2. 福建农林大学国家菌草工程技术研究中心, 福州 350002
2. China National Engineering Research Center of Juncao Technology, Fujian Agriculture and Forestry University, Fuzhou 350002, China
新生仔猪因免疫力低下、肠道微生物系统发育不完善[1]、易受大肠杆菌感染,导致腹泻,引起肠道炎症,肠道屏障功能遭到破坏[2]。其中肠出血性大肠杆菌(enterohemorrhagic Escherichia coli,EHEC)可提高促炎因子白细胞介素-6(IL-6)、白细胞介素-1β(IL-1β)、肿瘤坏死因子-α(TNF-α)基因表达量,造成肠道损伤,细胞膜通透性升高,水盐代谢失衡[3-4],造成仔猪腹泻。另外,肠道疾病的发生与紧密连接蛋白磷酸化[5]及相关蛋白表达下调有关。研究表明,当细菌感染肠道上皮细胞时,Toll样受体4(TLR4)识别细胞外的致病菌,促进下游炎症因子转录,激活肠道炎症信号通路,引起炎症反应[6]。相关文献报道灵芝多糖(Ganoderma lucidum polysaccharides)可促进细胞增殖、降低丙二醛含量、提高血清抗氧化酶活力、促进肿瘤细胞凋亡和降低促炎因子表达[7-9],具有提高免疫、抗氧化、抗菌和抗肿瘤等功能[10-12],在畜牧业中应用前景可观。本试验选用的灵芝多糖来自于福建农林大学菌草所研发的“混合菌草培养基”栽培的灵芝,其多糖含量远高于(2.5倍以上)传统的棉籽壳和木屑培养基所栽培的灵芝,该方法降低了灵芝生产成本[13]。研究报道,在猪饲粮中添加灵芝多糖可提高血清白细胞介素-10(IL-10)含量,降低IL-1β含量,提高仔猪抗病力,缓解免疫应激[14-15]。在鸡饲粮中添加灵芝多糖可促进外周血淋巴细胞和脾淋巴细胞的增殖,显著提高新城疫(ND)和传染性法氏囊(IBD)抗体阳性水平[16-17]。此外,植物类多糖能改善肠道菌群、脂肪酸组成、肠道免疫功能和修复肠道损伤。赵玉蓉等[18]报道,牛膝多糖(ABPS)可显著抑制沙门氏菌侵染IPEC-1细胞,提高紧密连接蛋白转录水平。灵芝多糖可通过影响机体细胞活力、细胞因子含量调节机体免疫,但灵芝多糖如何调节猪小肠上皮细胞IPEC-1免疫功能的研究甚少。本试验旨在通过建议体外IPEC-1细胞炎症模型,探讨灵芝多糖对IPEC-1细胞增殖、周期、抑菌作用以及免疫功能的影响,为灵芝多糖改善动物肠道健康提供新的理论依据。
1 材料与方法 1.1 试验材料灵芝多糖:由福建农林大学菌草研究所提供,从菌草栽培灵芝中提取、纯化所得,深褐色粉末,易溶于水,平均相对分子质量为5.1×105,多糖含量为94.84%。
猪小肠上皮细胞IPEC-1:购自福州泽叶生物公司。
EHEC O157 ∶ H7:购自BNCC公司。
试剂:DMEM/F12培养基、胎牛血清(FBS)和EDTA-胰蛋白酶,购自美国Gibco公司;磷酸盐缓冲液(PBS)、青链霉素混合液购自美国Hyclone公司;CCK-8试剂盒和LDH释放率检测试剂盒购自日本dojindo公司;细胞周期检测试剂盒购自江苏凯基生物技术股份有限公司;庆大霉素购自Hyclone公司;IL-6、IL-1β、TNF-α、干扰素-γ(IFN-γ)和IL-10酶联免疫吸附测定(ELISA)试剂盒购自上海酶联生物公司;Trizol、PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒购自日本TaKaRa公司;SYBR qPCR试剂盒购自南京诺唯赞生物有限公司;LB培养基购自北京索莱宝公司;无水乙醇、氯仿、异丙醇购自广州鼎国生物有限公司。
主要仪器:MCO-170AICUVL-PC CO2培养箱购自日本PHCbi公司;恒温培养箱购自美国Thermo公司;恒温摇床、低速离心机购自上海安亭科学仪器厂;Ts2倒置生物显微镜购自日本Nikon公司;SW-CJ-1FD洁净工作台购自苏州安泰空气技术有限公司;5424R高速冷冻离心机购自德国Effedorf公司;酶标仪、T100TM Thermal Cycler、荧光定量PCR仪购自美国Bio-Rad公司;NovoCyte 1030型流式细胞仪购自美国ACEA公司。
1.2 试验方法 1.2.1 灵芝多糖对IPEC-1细胞活力的影响待细胞融合生长至70%~80%,消化离心后进行细胞计数,以2×104个细胞/孔的浓度接种于96孔培养板,培养过夜;加入100 μL含有不同浓度(5、10、20、50、100、200、400和800 μg/mL)灵芝多糖的培养基,同时设置空白孔(不含细胞和灵芝多糖的培养基、加CCK-8)和对照孔(含细胞无灵芝多糖的培养基,加CCK-8),每种处理设置6个重复,在37 ℃、CO2培养箱中分别培养12、24、48 h后,加入10 μL CCK-8,37 ℃、CO2培养箱培养2 h,于450 nm处测定吸光度(OD450 nm)。

|
待细胞融合生长至70%~80%,消化离心后接种于6孔细胞培养板,于37 ℃、CO2培养箱培养过夜;由灵芝多糖对细胞活力的试验结果,选用终浓度分别0(空白组)、5、10、20、50和100 μg/mL灵芝多糖处理24 h,收集细胞;用PBS清洗后,2 000 r/min离心5 min,收集细胞;70%乙醇固定过夜后,用PBS洗去固定液;细胞悬液用200目筛网过滤1次;加入提前配制好的500 μL PI/RNase A染色工作液,室温避光30~60 min,流式细胞仪检测,记录激发波长488 nm处红色荧光。G0期为暂时离开细胞周期,停止细胞分裂,去执行一定生物学功能的细胞所处的时期(细胞静止期);G1期为合成前期;G2期为合成后期;S期为DNA合成时期;M期为细胞分裂期。增殖指数(PI)的计算公式为:

|
将EHEC菌液接种于LB液体培养基中,37 ℃、180 r/min振荡培养过夜,连续接种活化3代。取10 μL菌悬液接种于10 mL LB培养基中,37 ℃、180 r/min振荡培养至OD600 nm=0.5,浓度约为2.5×109 CFU/mL,细菌计数。菌液4 ℃、4 000 r/min离心10 min,弃掉上清,PBS重悬,用不含抗生素的DMEM/F12培养基稀释至合适浓度(2×108 CFU/mL),4 ℃备用。
1.2.4 EHEC增殖的测定向96孔培养板中加入180 μL含1 mmol/L的NaH2PO4和25 mmol/L的NaHCO3的LB培养基;向96孔培养板中加入灵芝多糖,灵芝多糖的终浓度分别为0、5、10、20、50和100 μg/mL,接种EHEC至96孔板,每孔接种数量为2×104 CFU;将96孔培养板置于37 ℃恒温培养箱培养12 h;取出96孔培养板,酶标仪测定590 nm处吸光度(OD590 nm),OD590 nm可反映EHEC的增殖情况。
