肠道是猪、禽消化和吸收营养物质的主要组织器官,也是抵御病原性微生物感染的一道重要屏障系统,在维持猪、禽机体内环境稳态中扮演重要角色[1-2]。在养殖业,引起猪、禽肠道健康问题的因素很多,其中由病原微生物引发的猪、禽肠道损伤问题一直是困扰猪、禽业健康发展的重要因素之一[3]。病原微生物主要通过破坏肠道上皮屏障和生物屏障以及抑制肠道免疫功能引起肠道损伤,感染病原微生物的猪、禽肠道组织通常表现为肠黏膜组织形态和结构急性损伤、通透性增强、消化吸收功能下降、炎症反应加剧、氧化应激、肠道菌群结构改变及对营养物质(包括氨基酸、葡萄糖和脂类)的代谢改变,继而导致猪、禽肠道继发性损伤和功能紊乱[4]。当前,已有多项研究探明了不同营养调控物质在调控猪、禽病原性肠道损伤中的作用,这些营养调控物质主要包括:1)天然植物提取物,包括黄酮类植物活性成分、植物精油、多酚类植物化合物和多糖类植物化合物等[5];2)微生态制剂,包括鼠李糖乳杆菌、植物乳杆菌和凝结芽孢杆菌等[6];3)功能性氨基酸,包括谷氨酰胺、L-精氨酸、N-乙酰半胱氨酸和支链氨基酸等[7-8]。此外,还包括其他营养调控剂,如氧化锌、鱼油、壳聚糖和维生素等[9-12]。本文综述了各类猪、禽病原性肠道损伤机理和多种营养调控物质在修复猪、禽病原性肠道损伤中的作用和机制,旨在为建立高效修复猪、禽病原性肠道损伤多靶标营养调控技术的研究和应用提供参考。
1 猪、禽病原性肠道损伤及机理 1.1 猪病毒性肠道损伤及机理生猪养殖过程中,引起猪群腹泻的病毒主要为肠道冠状病毒,包括猪流行性腹泻病毒(porcine epidemic diarrhea virus,PEDV)、传染性胃肠炎病毒(transmissible gastroenteritis virus,TGEV)以及新发的猪三角洲冠状病毒(porcine deltacoronavirus,PDCoV)和猪急性腹泻综合征病毒(swine acute diarrhea syndrome coronavirus,SADS-CoV)[13]。此外,猪轮状病毒在猪群腹泻中也比较常见[14]。
冠状病毒引起的症状非常相似,主要表现为脱水、呕吐和水样腹泻。单纯依靠临床诊断很难区分这些病毒,因此需要实验室手段进行鉴别诊断。在以上4种冠状病毒中,PEDV的流行率最高,致病性也最强[13]。由于仔猪的免疫系统发育不完善,肠细胞更新速度慢,因此PEDV对仔猪的危害最大,死亡率可高达90%[15]。研究发现,PEDV不仅可以通过粪-口途径传播,也可以通过鼻腔途径传播[16]。小肠上皮细胞是PEDV入侵的主要靶细胞,尤其是小肠后段,感染PEDV后其绒毛会发生明显萎缩。肠细胞脱落和功能紊乱会导致消化酶如二糖酶、亮氨酸氨肽酶以及碱性磷酸酶活性的下降,进而引起仔猪消化不良,肠腔渗透压升高,最终导致仔猪腹泻[17]。PEDV感染仔猪后,仔猪小肠中杯状细胞的数量也会显著下降,进而降低黏液的分泌。肠嗜铬细胞分泌的5-羟色胺会诱导仔猪呕吐,并通过刺激炎性细胞因子的表达降低仔猪食欲,从而加重脱水[18]。由于碳酸氢盐的丢失,仔猪感染PEDV后会伴随高血钾和酸中毒。同时,肠道屏障的破坏会吸收肠腔中的食糜和细菌,引发过敏反应和共感染,并改变肠道菌群的结构[19]。
猪群感染PEDV 7~10 d左右,血清中会检测到抗PEDV的免疫球蛋白G(IgG)或免疫球蛋白A(IgA),且抗体水平可以持续半年左右[20]。粪便中分泌型IgA出现的时间较晚(感染后14 d左右),可以持续1~2个月[21-22]。研究发现,猪群抵抗PEDV不仅依赖机体的体液免疫,也离不开细胞介导的免疫应答反应,如炎性反应。PEDV感染新生仔猪,可上调干扰素和炎性细胞因子的表达水平。该作用一方面是由于病毒直接作用于细胞的模式识别受体,激活下游的信号通路;另一方面是由于病毒诱导的肠细胞凋亡或坏死,间接促进了细胞因子的表达。PEDV感染的急性期,细胞因子的表达有利于初始T细胞分化为细胞毒性细胞或辅助性T细胞,从而清除受感染的细胞或诱导中和抗体的产生[23]。然而,细胞因子的过度表达会降低食欲,损伤肠道的屏障功能。
