activated dendritic cells[J]. Immunity, 2015, 43(1): 65-79. DOI:10.1016/j.immuni.2015.06.010 |
| [23] |
LIU J M, FU Y Y, ZHANG H, et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice[J]. Food & Function, 2017, 8(11): 4042-4052. |
| [24] |
王倩, 李海花, 窦彩霞, 等. 一株丁酸梭菌的分离鉴定及其益生特性研究[J]. 中国畜牧兽医, 2020, 47(1): 258-266. |
| [25] |
YANG J, ZHU C, YE J L, et al. Protection of porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway[J]. Journal of Agricultural and Food Chemistry, 2019, 67(6): 1726-1735. DOI:10.1021/acs.jafc.8b03662 |
| [26] |
MENG Q W, GUO T, LI G Q, et al. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1[J]. Journal of Animal Science and Biotechnology, 2018, 9: 34. DOI:10.1186/s40104-018-0248-y |
| [27] |
ZHANG Y M, DONG Z L, YANG H S, et al. Effects of dose and duration of dietary copper administration on hepatic lipid peroxidation and ultrastructure alteration in piglets' model[J]. Journal of Trace Elements in Medicine and Biology, 2020, 61: 126561. DOI:10.1016/j.jtemb.2020.126561 |
| [28] |
WEN Z S, TANG Z, MA L, et al. Protective effect of low molecular weight seleno-aminopolysaccharide on the intestinal mucosal oxidative damage[J]. Marine Drugs, 2019, 17(1): 64. DOI:10.3390/md17010064 |
| [29] |
PENG C L, DING X D, ZHU L, et al. β-conglycinin-induced intestinal porcine epithelial cell damage via the nuclear factor κB/mitogen-activated protein kinase signaling pathway[J]. Journal of Agricultural and Food Chemistry, 2019, 67(32): 9009-9021. DOI:10.1021/acs.jafc.9b02784 |
| [30] |
GUO T T, HUANG J H, ZHANG H Y, et al. Abcb1 in pigs: molecular cloning, tissues distribution, functional analysis, and its effect on pharmacokinetics of enrofloxacin[J]. Scientific Reports, 2016, 6: 32244. DOI:10.1038/srep32244 |
| [31] |
QIAO J Y, SUN Z Y, LIANG D M, et al. Lactobacillus salivarius alleviates inflammation via NF-κB signaling in ETEC K88-induced IPEC-J2 cells[J]. Journal of Animal Science and Biotechnology, 2020, 11: 76. DOI:10.1186/s40104-020-00488-5 |
| [32] |
GORRINI C, HARRIS I S, MAK T W. Modulation of oxidative stress as an anticancer strategy[J]. Nature Reviews Drug Discovery, 2013, 12(12): 931-947. DOI:10.1038/nrd4002 |
| [33] |
FILOMENI G, DE ZIO D, CECCONI F. Oxidative stress and autophagy: the clash between damage and metabolic needs[J]. Cell Death & Differentiation, 2015, 22(3): 377-388. |
| [34] |
SIES H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress[J]. Redox Biology, 2017, 11: 613-619. DOI:10.1016/j.redox.2016.12.035 |
| [35] |
LI J Y, SHEN H K, ZHAO Z J, et al. Protective effects of Clostridium butyricum against oxidative stress induced by food processing and lipid-derived aldehydes in Caco-2 cells[J]. Applied Microbiology and Biotechnology, 2020, 104(21): 9343-9361. DOI:10.1007/s00253-020-10896-2 |
| [36] |
CASSIR N, BENAMAR S, KHALIL J B, et al. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates[J]. Clinical Infectious Diseases, 2015, 61(7): 1107-1115. DOI:10.1093/cid/civ468 |
| [37] |
LIU Y H, LIU C, HUANG L Q, et al. A discovery of relevant hepatoprotective effects and underlying mechanisms of dietary Clostridium butyricum against corticosterone-induced liver injury in Pekin ducks[J]. Microorganisms, 2019, 7(9): 358. DOI:10.3390/microorganisms7090358 |
| [38] |
SUN J, LING Z X, WANG F Y, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis[J]. Neuroscience Letters, 2016, 613: 30-35. DOI:10.1016/j.neulet.2015.12.047 |
| [39] |
WANG F Y, LIU J M, LUO H H, et al. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice[J]. World Journal of Gastroenterology, 2015, 21(27): 8340-8351. DOI:10.3748/wjg.v21.i27.8340 |
| [40] |
LEE J M, CALKINS M J, CHAN K M, et al. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis[J]. The Journal of Biological Chemistry, 2003, 278(14): 12029-12038. DOI:10.1074/jbc.M211558200 |