1.2.5 灵芝多糖预处理对IPEC-1细胞抵抗EHEC侵染能力的测定细胞贴壁生长至70%~80%,消化离心后接种于6孔细胞培养板,37 ℃、CO2培养箱培养过夜;弃去培养基,PBS清洗后添加灵芝多糖,使其终浓度分别为0、5、10、20、50和100 μg/mL,置于37 ℃、CO2培养箱培养24 h;弃去培养基,PBS清洗2遍,每孔加入200 μL浓度为l%的Triton X-100裂解液裂解细胞;收集细胞裂解液,4 ℃、8 000 r/min离心10 min。取160 μL裂解液上清加入96孔细胞培养板;每孔中加入提前配制好的EHEC菌悬液[感染复数(multiplicity of infection,MOI)=40]40 μL,将96孔培养板置于37 ℃恒温培养箱培养12 h,取出96孔培养板,酶标仪测定OD590 nm,OD590 nm可反映细菌的增殖情况。
1.2.6 细胞因子分泌量的检测待细胞融合生长至70%~80%,按1×105个细胞/孔加入12孔细胞培养板中,加入灵芝多糖(使其终浓度分别为0、5、10、20、50和100 μg/mL)培养24 h,然后用大肠杆菌(MOI=40)感染2 h,并设空白组,每种处理设6个重复,取培养上清液,按ELISA试剂盒说明书操作,使用酶标仪测定OD450 nm,计算INF-γ、IL-6、IL-10、TNF-α和IL-1β的分泌量。
1.2.7 IL-6、IL-1β、TNF-α和IL-10基因表达量的检测待细胞融合生长至70%~80%,按2×105个细胞/孔加入6孔细胞培养板中,加入灵芝多糖(使其终浓度分别为0、50和100 μg/mL)培养24 h,大肠杆菌(MOI=40)感染2 h,并设空白组,每种处理设6个重复。根据TaKaRa公司Trizol裂解法说明书提取总RNA,测定RNA纯度和浓度,逆转录合成cDNA,按照试剂盒说明,利用荧光定量PCR(qRT-PCR)技术检测IL-6、IL-1β、TNF-α和IL-10基因的表达情况。qRT-PCR反应程序:95 ℃预变性3 min;95 ℃变性20 s,60 ℃退火20 s,72 ℃延伸20 s,40个循环;熔解曲线收集:95 ℃ 15 s,60 ℃ 60 s,95 ℃ 15 s。qRT-PCR数据采用2-ΔΔCt方法计算目的基因的mRNA相对表达量。以甘油醛-3-磷酸脱氢酶(GAPDH)为内参基因,在NCBI上搜索猪IL-6、IL-1β、TNF-α和IL-10基因的全序列,使用Primer Premier 6.0软件设计引物(表 1),引物序列均由上海生工生物工程股份有限公司合成。
|
|
表 1 引物序列信息 Table 1 Primer sequence information |
使用Excel 2019对试验数据进行初步整理和统计,用GraphPad Prism 8软件中one-way ANOVA进行方差分析并用Duncan氏法进行多重比较。试验结果用平均值±标准误(mean±SE)表示,以P < 0.05表示差异显著,P>0.05表示差异不显著。
2 结果与分析 2.1 灵芝多糖对IPEC-1细胞活力的影响由图 1可知,灵芝多糖作用IPEC-1细胞48 h后细胞活力明显下降,且作用4 h时细胞活力优于作用12 h时,故选择24 h作为最佳作用时间。灵芝多糖作用24 h,浓度为5~100 μg/mL时细胞活力优于浓度为200~800 μg/mL。
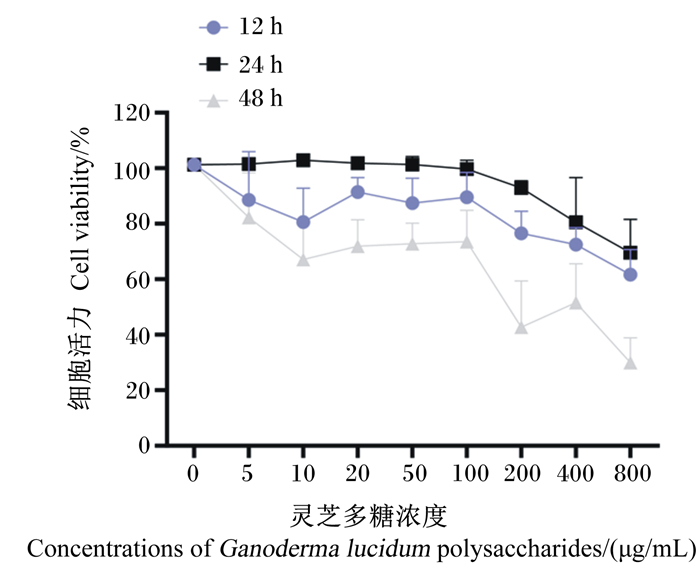
|
图 1 灵芝多糖对IPEC-1细胞活力的影响 Fig. 1 Effects of Ganoderma lucidum polysaccharides on cell viability of IPEC-1 cells |
由表 2可知,相比空白组相比,不同浓度灵芝多糖对G0/G1、S期、G2/M细胞分布和PI未产生显著影响(P < 0.05);不同浓度(5~100 μg/mL)灵芝多糖之间上述指标的差异亦不显著(P < 0.05)。
|
|
表 2 灵芝多糖对IPEC-1细胞周期的影响 Table 2 Effects of Ganoderma lucidum polysaccharides on cell cycle of IPEC-1 cells |
由图 2可知,浓度为5、10、20、50和100 μg/mL的灵芝多糖可显著抑制EHEC的增殖(P < 0.05),且浓度为20、50和100 μg/mL的灵芝多糖之间差异不显著(P>0.05)。
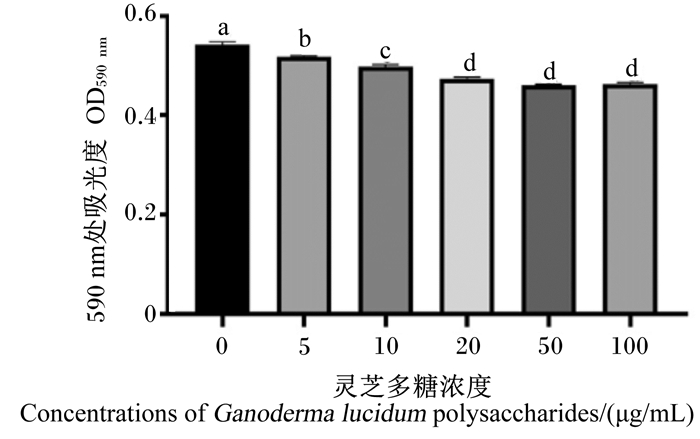
|
数据柱标注相同小写字母表示差异显著(P>0.05),不同小写字母表示差异显著(P < 0.05)。下图同。 Value columns with the small letters mean no significant difference (P>0.05), while with different small letters mean significant difference (P < 0.05). The same as below. 图 2 灵芝多糖对EHEC增殖的影响 Fig. 2 Effects of Ganoderma lucidum polysaccharides on EHEC proliferation |
由图 3可知,浓度为50和100 μg/mL的灵芝多糖处理IPEC-1细胞24 h可显著抑制EHEC增殖(P < 0.05),而浓度为5、10和20 μg/mL的灵芝多糖处理IPEC-1细胞24 h可显著促进EHEC增殖(P < 0.05)。
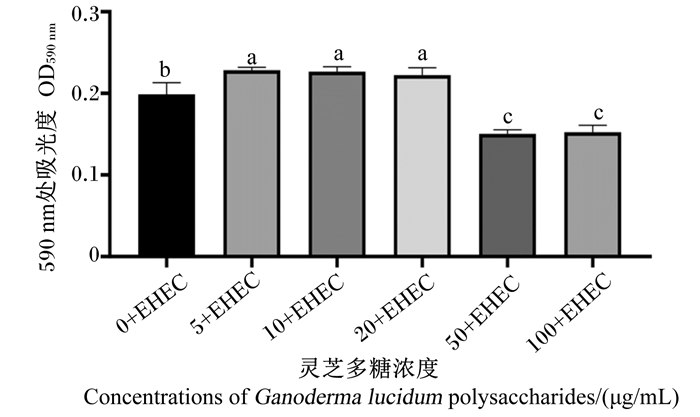
|
EHEC: 肠出血性大肠杆菌enterohemorrhagic Escherichia coli。下图同the same as below。 图 3 灵芝多糖对IPEC-1细胞抗EHEC活性的影响 Fig. 3 Effects of Ganoderma lucidum polysaccharides on anti-EHEC activity of IPEC-1 cells |
