碳水化合物是猪饲粮能量的主要来源,一般占饲粮组成的60%以上,其成本超饲粮成本50%[1]。碳水化合物也可以作为机体大分子物质合成的前体物,在猪生长与健康中发挥重要作用[2]。碳水化合物结构复杂、种类繁多,根据其化学分子聚合度(degree of polymerization, DP),可分为单糖(DP=1)、寡糖(DP=2~9)和多聚糖(DP≥10),而多聚糖包括淀粉和非淀粉多糖(non-starch polysaccharide, NSP)[3-4]。当前,对碳水化合物营养性与非营养性生理功效的认识仍不全面、不充分,已成为动物营养与饲料科学理论与技术的短板。过去对猪碳水化合物营养的研究多集中在单一种类及添加水平上,对猪饲粮碳水化合物结构平衡的研究鲜有报道。然而,平衡的饲粮营养结构是保障动物健康和获得理想生产性能的核心条件之一。本文主要以本实验室前期的相关工作为基础,就仔猪主要碳水化合物营养及碳水化合物结构平衡与肠道健康的相关研究进行总结,旨在为猪饲粮碳水化合物的科学利用提供理论依据和技术支持。
1 猪肠道健康的概念与意义肠道不仅是养分消化吸收的主要器官,也是机体防御的重要屏障,其健康状况影响着猪整体健康和生产水平。当前,肠道健康问题仍然是制约养猪业发展的瓶颈,因此,深入认识肠道健康的概念对于寻找保障猪肠道健康的营养技术手段至关重要。维持正常的肠道消化吸收与屏障功能是保障猪肠道健康的关键。在猪肠道中,饲粮各种养分被分解成小分子物质,经肠道绒毛吸收入血液和淋巴,供机体组织利用[5]。肠道分泌的消化酶活性显著影响养分利用,消化酶的分泌量可作为评判猪肠道健康的标识。猪肠壁可分为4层,由内向外依次是黏膜层、黏膜下层、肌层及浆膜层。成年猪的小肠黏膜层表面有许多的绒毛与皱褶,可进一步增加肠道的吸收面积,据报道,成年猪肠道总表面积可达1 000 m2[5]。
肠道黏膜绒毛高度与其上皮细胞数量呈显著正相关,其高度越高,成熟的上皮细胞越多,小肠吸收能力越强。肠道黏膜隐窝深度是细胞成熟度的反映,隐窝越浅表明上皮细胞越成熟,分泌功能越强[6]。猪肠道屏障主要由物理屏障、化学屏障、免疫屏障及微生物屏障4部分组成[7]。物理屏障是保证肠上皮屏障功能与选择通透性的结构基础,紧密连接蛋白则是影响肠上皮细胞通透性大小的重要因素[8]。肠道黏膜化学屏障主要由肠道分泌的黏液、消化酶、胆汁酸与黏蛋白等物质构成,可避免肠黏膜受酸、碱和酶的侵蚀。肠道免疫屏障由肠上皮细胞间的免疫分子、免疫细胞及相关淋巴组织构成,可中和肠道中的抗原,避免病原微生物侵袭进入机体内环境。猪肠道内也栖息着种类繁多、数量庞大的微生物,其中以盲肠和结肠的微生物最为丰富。肠道微生物与宿主在历史进化过程中形成相互依赖的共生关系,因此维持肠道微生物系统的稳态对促进猪肠道健康也十分重要。
2 碳水化合物对猪肠道健康的影响 2.1 淀粉淀粉是植物碳水化合物的最主要形式,由葡萄糖单位构成,是猪最主要能量来源[9]。淀粉按其化学结构可分为直链淀粉(amylose, AM)和支链淀粉(amylopectin, AP)。AM主要由葡萄糖分子经α-(1, 4)糖苷键形成线形直链多糖[3]。AP是不规则呈树枝状的大分子,主要由葡萄糖经α-(1, 4)和α-(1, 6)糖苷键连接构成[10]。研究指出,淀粉的形态、粒径大小及AM/AP值等决定淀粉的物理化学特性[11]。AM结构紧凑表面积小而不利于消化;但AP表面积大,利于与消化酶接触,易被机体消化吸收。饲粮AM/AP值越低,淀粉可消化性越强[12]。此外,饲喂低AM/AP值的淀粉可提高仔猪空肠蔗糖酶与麦芽糖酶活性[13]。另有研究发现,饲喂高AM/AP值的淀粉仔猪小肠绒毛高度及绒隐比增加而凋亡率降低,且可上调肠道屏障功能相关基因表达,同时降低后肠食糜pH[14];然而,饲粮AM与AP满足更平衡的比例时不仅可提高仔猪养分消化率,还可促进肠道形态发育[15]。碳水化合物是肠道微生物的主要底物来源,故其结构与种类不同,肠道微生物的响应模式不同。豌豆淀粉AM/AP值高于小麦淀粉、玉米淀粉和木薯淀粉,采食豌豆淀粉仔猪小肠和大肠双歧杆菌、芽孢杆菌和乳酸杆菌丰度提高,肠道大肠杆菌丰度降低[16]。饲喂高AM/AP值淀粉能够显著提高育肥猪回肠梭菌属、乳杆菌属、拟杆菌门及链球菌丰度[17]。饲粮淀粉AM含量增加,仔猪后肠微生物发酵底物增加,短链脂肪酸(short chain fatty acids, SCFAs)产量提高,肠道pH降低,同时促进后肠双歧杆菌增殖[18];而采食高AP/AM值淀粉,肥育猪后肠双歧杆菌数量显著减少,SCFAs含量也降低[19]。
2.2 纤维饲粮纤维主要由NSP组成,是影响仔猪肠道功能的重要组分,饲粮添加纤维可提高猪回肠蔗糖酶与麦芽糖酶活性[20]。采食小麦麸纤维饲粮比玉米纤维和大豆纤维可上调肠道Toll样受体2(TLR2)基因表达及改变肠道微生物结构而增强仔猪肠道屏障功能[21],且长期饲喂豌豆纤维显著改善生长猪结肠屏障与免疫功能[22]。NSP组分不同,对肠道功能产生的效应不同。饲粮添加木聚糖和葡聚糖可促进肠道屏障功能,改善微生态平衡,利于仔猪肠道健康;而添加纤维素却未有以上促进作用[23]。然而,添加可溶性纤维(菊粉)和不可溶性纤维(木质纤维素)比单一纤维添加显著促进仔猪养分消化及肠道屏障功能[24],且复合添加可溶与不可溶纤维显著增加大鼠回肠上皮杯状细胞数量[25]。本实验室进一步研究发现,可溶与不溶性纤维满足适宜比例的同时且维持合理水平,可改善仔猪肠道形态发育[15]。相比玉米和大豆纤维,饲喂小麦和豌豆纤维仔猪后肠大肠杆菌数量减少,双歧杆菌数量显著增加[21]。采食豌豆纤维可增加生长猪结肠乳酸菌数量[22],提高后肠纤维分解菌属、乳酸菌属和毛螺菌属丰度[26]。饲粮添加菊粉可增加仔猪和肥育猪后肠双歧杆菌数量,减少大肠杆菌数量[27]。研究肠道微生物对不同NSP组分的响应,发现饲喂葡聚糖和纤维素仔猪后肠乳酸杆菌属等有益菌数量明显增加,但木聚糖组仔猪肠道变形菌门等有害菌数量显著增加[28]。