1.2 猪细菌性肠道损伤及机理大肠杆菌是引起仔猪肠道损伤的主要细菌性微生物[24]。它主要通过被污染的饲料和饮水进入仔猪肠道,可破坏仔猪肠道黏膜屏障。产肠毒素大肠杆菌(ETEC)是流行率较高的一种大肠杆菌,主要依靠黏附因子定植于仔猪肠上皮细胞,通过大量繁殖破坏肠道正常微生态平衡,并释放多种肠毒素,如耐热肠毒素a(STa)、耐热肠毒素b(STb)、不耐热肠毒素(LT)[25]。这些肠毒素与肠道上皮细胞表面的鸟苷酸环化酶受体的胞外部分结合,激活其胞内的功能结构域,增加细胞内环磷酸鸟苷(cGMP)含量,并激活cGMP依赖的蛋白激酶,导致肠道水分和电解质代谢失衡,最终引起仔猪腹泻[26]。STa也可以通过调节肠道上皮细胞的紧密连接引起腹泻,即通过抑制紧密连接相关蛋白的表达,增加肠道上皮细胞的通透性。STa还可以介导先天性免疫应答反应,促进炎性细胞因子的分泌[27]。通过基因表达分析,发现表达STa重组大肠杆菌感染可下调仔猪空肠营养代谢相关基因胰岛素受体(INSR)和磷酸烯醇式丙酮酸羧激酶1(PCK1)及回肠脂蛋白脂肪酶(LPL)相对表达量,提示ETEC感染可以影响肠道正常的糖代谢和脂质代谢[27]。ETEC感染还可以改变肠道微生态环境,如提高仔猪回肠和结肠中变形菌门比例,降低空肠厚壁菌门和拟杆菌门数量以及回肠厚壁菌门数量[28]。
1.3 禽病原性肠道损伤及机理 1.3.1 家禽球虫病和坏死性肠炎艾美耳球虫是专性细胞内寄生原虫,球虫卵囊可以通过粪口传播。寄生在鸡肠道的球虫主要有7种: 堆型艾美耳球虫、毒害艾美耳球虫、巨型艾美耳球虫和早熟艾美耳球虫寄生在小肠前段(十二指肠和空肠),柔嫩艾美耳球虫、和缓艾美耳球虫和布氏艾美耳球虫寄生于小肠后段、盲肠和结直肠近端,其中流行最广的是柔嫩艾美耳球虫[29]。球虫子孢子侵入宿主后,在裂殖生殖阶段破坏肠道黏膜细胞,导致肠道通透性增加、消化吸收功能降低、电解质代谢紊乱,诱导炎症反应,进而发生临床和亚临床症状的球虫病[30]。球虫感染是诱导家禽坏死性肠炎的主要因素之一,球虫感染造成的肠道黏膜损伤,使血液等体内的蛋白质渗透到肠腔,为产气荚膜梭菌的增殖提供了丰富的蛋白质底物[31]。A型和G型产气荚膜梭菌是家禽坏死性肠炎的主要致病菌[32],细菌可以分泌大量的水解酶类降解肠道的黏液层,为其快速增殖提供营养物质来源,当细菌增殖到一定数量后形成菌落,引发群体感应系统(quorum sensing system,QS系统),诱导毒素等毒力因子的基因表达,毒素的细胞毒性作用造成肠道黏膜的坏死,细菌表达黏附素附着到肠道黏膜下层,为其深入感染和定植创造条件,此时肠道黏膜大量坏死和脱落[33]。临床症状的坏死性肠炎表现为小肠肠壁变薄变脆或出现大范围可见的溃疡和坏死性损伤,死亡率高达30%,亚临床症状的坏死性肠炎表现为生长性能下降[34],该病每年给全球带来约6亿美金的经济损失[35]。
1.3.2 其他家禽病原性肠道损伤禽致病性大肠杆菌(avian pathogenic Escherichia coli,APEC)是阻碍家禽养殖业的重要病原菌之一,可导致家禽肝周炎、气囊炎、心包炎、腹膜炎、蜂窝组织炎、关节炎等,统称为大肠杆菌病[36]。APEC致病机理复杂,多种毒力因子参与致病过程,包括但不限于黏附素、侵袭素、保护素、铁离子摄取系统、毒素、双组分系统、QS系统、转录调控因子、分泌系统等。它们参与了APEC的黏附、入侵、定植、增殖、细胞裂解和损伤、运动和生物膜形成等[37]。APEC在家禽生产中可垂直传播,对雏鸡的致死率高达53.5%,且有随食物链传播的风险,威胁食品安全和人类健康[38]。肠炎沙门氏菌可引起雏鸡的急性全身性感染,成年鸡通常不表现出临床症状,但是存在食物链传播的风险。肠炎沙门氏菌的多种毒力因子和分泌系统参与肠道上皮细胞损伤,诱导巨噬细胞和中性粒细胞参与炎症反应[39]。空肠弯曲杆菌是家禽养殖中普遍流行的主要致病菌之一,它通过Toll样受体(TLRs)介导的信号通路激活非特异性免疫反应,破坏肠道上皮细胞之间的紧密连接蛋白,增加肠道的通透性,造成细菌移位、肠道吸收功能降低、肠道菌群结构改变[40]。空肠弯曲杆菌的传播速度很快,虽然家禽通常无明显症状,但是生产性能降低带来的经济损失以及随食物链传播的风险不容忽视。