由图 4可知,通过对IPEC-1炎性因子分泌量进行检测发现,与空白组相比,EHEC组的IL-6、IL-1β和IFN-γ分泌量显著上升(P < 0.05),IL-10分泌量显著下降(P < 0.05);与EHEC组相比,5、10、20、50和100 μg/mL灵芝多糖+EHEC组的IL-6、TNF-α和IFN-γ分泌量显著降低(P < 0.05),20、50和100 μg/mL灵芝多糖+EHEC组的IL-1β分泌量显著下降,5和50 μg/mL灵芝多糖+EHEC组的IL-10分泌量显著上升(P < 0.05)。
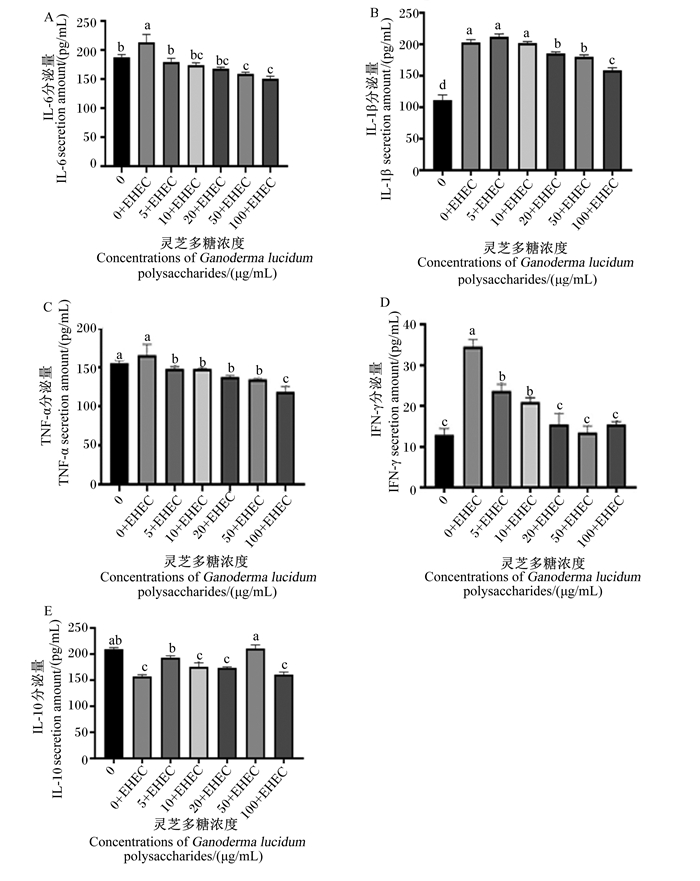
|
图 4 灵芝多糖对IPEC-1细胞炎性因子分泌量的影响 Fig. 4 Effects of Ganoderma lucidum polysaccharides on inflammatory factor secretion amounts of IPEC-1 cells |
由图 5可知,与空白组相比,EHEC组的TNF-α和IL-1β)mRNA相对表达量显著升高(P < 0.05),IL-6 mRNA相对表达量升高(P>0.05),IL-10 mRNA相对表达量下降(P>0.05);50和100 μg/mL灵芝多糖组的IL-6、IL-1β、TNF-α和IL-10 mRNA相对表达量与空白组差异不显著(P>0.05)。与EHEC组相比,50和100 μg/mL灵芝多糖+EHEC组的IL-1β mRNA相对表达量显著降低(P < 0.05),IL-6和TNF-α mRNA相对表达量下降(P>0.05),IL-10 mRNA相对表达量上升(P>0.05)。
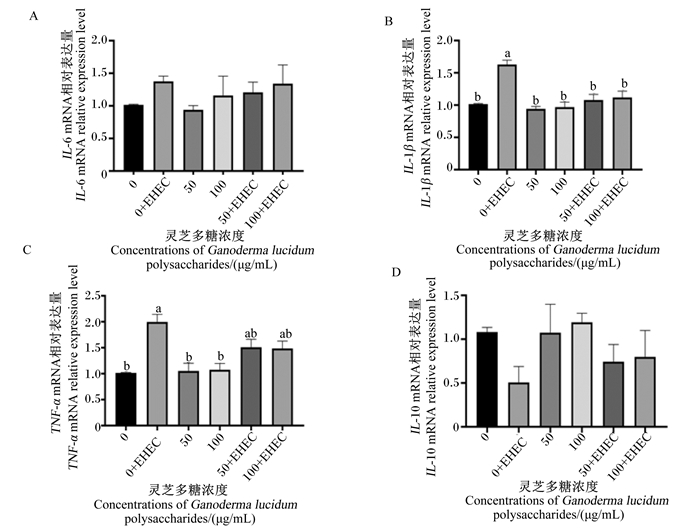
|
图 5 灵芝多糖对IPEC-1细胞IL-6、TNF-α、IL-1β、IL-10基因表达的影响 Fig. 5 Effects of Ganoderma lucidum polysaccharides on gene expression of IL-6, TNF-α, IL-1β)and IL-10 of IPEC-1 cells |
国家菌草工程技术研究中心在人、小鼠和奶牛等动物上的研究证实灵芝多糖具有促进免疫细胞增殖、清除氧化自由基、免疫增强等功能[19-20],Huang等[21]研究发现灵芝多糖200、100 mg/(kg·d)剂量组可提升小鼠自然杀伤细胞活性。王斌等[22]研究发现灵芝多糖可明显刺激小鼠脾脏树突状细胞增殖,且与细胞因子有显著的协同作用,具有类生长因子和协同生长因子的作用。肠道上皮细胞的增殖与肠道健康密切相关,小肠上皮细胞的增殖可提高绒毛高度,促进消化吸收[23]和免疫应激反应应答。CCK-8是一种综合指标比较好的检测细胞活力的试剂,是通过检测活细胞中脱氢酶活性来确定活细胞数,操作简便、稳定性强、灵敏度高[24-26]。本试验通过细胞活力验证灵芝多糖对IPEC-1细胞的存活率影响的结果表明,灵芝多糖作用24 h优于作用12、48 h,且灵芝多糖浓度为5~100 μg/mL时细胞活力优于其他浓度,可能是因为其多糖成分不能完全降解,糖酵解途径未完全发挥作用,且呈药剂依赖效应。而灵芝多糖作用48 h时细胞活力下降,则说明灵芝多糖作用时间过久会产生毒副作用,导致细胞损伤。
3.2 灵芝多糖对IPEC-1细胞周期的影响细胞周期是反映细胞增殖与凋亡的重要指标,细胞凋亡是清除异常细胞或损伤细胞,维持肠上皮细胞更新与正常存活的生理过程。其中G2/M期+S期反映细胞增殖能力。研究表明,G0/G1期停滞和S期降低,凋亡率增加[27-28],是对细胞抑制的表现,G2/M期下降可促进细胞凋亡,Jun氨基末端激酶(JNK)磷脂酸化水平增加。本研究结果表明,5~100 μg/mL灵芝多糖对细胞增殖无显著作用,细胞周期结果表明,G0/G1期、S期和G2/M期细胞分布各浓度灵芝多糖之间差异不显著,细胞周期试验结果与细胞增殖结果相一致。Bai等[29]研究发现,灵芝多糖(150和300 μg/mL)给药24 h显著抑制人类结直肠腺癌细胞HCT-116的增殖,促进细胞凋亡。Jin等[30]研究发现,200 g/mL的灵芝多糖作用宫颈癌细胞48 h能减弱宫颈癌细胞的侵袭和迁移能力,促进宫颈癌细胞凋亡,限制宫颈癌细胞周期。因此,我们推测机体处于发病状态时灵芝多糖可发挥其药理作用。本研究在正常细胞状态下给药,对细胞无显著作用,说明5~100 μg/mL灵芝多糖对细胞毒副作用小。
3.3 灵芝多糖对EHEC增殖的影响肠道微生物的组成变化会影响宿主代谢网络,影响肠道内微生物的代谢水平。一定水平的短链脂肪酸能够维持肠道屏障功能,抑制全身炎症。大量研究证明,多糖和植物提取物可以改善肠道微生物的组成,促进有益菌的生长[31-32],抑制有害菌(如大肠杆菌、金黄色葡萄球菌、沙门氏菌等革兰氏阴性菌)的生长[33-36]。Tong等[37]和Guo等[38]研究发现,灵芝多糖可促进瘤胃球菌、双歧杆菌和短链脂肪酸产生菌等良性细菌的生长,从而改善机体代谢。本研究通过体外抑菌试验验证灵芝多糖是否有一定程度抑菌作用,结果表明,灵芝多糖对EHEC在体外有一定抑制作用,原因可能是,灵芝多糖参与破坏细菌细胞壁,从而起到抑菌作用。但灵芝多糖抑制大肠杆菌的具体作用机理还需进一步探究。
3.4 灵芝多糖预处理对IPEC-1细胞抵抗EHEC侵染能力的影响小肠上皮细胞由肠上皮细胞膜和肠上皮细胞紧密连接组成[39],因而小肠上皮细胞是抵御病原菌的第1道防线[40]。本研究就灵芝多糖能否加强IPEC-1细胞抵抗EHEC侵染能力以及细胞自身免疫功能进行了探究。革兰氏阴性菌细胞壁比较薄且化学组成较单一,只含有90%的肽聚糖和10%的磷壁酸。有研究发现,中草药对细菌的抑菌作用机制和靶位点有可能不同于抗生素或疫苗,其在一定程度上对细菌细胞壁的完整性有破坏作用,增强抑菌物质的亲和度,起到抑菌杀菌效果[41]。综上所述,灵芝多糖可通过提高细胞内活性物质的分泌,从而达到抑菌杀菌效果。谢红兵[42]研究表明多糖对大肠杆菌、沙门氏菌和金黄色葡萄球菌有显著抑制作用。陈洪亮[43]在体外抑菌试验中也得到相同结果。毛晓峰[44]和赵露露等[45]报道黄芪多糖有一定程度抑菌效果,可缓解断奶仔猪腹泻。上述研究结果与本研究结果相符,浓度为50和100 μg/mL的灵芝多糖处理IPEC-1细胞24 h,可显著抑制EHEC增殖。灵芝多糖可能通过影响核因子-κB(NF-κB) 基因和蛋白的表达,进一步调控机体炎性因子分泌量或基因表达量,从而发挥免疫调节作用,提高抗菌能力。