2.3 寡糖寡糖是小分子碳水化合物,因其可作为微生物发酵底物、促进有益菌生成而被定义为益生元[29]。饲粮添加寡糖可促进仔猪和生长肥育猪生长[30-31],提高母猪繁殖性能[32-33]。饲粮添加果寡糖可增加仔猪肠黏膜厚度,促进上皮细胞增殖,提高丁酸产生量[34]。饲粮添加苹果果胶寡糖可缓解仔猪遭受轮状病毒引起的肠道损伤[35]。饲粮添加褐藻寡糖也显著促进仔猪肠道黏膜生长,上调肠道屏障功能相关基因表达[36]。此外,饲粮添加果寡糖显著增加仔猪直肠梭菌和双歧杆菌数量,减少沙门氏菌、大肠杆菌、梭菌和志贺氏杆菌数量等[37-38]。饲粮添加纤维寡糖则可降低结肠链球菌和大肠杆菌丰度,提高双歧杆菌丰度[39]。半乳寡糖和甘露寡糖均可抑制鼠伤寒沙门氏菌和致病性大肠杆菌产生,促进双歧杆菌和乳酸杆菌增殖[40-41]。
以上研究表明,饲粮AM/AP值和可溶与不溶性纤维间的比例适宜,更有利于猪的肠道健康。研究发现,在患糖尿病妇女饮食中提高纤维占淀粉的比例,有利于2型糖尿病人的健康[42]。在猪饲粮中淀粉占比过高而非淀粉多糖含量不足将致使后肠微生物发酵所需的底物减少,不利于后肠微生物繁殖。若猪饲粮淀粉含量不足而非淀粉多糖占比增加将导致饲粮能量浓度降低,抗营养因子增多,不利于营养物质的消化吸收和机体生长发育。因此碳水化合物营养的内涵不应局限于营养素与营养水平,维持饲粮不同种类碳水化合物间的平衡对猪生长与肠道健康也尤为重要。
3 碳水化合物平衡对猪肠道健康的影响及机制饲粮营养结构平衡的概念已得到学术界的广泛关注。例如,必需氨基酸之间以及必需氨基酸与非必需氨基酸间的平衡,矿物质元素间的适宜比例,甚至饲粮中各营养要素(蛋白质、碳水化合物、脂肪、矿物质和维生素等)的整体平衡等,然而有关碳水化合物平衡的概念尚属空白。本实验室通过研究和总结认为,碳水化合物平衡的内涵至少包括淀粉内AM与AP比例的平衡,NSP间可溶性NSP与不溶性NSP比例与添加水平的平衡,以及淀粉、NSP与寡糖间的平衡等。
3.1 饲粮碳水化合物平衡模式初探本实验室研究发现,饲粮淀粉AM/AP值与NSP水平满足合适的组合时可促进仔猪肠道发育、消化吸收与屏障功能;然而甘露寡糖与不同AM/AP值淀粉及不同水平NSP以不同组合模式供给仔猪时,低剂量的甘露寡糖有利于提高仔猪的生长性能并促进肠道功能[15, 43]。这提示了饲粮淀粉、NSP与寡糖之间存在互作,因此满足饲粮淀粉AM/AP值、可溶性NSP与不溶性NSP的比例与水平、寡糖剂量达到平衡关系时会进一步促进仔猪肠道功能。本实验室进一步采用营养学、分子生物学和生物信息学等研究手段,以生长性能、养分消化率、血清生化、肠道功能及微生物结构等为联合效应指标,发现当饲粮碳水化合物组合满足淀粉AM/AP值为1∶1,外源NSP复合物(菊粉与纤维素1∶1)添加3%,甘露寡糖供给400 mg/kg时,与其他组合比较,可通过上调肠道发育屏障功能基因表达、提高消化酶活性而增强仔猪肠道功能,且可提高后肠纤维降解菌、产丁酸菌及SCFAs的产量;同时显著改善仔猪机体糖类、脂肪及蛋白质代谢和提高生长性能[15]。以上研究显示,对7~18 kg仔猪而言,饲粮碳水化合物平衡模式为AM/AP值1∶1、外源NSP复合物3%及甘露寡糖400 mg/kg,该模式更有利于仔猪生长和肠道健康达到最佳状态。
3.2 碳水化合物平衡影响猪肠道健康的微生物学机制饮食是影响哺乳和断奶期间仔猪肠道微生物结构与功能的重要因素[44]。而人肠道微生物是调控代谢与健康的关键靶点;肠道微生物可编码产生相关酶降解植物多糖,以单糖与寡糖进入微生物细胞而被利用[45]。抗生素干预会破坏小鼠肠道微生物组成结构。易诱发参与碳水化合物代谢的关键基因表达异常,进而引起机体肥胖[46]。然而提高肠道微生物多样性,可促进婴儿肠道利用碳水化合物的基因表达[47]。饲喂不同碳水化合物组合显著影响仔猪肠道微生物结构与代谢。当饲喂碳水化合物平衡组合饲粮时,与最差组合相比,能够显著提高仔猪结肠厚壁菌门的丰度而明显降低拟杆菌门的丰度,同时促进产丁酸菌与丁酸的生成[15]。据报道,肠道微生物调节机体代谢尤其是糖和脂代谢的机制,主要是通过其代谢产物与单磷酸腺苷活化蛋白激酶(AMP-activated protein kinase, AMPK)信号途径实现的[48-49]。未被猪前肠利用的碳水化合物,经后肠微生物发酵产生的代谢产物主要是SCFAs。由此推测碳水化合物平衡作用猪肠道健康受微生物影响,且可能机制与其代谢产物SCFAs有关。在无菌仔猪、无菌仔猪移植粪菌及无菌仔猪饲粮拌喂SCFAs试验中,微生物缺失显著降低了碳水化合物平衡组合对仔猪生长、消化吸收与SCFAs产生的效应,表明仔猪碳水化合物平衡组合依赖肠道微生物发挥效用;同时发现肠道微生物结构破坏时,将损害碳水化合物平衡组合对仔猪后肠微生物与代谢的有益影响,表明仔猪碳水化合物平衡组合要依赖合理结构的肠道微生物[50];此外,肠道微生物介导仔猪碳水化合物平衡模式的机制可能与SCFAs-G蛋白偶联受体(G-protein-coupled receptors,GPCRs)-AMPK-乙酰辅酶A羧化酶(ACC)信号通路有关[51]。