| [41] |
TARANTINI S, VALCARCEL-ARES M N, YABLUCHANSKIY A, et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype[J]. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences, 2008, 73(7): 853-863. |
| [42] |
LEE J K, KIM N J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer's disease[J]. Molecules, 2017, 22(8): 1287. DOI:10.3390/molecules22081287 |
| [43] |
UWADA J, YAZAWA T, ISLAM M T, et al. Activation of muscarinic receptors prevents TNF-α-mediated intestinal epithelial barrier disruption through p38 MAPK[J]. Cellular Signalling, 2017, 35: 188-196. DOI:10.1016/j.cellsig.2017.04.007 |
| [44] |
KRYSAN P J, COLCOMBET J. Cellular complexity in MAPK signaling in plants: questions and emerging tools to answer them[J]. Frontiers in Plant Science, 2018, 9: 1674. DOI:10.3389/fpls.2018.01674 |
| [45] |
GUO Y J, PAN W W, LIU S B, et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Experimental and Therapeutic Medicine, 2020, 19(3): 1997-2007. |
| [46] |
KIM E K, CHOI E J. Pathological roles of MAPK signaling pathways in human diseases[J]. Biochimica et Biophysica Acta: Molecular Basis of Disease, 2010, 1802(4): 396-405. DOI:10.1016/j.bbadis.2009.12.009 |
| [47] |
LEA S, HARBRON C, KHAN N, et al. Corticosteroid insensitive alveolar macrophages from asthma patients; synergistic interaction with a p38 mitogen-activated protein kinase (MAPK) inhibitor[J]. British Journal of Clinical Pharmacology, 2015, 79(5): 756-766. DOI:10.1111/bcp.12536 |
| [48] |
YOKOTA T, WANG Y B. p38 MAP kinases in the heart[J]. Gene, 2016, 575(2): 369-376. DOI:10.1016/j.gene.2015.09.030 |
| [49] |
SAXENA A, LOPES F, MCKAY D M. Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli[J]. Inflammation Research, 2018, 67(10): 829-837. DOI:10.1007/s00011-018-1172-5 |
| [50] |
ZIPPER L M, MULCAHY R T. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes[J]. Biochemical and Biophysical Research Communications, 2000, 278(2): 484-492. DOI:10.1006/bbrc.2000.3830 |
| [51] |
WYDAU-DEMATTEIS S, LOUIS M, ZAHR N, et al. The functional dlt operon of Clostridium butyricum controls the D-alanylation of cell wall components and influences cell septation and vancomycin-induced lysis[J]. Anaerobe, 2015, 35(Pt.B): 105-114. |
| [52] |
WANG J B, QI L L, MEI L H, et al. C. butyricum lipoteichoic acid inhibits the inflammatory response and apoptosis in HT-29 cells induced by S. aureus lipoteichoic acid[J]. International Journal of Biological Macromolecules, 2016, 88: 81-87. DOI:10.1016/j.ijbiomac.2016.03.054 |
| [53] |
ZHAO X, GUO Y M, LIU H B, et al. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate[J]. Applied Microbiology and Biotechnology, 2014, 98(17): 7549-7557. DOI:10.1007/s00253-014-5829-x |
| [54] |
CHEN D F, JIN D C, HUANG S M, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota[J]. Cancer Letters, 2020, 469: 456-467. DOI:10.1016/j.canlet.2019.11.019 |
| [55] |
SIVAPRAKASAM S, PRASAD P D, SINGH N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis[J]. Pharmacology & Therapeutics, 2016, 164: 144-151. |
| [56] |
THANGARAJU M, CRESCI G A, LIU K B, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon[J]. Cancer Research, 2009, 69(7): 2826-2832. DOI:10.1158/0008-5472.CAN-08-4466 |
| [57] |
DETMAN A, MIELECKI D, CHOJNACKA A, et al. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors[J]. Microbial Cell Factories, 2019, 18(1): 36. DOI:10.1186/s12934-019-1085-1 |
| [58] |
COOK S I, SELLIN J H. Review article: short chain fatty acids in health and disease[J]. Alimentary Pharmacology & Therapeutics, 1998, 12(6): 499-507. |
| [59] |
Al BATRAN R, AL-BAYATY F, JAMIL AL-OBAIDI M M, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats[J]. PLoS One, 2013, 8(5): e64751. DOI:10.1371/journal.pone.0064751 |