3.5 灵芝多糖对IPEC-1细胞免疫功能的影响小肠上皮细胞主要利用谷氨酰胺和葡萄糖提供能量,而IPEC-1细胞具有典型小肠上皮细胞的特征,并且小肠是动物机体消化、吸收的主要场所,因此小肠上皮细胞的免疫健康极其重要[46]。炎性因子TNF-α和IL-6在诱导炎症反应后迅速产生[47],在细菌感染性疾病的发病机制中起重要作用,导致炎症的发生[48],引起肠道上皮损伤和屏障障碍[49]。本试验通过应用EHEC,可致IPEC-1细胞发生损伤、免疫功能减弱,为后续体内研究提供依据。本试验预试验建立的验证模型(EHEC-IPEC-1细胞体外炎症模型)EHEC(MOI=40)侵染2 h,可使细胞存活率下降至60%左右,炎性因子分泌量上升。另已有文献证明,EHEC(MOI=10)浸染1 h,可使仔猪肠道形态发生变化,破坏仔猪屏障功能,造成炎症反应[50]。研究发现,黑灵芝多糖可明显抑制小鼠体内TNF-α分泌量[51-52]。在体外细胞试验中发现,灵芝多糖(5、10、20、50和100 μg/mL)处理RAW264.7细胞后,呈剂量依赖效应,降低促炎因子诱导型一氧化氮合酶(iNOS)、白细胞介素-4(IL-4)和TNF-α的表达量,而抗炎因子IL-10的表达量明显增加[53-54]。在脂多糖(LPS)刺激的人主动脉平滑肌细胞中,灵芝多糖(10 μg/mL)明显降低IL-1β的表达。上述研究表明,灵芝多糖可抑制炎症反应,增强机体免疫能力。
机体相关免疫细胞分泌细胞因子是细胞免疫系统关键的信号因子,细胞炎性因子分泌量与转录水平的高低是免疫功能正常与否的重要标志,炎性因子IL-6、IL-1β、IL-10和IFN-γ等的改变与人和动物肠道的感染有关。崔小珍等[55]研究表明松针多糖显著抑制LPS应激下鸡巨噬细胞因子TNF-α、IL-6和TNF-α分泌。本试验中,在炎症状态下,灵芝多糖提高IL-10和降低IFN-γ、IL-6、IL-1β和TNF-α分泌量,证明灵芝多糖是通过调控炎性因子分泌量,来缓解由细菌感染引起的炎症疾病。Walsh等[56]报道,褐藻多糖显著抑制促炎因子IL-6和IL-1β基因表达量,缓解仔猪肠道炎症。Khan等[57]用蘑菇多糖治疗小鼠,发现与M1表型相关的细胞因子(IFN-γ、IL-1β和TNF-α)基因表达下调,与M2表型相关的细胞因子(IL-4、IL-10、IL-12和IL-13)基因表达上调。研究报道,当机体发生炎症反应,细胞因子分泌量和基因表达会激活免疫应答系统,促进药物作用于机体[51, 58-59],通过免疫信号通路介导下游基因变化,从而发挥免疫作用,但调节炎症反应的免疫机制还需探究。本试验结果表明,在EHEC侵染IPEC-1细胞后,20、50和100 μg/mL灵芝多糖+EHEC组IL-6、IL-1β、IFN-γ和TNF-α的分泌量显著降低,5和10 μg/mL灵芝多糖+ EHEC组IL-1β的分泌量减少,5和50 μg/mL灵芝多糖+EHEC组IL-10的分泌量显著升高。通过转录水平测定,50和100 μg/mL灵芝多糖+EHEC组IL-1β)mRNA相对表达量显著降低,IL-6和TNF-α mRNA相对表达量下降,IL-10 mRNA相对表达量上升。综上表明,浓度为50和100 μg/mL的灵芝多糖可缓解EHEC诱发的炎症反应。
4 结论5~100 μg/mL灵芝多糖作用IPEC-1细胞24 h细胞活力均在100%左右,且对细胞毒副作用小,有抑菌作用,可增强IPEC-1细胞抗EHEC感染的能力。大肠杆菌感染IPEC-1细胞可使其发生炎症反应,灵芝多糖预处理能够缓解大肠杆菌诱导的促炎性因子表达的上调及抑炎性因子表达的下调。
| [1] |
黄静. ETEC感染对猪肠道上皮细胞的炎性损伤作用及甘草多糖的干预效果研究[D]. 硕士学位论文. 重庆: 西南大学, 2020: 12-15. HUANG J. Effect of ETEC infection on inflammatory injury of porcine intestinal epithelial cells and the intervention effect of glycyrrhiza polysaccharide[D]. Master's Thesis. Chongqing: Southwest University, 2020: 12-15. (in Chinese) |
| [2] |
REN X, ZHU Y, GAMALLAT Y, et al. E.coli O124 K72 alters the intestinal barrier and the tight junctions proteins of Guinea pig intestine[J]. Biomedicine & Pharmacotherapy, 2017, 94: 468-473. |
| [3] |
FLYNN A, BURET A G. Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E.coli but are dispensable for increased paracellular permeability[J]. Microbial Pathogenesis, 2008, 44(4): 311-319. DOI:10.1016/j.micpath.2007.10.007 |
| [4] |
BRAICU C, SELICEAN S, COJOCNEANU-PETRIC R, et al. Evaluation of cellular and molecular impact of zearalenone and Escherichia coli co-exposure on IPEC-1 cells using microarray technology[J]. BMC Genomics, 2016, 17: 576. DOI:10.1186/s12864-016-2830-z |
| [5] |
RAO R. Occludin phosphorylation in regulation of epithelial tight junctions[J]. Annals of the New York Academy of Sciences, 2009, 1165(1): 62-68. DOI:10.1111/j.1749-6632.2009.04054.x |
| [6] |
GABRILSKA R A, RUMBAUGH K P. Biofilm models of polymicrobial infection[J]. Future Microbiology, 2015, 10(12): 1997-2015. DOI:10.2217/fmb.15.109 |
| [7] |