4 小结与展望碳水化合物数量大、种类多、结构复杂,寻找促进碳水化合物高效利用的营养技术非常重要。本实验室研究发现,饲粮不同碳水化合物间应满足适宜的平衡关系,当饲粮淀粉AM/AP值、NSP水平及寡糖剂量的组合达到平衡时可进一步增强仔猪的肠道健康,而碳水化合物平衡影响猪肠道健康的机制与微生物及其代谢产物有关(图 1)。传统的猪营养问题多关注碳水化合物的能值与抗营养作用,但忽视了不同种类与结构碳水化合物营养生理效应的差异及碳水化合物间的比例平衡。研究碳水化合物间的平衡模式有利于认识其代谢的复杂性,同时有助于饲粮配方优化及动物遗传潜力的发挥。碳水化合物平衡模式也受猪品种、生理状态、饲养环境与饲养管理等多种因素影响。今后还需进一步开展系统研究,以建立适合不同养殖条件下的猪饲粮碳水化合物平衡模式。
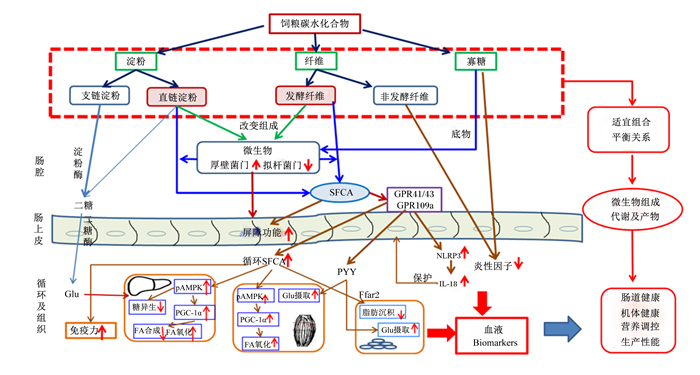
|
SFCA:短链脂肪酸short chain fatty acid;GPR:G蛋白偶联受体G-protein-coupled receptor;Glu:葡萄糖glucose;pAMPK:磷酸化单磷酸腺苷活化蛋白激酶phosphorylated adenosine monophosphate activated protein kinase;PGC-1α:过氧化物酶体增殖物激活受体γ共激活因子-1α peroxisome proliferator activated receptor γ coactivator-1α;FA:脂肪酸fatty acid;PYY:酪酪肽peptide YY;Ffar2:游离脂肪酸受体2 free fatty acid receptor 2;NLRP3:NOD样受体热蛋白结构域相关蛋白3 NOD-like receptor thermal protein domain associated protein 3;IL-18:白细胞介素-18 interleukin-18;Biomarkers:生物标记。 图 1 饲粮碳水化合物平衡效应及机制 Fig. 1 Effects and mechanisms of dietary carbohydrate balance |
| [1] |
BACH KNUDSEN K E, HEDEMANN M S, LÆRKE H N. The role of carbohydrates in intestinal health of pigs[J]. Animal Feed Science and Technology, 2012, 173(1/2): 41-53. |
| [2] |
印遇龙, 吴信, 李铁军. 猪日粮碳水化合物营养研究进展[J]. 动物营养学报, 2007, 19(增刊): 435-440. YIN Y L, WU X, LI T J. Research on carbohydrates in swine nutrition[J]. Chinese Journal of Animal Nutrition, 2007, 19(Suppl.): 435-440 (in Chinese). |
| [3] |
CUMMINGS J H, STEPHEN A M. Carbohydrate terminology and classification[J]. European Journal of Clinical Nutrition, 2007, 61(Suppl.1): S5-S18. |
| [4] |
ENGLYST K N, LIU S, ENGLYST H N. Nutritional characterization and measurement of dietary carbohydrates[J]. European Journal of Clinical Nutrition, 2007, 61(Suppl.1): S19-S39. |
| [5] |
余冰, 张克英, 郑萍, 等. 猪营养与肠道健康[J]. 中国畜牧杂志, 2010, 46(15): 73-76. YU B, ZHANG K Y, ZHENG P, et al. Research advances of nutrition on intestinal health of pigs[J]. Chinese Journal of Animal Science, 2010, 46(15): 73-76 (in Chinese). |
| [6] |
任善茂, 陶勇. 精氨酸对断奶仔猪肠道健康的影响及相关机制[J]. 动物营养学报, 2014, 26(8): 2035-2039. REN S M, TAO Y. Effect of arginine on intestinal health of weaner piglets and related mechanisms[J]. Chinese Journal of Animal Nutrition, 2014, 26(8): 2035-2039 (in Chinese). DOI:10.3969/j.issn.1006-267x.2014.08.001 |
| [7] |
周华, 陈代文, 毛湘冰, 等. 低蛋白质平衡氨基酸饲粮对断奶仔猪肠道健康影响的研究进展[J]. 动物营养学报, 2015, 27(4): 1041-1046. ZHOU H, CHEN D W, MAO X B, et al. Effects of a low protein diet with balanced amino acids on intestinal health in weaning piglets[J]. Chinese Journal of Animal Nutrition, 2015, 27(4): 1041-1046 (in Chinese). DOI:10.3969/j.issn.1006-267x.2015.04.006 |