CHENY G, SHEN Z J, CHEN X P. Modulatory effect of Ganoderma lucidum polysaccharides on serum antioxidant enzymes activities in ovarian cancer rats[J]. Carbohydrate Polymers, 2009, 78(2): 258-262. DOI:10.1016/j.carbpol.2009.03.030 |
| [8] |
ZHANG K, LIU Y F, ZHAO X L, et al. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum[J]. International Journal of Biological Macromolecules, 2018, 107(Pt.A): 486-493. |
| [9] |
XU Y N, ZHONG J J. Impacts of Calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum[J]. Biotechnology Advances, 2012, 30(6): 1301-1308. DOI:10.1016/j.biotechadv.2011.10.001 |
| [10] |
易丹, 侯永清. 天然植物活性成分与猪肠道健康的研究进展[J]. 动物营养学报, 2020, 32(10): 4518-4524. YI D, HOU Y Q. Research progress on natural plant bioactive compounds and swine intestinal health[J]. Chinese Journal of Animal Nutrition, 2020, 32(10): 4518-4524 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.10.005 |
| [11] |
GAO Y H, GAO H, CHAN E I, et al. Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice[J]. Immunological Investigations, 2005, 34(2): 171-198. DOI:10.1081/IMM-55813 |
| [12] |
SKALICKA-WOŹNIAK K, SZYPOWSKI J, ŁOŚ R, et al. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P.Karst.strains cultivated on different wood type substrates[J]. Acta Societatis Botanicorum Poloniae, 2012, 81(1): 17-21. DOI:10.5586/asbp.2012.001 |
| [13] |
李伟文. 菌草鹿角灵芝多糖含量研究及其菌糠多糖应用初探[D]. 硕士学位论文. 福州: 福建农林大学, 2010. LI W W. Analysis of the content of polysaccharide in Juncao antelope glucidum and applied study on polysaccharide in spent substrate form Juncao antelope-shape glucidum culture[D]. Master's Thesis. Fuzhou: Fujian Agriculture and Forestry University, 2010. (in Chinese) |
| [14] |
易霞, 杨沁洁, 李清亮. 灵芝多糖对内江猪免疫功能的影响[J]. 中国畜牧兽医文摘, 2017, 33(10): 217-218. YI X, YANG Q J, LI Q L. Effects of polysaccharide from Ganoderma lucidum on immune function of Neijiang pigs[J]. Chinese Animal Husbandry and Veterinary Abstracts, 2017, 33(10): 217-218 (in Chinese). |
| [15] |
马进文. 灵芝多糖在影响猪瘟疫苗效价中的研究[J]. 畜牧兽医杂志, 2016, 35(2): 132-133. MA J W. Study on the effect of Ganoderma lucidum polysaccharide on the titer of swine fever vaccine[J]. Journal of Animal Science and Veterinary Medicine, 2016, 35(2): 132-133 (in Chinese). DOI:10.3969/j.issn.1004-6704.2016.02.049 |
| [16] |
张萍, 刘海侠, 江善祥. 赤灵芝多糖对鸡淋巴细胞增殖的影响[J]. 中国家禽, 2015, 37(20): 54-56. ZHANG P, LIU H X, JIANG S X. Effect of Ganoderma lucidum polysaccharide on chicken lymphocyte proliferation[J]. China Poultry, 2015, 37(020): 54-56 (in Chinese). |
| [17] |
丁蓉龙. 灵芝多糖对鸡免疫增强作用的初步研究[D]. 硕士学位论文. 南京: 南京农业大学, 2014. DING R L. The study on immune-enhancing of Ganoderma lucidum poilysaccharide in chicken[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2014. (in Chinese) |
| [18] |
赵玉蓉, 王耀东. 牛膝多糖抑制沙门氏菌侵染猪小肠上皮细胞的研究[J]. 动物营养学报, 2018, 30(12): 5083-5088. ZHAO Y R, WANG Y D. Study on inhibitory effects of achyranthes bidentata polysaccharides on salmonella infection in intestinal porcine epithelial cells-1[J]. Chinese Journal of Animal Nutrition, 2018, 30(12): 5083-5088 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.12.036 |
| [19] |
WANG J, CAO B, ZHAO H, et al. Emerging roles of Ganoderma lucidum in anti-aging[J]. Aging and Disease, 2017, 8(6): 691-707. DOI:10.14336/AD.2017.0410 |
| [20] |
游育红, 林志彬. 灵芝多糖肽对小鼠巨噬细胞自由基的清除作用[J]. 中国临床药理学与治疗学, 2004, 9(1): 52-55. YOU Y H, LIN Z B. Free radical scavenging activity by Ganoderma lucidum polysaccharides peptide on peritoneal macrophages in mice[J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2004, 9(1): 52-55 (in Chinese). DOI:10.3969/j.issn.1009-2501.2004.01.012 |
| [21] |
HUANG S Q, LI J W, WANG Z, et al. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice[J]. Molecules (Basel, Switzerland), 2010, 15(5): 3694-3708. DOI:10.3390/molecules15053694 |
| [22] |
王斌, 萱垣昇, 李杰芬. 灵芝多糖对小鼠脾脏树突状细胞的增殖作用[J]. 免疫学杂志, 2006, 22(3): 55-57. WANG B, XUAN Y S, LI J F. Effects of ganoderma polysaccharides on proliferation of dendritic cells in mouse spleen[J]. Immunological Journal, 2006, 22(3): 55-57 (in Chinese). |
| [23] |
李杰, 伍树松, 熊兴耀, 等. 博落回生物碱对猪肠上皮细胞增殖的影响[J]. 动物营养学报, 2014, 26(6): 1632-1637. LI J, WU S S, XIONG X Y, et al. Effects of macleaya cordata alkaloids on intestinal porcine EPithelial cells proliferation[J]. Chinese Journal of Animal Nutrition, 2014, 26(6): 1632-1637 (in Chinese). DOI:10.3969/j.issn.1006-267x.2014.06.026 |
| [24] |
谢光洪, 许远靖, 田武林, 等. β-HB对牛皱胃平滑肌细胞活性影响-MTT法和CCK-8法的比较研究[J]. 中国兽医学报, 2013, 33(3): 436-438, 449. XIE G H, XU Y J, TIAN W L, et al. Effect of β-HB on the abomasum smooth muscle cell viability of dairy cow-comparative studies on the MTT and CCK-8 assay[J]. Chinese Journal of Veterinary Science, 2013, 33(3): 436-438, 449 (in Chinese). |
| [25] |
王瑾, 马肖容, 张王刚. CCK-8法在淋巴细胞增殖检测中最佳实验条件的筛选[J]. 中国医药导报, 2018, 15(23): 13-16. WANG J, MA X R, ZHANG W G. Optimization of the experimental conditions of CCK-8 in lymphocyte proliferation assays[J]. China Medical Herald, 2018, 15(23): 13-16 (in Chinese). |
| [26] |
LOU J, CHU G, ZHOU G, et al. Comparison between two kinds of cigarette smoke condensates (CSCs) of the cytogenotoxicity and protein expression in a human B-cell lymphoblastoid cell line using CCK-8 assay, comet assay and protein microarray[J]. Mutation Research, 2010, 697(1/2): 55-59. |
| [27] |
CHEN J, XIE H, CHEN D, et al. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs[J]. ACS Omega, 2018, 3(2): 2211-2219. DOI:10.1021/acsomega.7b01971 |
| [28] |
LIU Y, WANG C, LI J, et al. Phellinus linteus polysaccharide extract improves insulin resistance by regulating gut microbiota composition[J]. FASEB Journal, 2020, 34(1): 1065-1078. DOI:10.1096/fj.201901943RR |
| [29] |