| [8] |
DAVILA A M, BLACHIER F, GOTTELAND M, et al. Re-print of "Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host"[J]. Pharmacological Research, 2013, 69(1): 114-126. DOI:10.1016/j.phrs.2013.01.003 |
| [9] |
YIN Y L, DENG Z Y, HUANG H L, et al. Nutritional and health functions of carbohydrate for pigs[J]. Journal of Animal and Feed Sciences, 2004, 13(4): 523-538. DOI:10.22358/jafs/67623/2004 |
| [10] |
TESTER R F, KARKALAS J, QI X. Starch-composition, fine structure and architecture[J]. Journal of Cereal Science, 2004, 39(2): 151-165. DOI:10.1016/j.jcs.2003.12.001 |
| [11] |
DONA A C, PAGES G, GILBERT R G, et al. Digestion of starch: in vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release[J]. Carbohydrate Polymers, 2010, 80(3): 599-617. DOI:10.1016/j.carbpol.2010.01.002 |
| [12] |
SINGH J, DARTOIS A, KAUR L. Starch digestibility in food matrix: a review[J]. Trends in Food Science & Technology, 2010, 21(4): 168-180. |
| [13] |
GAO X Q, YU B, YU J, et al. Influences of dietary starch structure on intestinal morphology, barrier functions, and epithelium apoptosis in weaned pigs[J]. Food & Function, 2020, 11(5): 4446-4455. |
| [14] |
GAO X Q, YU B, YU J, et al. Effects of dietary starch structure on growth performance, serum glucose-insulin response, and intestinal health in weaned piglets[J]. Animals: an Open Access Journal from MDPI, 2020, 10(3): 543. |
| [15] |
ZHOU H, YU B, HE J, et al. The optimal combination of dietary starch, non-starch polysaccharides, and mannan-oligosaccharide increases the growth performance and improves butyrate-producing bacteria of weaned pigs[J]. Animals: an Open Access Journal from MDPI, 2020, 10(10): 1745. |
| [16] |
XIANG Z T, Q H W, HAN G Q, et al. Real-time TaqMan polymerase chain reaction to quantify the effects of different sources of dietary starch on Bifidobacterium in the intestinal tract of piglets[J]. African Journal of Biotechnology, 2011, 10(25): 5059-5067. |
| [17] |
LUO Y H, YANG C, WRIGHT A D G, et al. Responses in ileal and cecal bacteria to low and high amylose/amylopectin ratio diets in growing pigs[J]. Applied Microbiology and Biotechnology, 2015, 99(24): 10627-10638. DOI:10.1007/s00253-015-6917-2 |
| [18] |
FOUHSE J M, GÄNZLE M G, REGMI P R, et al. High amylose starch with low in vitro digestibility stimulates hindgut fermentation and has a bifidogenic effect in weaned pigs[J]. The Journal of Nutrition, 2015, 145(11): 2464-2470. DOI:10.3945/jn.115.214353 |