BAI J H, XU J, ZHAO J, et al. Ganoderma lucidum polysaccharide enzymatic hydrolysate suppresses the growth of human colon cancer cells via inducing apoptosis[J]. Cell Transplantation, 2020, 29(1): 963689720931435. |
| [30] |
JIN H, SONG C, ZHAO Z, et al. Ganoderma lucidum polysaccharide, an extract from Ganoderma lucidum, exerts suppressive effect on cervical cancer cell malignancy through mitigating epithelial-mesenchymal and JAK/STAT5 signaling pathway[J]. Pharmacology, 2020, 105(7/8): 461-470. |
| [31] |
张金洲, 苗志国, 赵伟鑫, 等. 山药多糖对仔猪生长性能与肠道菌群的影响[J]. 中国饲料, 2020, 667(23): 57-61. ZHANG J Z, MIAO Z G, ZHAO W X, et al. Effect of yam polysaccharide on growth performanceand intestinal microflora of piglets[J]. China Feed, 2020, 667(23): 57-61 (in Chinese). |
| [32] |
ZHOU H, YU B, HE J, et al. The optimal combination of dietary starch, non-starch polysaccharides, and mannan-oligosaccharide increases the growth performance and improves butyrate-producing bacteria of weaned Pigs[J]. Animals, 2020, 10(10): 1745. DOI:10.3390/ani10101745 |
| [33] |
张百刚, 钟旭美, 刘晓风, 等. 甘草多糖的提取及其抑菌试验的研究[J]. 粮油加工, 2010(8): 137-140. ZHANG B G, ZHONG X M, LIU X F, et al. Reserch on extraction and anti-microbial of glycyrrhiza polysaccharide[J]. Cereals and Oils Processing, 2010(8): 137-140 (in Chinese). |
| [34] |
姬丽娜, 张为民, 卿素珠, 等. 朱砂七多糖的提取分离及体外抑菌试验[J]. 黑龙江畜牧兽医, 2013, 1(7): 117-119. JI L N, ZHANG W M, QING S Z, et al. Extraction isolation and in vitro bacteriostatic test of polysaccharide from cinnabar[J]. Heilongjiang Animal Science and Veterinary Medicine, 2013, 1(7): 117-119 (in Chinese). |
| [35] |
高玉杰, 吕海涛. 浒苔多糖和硒化浒苔多糖抑菌作用研究[J]. 食品科技, 2013, 38(1): 195-198, 205. GAO Y J, LV H T. Antimibacterial activities of Enteromorpha polysaccharide and selenium Enteromorpha polysaccharide[J]. Food Science and Technology, 2013, 38(1): 195-198, 205 (in Chinese). |
| [36] |
周连玉, 钟睿, 焦璐, 等. 黄绿蜜环菌硒多糖抗氧化活性及抑菌活性研究[J]. 食品研究与开发, 2020, 41(15): 6-10. ZHOU L Y, ZHONG R, JIAO L, et al. Antioxidant and antibacterial activities of selenium-containing polysaccharide extracted from Armillaria luteo-virens[J]. Food Research and Development, 2020, 41(15): 6-10 (in Chinese). DOI:10.12161/j.issn.1005-6521.2020.15.002 |
| [37] |
TONG A J, HU R K, WU L X, et al. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters[J]. Journal of Food Biochemistry, 2020, 44(1): e13109. |
| [38] |
GUO W L, PAN Y Y, LI L, et al. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats[J]. Food & Function, 2018, 9(6): 3419-3431. |
| [39] |
BERG R D. Inhibition of bacterial translocation from the gastrointestinal tract to the mesenteric lymph nodes in specific pathogen-free mice but not gnotobiotic mice by non-specific macrophage activation[M]//MESTECKY J, RUSSELL M W, JACKSON S, et al. Advances in experimental medicine and biology, vol 371. Boston: Springer US, 1995: 149-154.