| [19] |
王华杰, 陈代文, 田刚, 等. 不同直链支链比玉米淀粉饲粮对生长育肥猪生长性能和肉品质的影响[C]//中国畜牧兽医学会动物营养学分会第十二次动物营养学术研讨会论文集. 武汉: 中国畜牧兽医学会, 2016. WANG H J, CHEN D W, TIAN G, et al. Effects of corn starch diets with different amylopectin amylopectin ratio on growth performance and meat quality of growing finishing pigs[D]//Proceedings of the 12th Symposium on Animal Nutrition, Animal Nutrition Branch, Association of Animal Science and Veterinary Medicine. Wuhan: Chinese Association of Animal Science and Veterinary Medicine, 2016. (in Chinese) |
| [20] |
CHEN H, MAO X B, CHE L Q, et al. Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs[J]. Animal Feed Science and Technology, 2014, 195: 101-111. DOI:10.1016/j.anifeedsci.2014.06.002 |
| [21] |
CHEN H, CHEN D W, MICHIELS J, et al. Dietary fiber affects intestinal mucosal barrier function by regulating intestinal bacteria in weaning piglets[J]. Communications in Agricultural and Applied Biological Sciences, 2013, 78(1): 71-78. |
| [22] |
CHE L Q, CHEN H, YU B, et al. Long-term intake of pea fiber affects colonic barrier function, bacterial and transcriptional profile in pig model[J]. Nutrition and Cancer, 2014, 66(3): 388-399. DOI:10.1080/01635581.2014.884229 |
| [23] |
WU X Y, CHEN D W, YU B, et al. Effect of different dietary non-starch fiber fractions on growth performance, nutrient digestibility, and intestinal development in weaned pigs[J]. Nutrition, 2018(51/52): 20-28. |
| [24] |
CHEN T T, CHEN D W, TIAN G, et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet[J]. Animal Feed Science and Technology, 2020, 260: 114335. DOI:10.1016/j.anifeedsci.2019.114335 |
| [25] |
HINO S, TAKEMURA N, SONOYAMA K, et al. Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats[J]. The Journal of Nutrition, 2012, 142(8): 1429-1436. DOI:10.3945/jn.112.159731 |
| [26] |
赵瑶. 日粮纤维源对生长猪生产性能和肠道健康的影响机制及肠道微生物的媒介作用[D]. 硕士学位论文. 雅安: 四川农业大学, 2018. ZHAO Y. The influence of different dietary fibers on the production performance and intestinal health of growing pigs and the role of intestinal microflora as a medium[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2018. (in Chinese) |
| [27] |
LYNCH M B, SWEENEY T, CALLAN J J, et al. The effect of dietary crude protein concentration and inulin supplementation on nitrogen excretion and intestinal microflora from finisher pigs[J]. Livestock Science, 2007, 109(1/2/3): 204-207. |