|
| [40] |
UIL J J, VAN ELBURG R M, VAN OVERBEEK F M, et al. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function[J]. Scandinavian Journal of Gastroenterology Supplement, 1997, 223(223): 70-78. |
| [41] |
李思聪, 曾富强, 聂健, 等. 七种中药提取物对大肠埃希菌和金黄色葡萄球菌的体外抑菌试验[J]. 四川畜牧兽医, 2013, 40(2): 29-31. LI S C, ZENG F Q, NIE J, et al. Antimicrobial effects of 7 Chinese herbel medicine extracts on Escherichia coli and Staphylococcus aurous in vitro[J]. Sichuan Animal & Veterinary Sciences, 2013, 40(2): 29-31 (in Chinese). DOI:10.3969/j.issn.1001-8964.2013.02.010 |
| [42] |
谢红兵. 植物多糖对断奶仔猪肠道生理特征和免疫机能的影响及机理研究[D]. 博士学位论文. 长沙: 湖南农业大学, 2018. XIE H B. Effects and mechanism of botanical polysaccharides on intestinal physiological characteristics and immune function of weaned piglets[D]. Ph. D. Thesis. Changsha: Hunan Agricultural University, 2018. (in Chinese) |
| [43] |
陈洪亮. 植物多糖的制备及对肉仔鸡免疫功能影响的研究[D]. 博士学位论文. 北京: 中国农业科学院, 2002. CHEN H L. Studies on the extraction, immunomodulating activities of herbal polysaccharides and approach to the mechansim[D]. Ph. D. Thesis. Bejing: Chinese Academy of Agricultural Sciences, 2002. (in Chinese) |
| [44] |
毛晓峰. 黄芪多糖影响断奶仔猪免疫功能及其作用机理的研究[D]. 硕士学位论文. 北京: 中国农业大学, 2004. MAO X F. Effects of Astragalus polysaccharide on the immunodulatory activities and its mechanism in weaned pigs[D]. Master's Thesis. Beijing: China Agricultural University, 2004. (in Chinese) |
| [45] |
赵露露, 廖秀冬, 张丽阳, 等. 植物提取物及其复合物对鸡源致病菌的体外抑菌作用[J]. 动物营养学报, 2017, 29(9): 3277-3286. ZHAO L L, LIAO X D, ZHANG L Y, et al. Bacteriostatic effects of plant extracts and their compounds on chicken pathogenic bacteria in vitro[J]. Chinese Journal of Animal Nutrition, 2017, 29(9): 3277-3286 (in Chinese). DOI:10.3969/j.issn.1006-267x.2017.09.031 |
| [46] |
李佩, 王树辉, 陈少魁, 等. 肿瘤坏死因子-α刺激对IPEC-1细胞活力, 物理屏障以及炎症相关基因表达的影响[J]. 动物营养学报, 2020, 32(9): 4337-4344. LI P, WANG S H, CHEN S K, et al. Effects of tumor necrosis factor-α stimulation on viability, physical barrier and inflammation-related gene expression of IPEC-1 cells[J]. Chinese Journal of Animal Nutrition, 2020, 32(9): 4337-4344 (in Chinese). |
| [47] |
TATAD A M F, NESIN M, PEOPLES J, et al. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes[J]. Neonatology, 2008, 94: 8-15. DOI:10.1159/000112541 |
| [48] |
CHOI C, KWON D, JUNG K, et al. Expression of inflammatory cytokines in pigs experimentally infected with Mycoplasma hyopneumoniae[J]. Journal of Comparative Pathology, 2006, 134(1): 40-46. DOI:10.1016/j.jcpa.2005.06.009 |
| [49] |
张莘莘, 李文娟, 聂少平, 等. 黑灵芝多糖对体外培养的小鼠腹腔巨噬细胞功能的影响[J]. 中国药理学通报, 2010, 26(9): 1139-1142. ZHANG S S, LI W J, NIE S P, et al. Effects of polysaccharide of Ganoderma atrum on the function of mouse peritoneal macrophages in vitro[J]. Chinese Pharmacological Bulletin, 2010, 26(9): 1139-1142 (in Chinese). |
| [50] |
熊海涛. 丁酸钠对仔猪抗大肠杆菌感染的作用及其机制研究[D]. 博士学位论文. 杭州: 浙江大学, 2016. XIONG H T. Effects and mechannnism of butyrate on disease resistant in piglets[D]. Ph. D. Thesis. Hangzhou: Zhejiang University, 2016. (in Chinese) |
| [51] |
LIANG C J, LEE C W, SUNG H C, et al. Ganoderma lucidum polysaccharides reduce lipopolysaccharide-induced interleukin-1β expression in cultured smooth muscle cells and in thoracic aortas in mice[J]. Evidence-based Complementary and Alternative Medicine, 2014(6): 305149. |
| [52] |
LI H R, XIAO Y H, HAN L, et al. Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system[J]. Brain Research Bulletin, 2021, 171(1): 16-24. |
| [53] |
STRUGNELL R A, WIJBURG O L. The role of secretory antibodies in infection immunity[J]. Nature Reviews Microbiology, 2010, 8(9): 656-667. DOI:10.1038/nrmicro2384 |
| [54] |
LEHNARDT S, MASSILLON L, FOLLETT P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(14): 8514-8519. DOI:10.1073/pnas.1432609100 |
| [55] |
崔小珍, 栾艳, 李婷婷, 等. 松针多糖对鸡巨噬细胞HD11的天然免疫调节[J]. 中国农业科学, 2020, 53(15): 3180-3186. CUI X Z, LUAN Y, LI T T, et al. Innate immunomodulatory effect of pine needle polysaccharide on chicken macrophage HD11[J]. Scientia Agricultura Sinica, 2020, 53(15): 3180-3186 (in Chinese). DOI:10.3864/j.issn.0578-1752.2020.15.017 |
| [56] |
WALSH A M, SWEENEY T, O'SHEA C J, et al. Effects of supplementing dietary laminarin and fucoidan on intestinal morphology and the immune gene expression in the weaned pig[J]. Journal of Animal Science, 2012, 90(Suppl.4): 284-286. |
| [57] |
KHAN I, HUANG G, LI X A, et al. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in ApcMin/+ mice[J]. Pharmacological Research, 2019, 148: 104448. DOI:10.1016/j.phrs.2019.104448 |
| [58] |
WEI B, ZHANG R, ZHAI J, et al. Suppression of Th17 cell response in the alleviation of dextran sulfate Sodium-Induced colitis by Ganoderma lucidum polysaccharides[J]. Journal of Immunology Research, 2018(4): 2906494. |
| [59] |
YU Q, NIE S P, WANG J L. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice[J]. Journal of Functional Foods, 2015, 15: 52-60. DOI:10.1016/j.jff.2015.03.015 |