| [28] |
TIAN G, WU X Y, CHEN D W, et al. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model[J]. Molecular Nutrition & Food Research, 2017, 61(10): 1700012. |
| [29] |
MUSSATTO S I, MANCILHA I M. Non-digestible oligosaccharides: a review[J]. Carbohydrate Polymers, 2007, 68(3): 587-597. DOI:10.1016/j.carbpol.2006.12.011 |
| [30] |
DUAN X D, CHEN D W, ZHENG P, et al. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring[J]. Animal Feed Science and Technology, 2016, 218: 17-25. DOI:10.1016/j.anifeedsci.2016.05.002 |
| [31] |
KIM W T, SHINDE P, CHAE B J. Effect of lecithin with or without chitooligosaccharide on the growth performance, nutrient digestibility, blood metabolites and pork quality of finishing pigs[J]. Canadian Journal of Animal Science, 2008, 88(2): 283-292. DOI:10.4141/CJAS07079 |
| [32] |
WAN J, YANG K Y, XU Q S, et al. Dietary chitosan oligosaccharide supplementation improves foetal survival and reproductive performance in multiparous sows[J]. RSC Advances, 2016, 6(74): 69670-70722. DOI:10.1039/C6RA09274H |
| [33] |
CHENG L K, WANG L X, XU Q S, et al. Chitooligosaccharide supplementation improves the reproductive performance and milk composition of sows[J]. Livestock Science, 2015, 174: 74-81. DOI:10.1016/j.livsci.2015.02.003 |
| [34] |
TSUKAHARA T, IWASAKI Y, NAKAYAMA K, et al. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa[J]. Journal of Nutritional Science and Vitaminology, 2003, 49(6): 414-421. DOI:10.3177/jnsv.49.414 |
| [35] |
MAO X B, XIAO X J, CHEN D W, et al. Dietary Apple pectic oligosaccharide improves gut barrier function of rotavirus-challenged weaned pigs by increasing antioxidant capacity of enterocytes[J]. Oncotarget, 2017, 8(54): 92420-92430. DOI:10.18632/oncotarget.21367 |
| [36] |
WAN J, ZHANG J, CHEN D W, et al. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs[J]. Animal Feed Science and Technology, 2017, 234: 118-127. DOI:10.1016/j.anifeedsci.2017.09.006 |
| [37] |
SAULNIER D M A, GIBSON G R, KOLIDA S. In vitro effects of selected synbiotics on the human faecal microbiota composition[J]. FEMS Microbiology Ecology, 2008, 66(3): 516-527. DOI:10.1111/j.1574-6941.2008.00561.x |
| [38] |
TANNER S A, LACROIX C, DEL'HOMME C, et al. Effect of Bifidobacterium thermophilum RBL67 and fructo-oligosaccharides on the gut microbiota in Göttingen minipigs[J]. The British Journal of Nutrition, 2015, 114(5): 746-755. DOI:10.1017/S0007114515002263 |
| [39] |
JIAO L F, KE Y L, XIAO K, et al. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs[J]. Journal of Animal Science, 2015, 93(3): 1157-1164. DOI:10.2527/jas.2014-8248 |
| [40] |
CASTILLO M, MARTÍN-ORÚ E S M, TAYLOR-PICKARD J A, et al. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function[J]. Journal of Animal Science, 2008, 86(1): 94-101. DOI:10.2527/jas.2005-686 |
| [41] |
TZORTZIS G, GOULAS A K, GEE J M, et al. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo[J]. The Journal of Nutrition, 2005, 135(7): 1726-1731. DOI:10.1093/jn/135.7.1726 |
| [42] |
ALESSA H B, BHUPATHIRAJU S N, MALIK V S, et al. Carbohydrate quality and quantity and risk of type 2 diabetes in US women[J]. The American Journal of Clinical Nutrition, 2015, 102(6): 1543-1553. DOI:10.3945/ajcn.115.116558 |
| [43] |
ZHOU H, CHEN D W, HE J F, et al. Effects of different combinations of dietary starch and non-starch polysaccharides on intestinal functions, and lipid and glucose metabolism in weaned pigs[J]. Journal of Animal and Feed Sciences, 2020, 29(3): 241-249. DOI:10.22358/jafs/127688/2020 |
| [44] |
FRESE S A, PARKER K, CALVERT C C, et al. Diet shapes the gut microbiome of pigs during nursing and weaning[J]. Microbiome, 2015, 3: 28. |
| [45] |
TANCA A, ABBONDIO M, PALOMBA A, et al. Potential and active functions in the gut microbiota of a healthy human cohort[J]. Microbiome, 2017, 5(1): 79. |
| [46] |
CHO I, YAMANISHI S, COX L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity[J]. Nature, 2012, 488(7413): 621-626. |
| [47] |
KOENIG J E, SPOR A, SCALFONE N, et al. Succession of microbial consortia in the developing infant gut microbiome[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(Suppl.1): 4578-4585. |
| [48] |
SONNENBURG J L, BÄCKHED F. Diet-microbiota interactions as moderators of human metabolism[J]. Nature, 2016, 535(7610): 56-64. |
| [49] |
DUMAS M E, BARTON R H, TOYE A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(33): 12511-12516. |
| [50] |
ZHOU H, SUN J, YU B, et al. Gut microbiota absence and transplantation affect growth and intestinal functions: an investigation in a germ-free pig model[J]. Animal Nutrition, 2021, 7(2): 295-304. |
| [51] |
ZHOU H, YU B, SUN J, et al. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 61. |