猪、禽肠道病原性损伤发生机理总结如图 1所示。
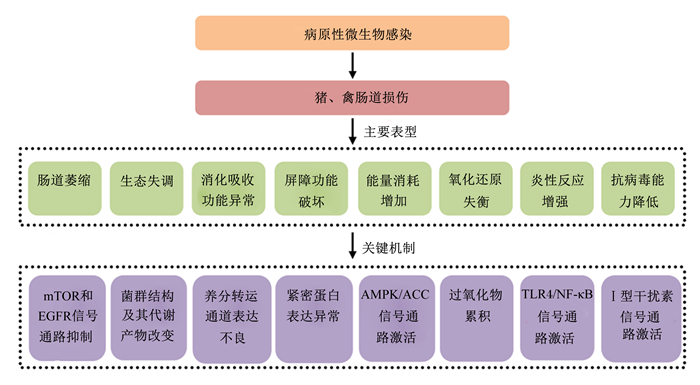
|
mTOR:哺乳动物雷帕霉素靶蛋白mammalian target of rapamycin;EGFR:上皮细胞生长因子受体epidermal growth factor receptor;AMPK/ACC:AMP活化的蛋白激酶/乙酰辅酶A羧化酶AMP-activated protein kinase/acetyl-CoA carboxylase;TLR4/NF-κB:Toll样受体4/核转录因子-κB Toll-like receptor-4/nuclear factor kappa B。 图 1 猪、禽病原性肠道损伤发生机理 Fig. 1 Mechanism of pathogenic intestinal injury in pigs and poultry |
在饲料全面禁抗的背景下,植物提取物作为一种绿色、安全的饲料添加剂,被认为是理想的抗生素替代品,可以用于改善畜禽生产性能与肠道健康。目前研究较多的天然植物提取物主要包括黄酮类植物活性成分、植物精油、多酚类植物化合物和多糖类植物化合物[41-42]。
2.1.1 黄酮类植物化合物黄酮类化合物广泛存在于多种植物中,其独特的化学结构使其对哺乳动物和其他类型的细胞具有许多重要的生物学功能。常见的黄酮类植物提取物包括黄芩、槲皮素和葛根素等。在猪生产中,饲粮中添加1 000 mg/kg黄芩提取物可以通过抑制核转录因子-κB(NF-κB)/p38信号通路发挥抗炎功效,缓解大肠杆菌感染引起的断奶仔猪腹泻[43]。本实验室研究发现,葛根素可以抑制肠道中PEDV的复制,增强PEDV感染仔猪的肠道吸收功能,并通过抑制NF-κB信号通路降低血浆和回肠中炎性细胞因子的表达;葛根素还具有调节肠道菌群和抗氧化的作用[44-45]。饲粮中添加槲皮素对仔猪肠道损伤的恢复也有促进作用,体外试验表明槲皮素可以与3C样蛋白酶结合,抑制PEDV的复制[46]。这些研究表明,黄酮类化合物具有抗氧化、抑菌、抗病毒、抗炎、改善肠道菌群结构等生物学作用,从而有助于猪群的健康生长。
2.1.2 植物精油植物精油包括牛至油、香芹酚、百里香酚、肉桂油等,在促进猪、禽肠道健康及提高其生产性能方面表现出明显的效果。植物精油在家禽抗病原感染的应用中非常广泛,对家禽肠道的沙门氏菌、大肠杆菌、弯曲杆菌和产气荚膜梭菌均有抑制作用[47]。饲粮中添加百里香酚和香芹酚可以改善肠道的屏障功能,缓解产气荚膜梭菌感染肉鸡引起的肠道损伤,并通过Toll样受体2(TLR2)介导的信号通路抑制肠道炎症反应,促进回肠卷曲乳酸杆菌(Lactobacillus crispatus)和敏捷乳杆菌(Lactobacillus agilis)的增殖,降低产气荚膜梭菌的致病性[48-49]。牛至、大蒜植物精油、复合植物精油有改善艾美耳球虫感染肉鸡生长性能的作用,主要通过调控肉鸡免疫功能和肠道菌群、提高肠道消化功能等发挥抗球虫作用[50-52]。香芹酚通过增加肉鸡盲肠菌群的多样性和乳酸杆菌数量,激活盲肠菌群抗菌合成相关信号通路,延迟和减少肉鸡肠道弯曲杆菌的定植[53-54]。这些研究表明,植物精油通过调节免疫、提高抗氧化和抗菌等功能促进禽类生长。植物精油在猪生产中的应用和研究也很多,然而其在猪抵抗病原感染中的研究比较缺乏。而且需要注意的是,饲喂含10 mg/kg辣椒油饲粮的断奶仔猪,在受到ETEC感染时,仔猪发生腹泻的频率更高,但生长性能并没有发生显著变化[55]。因此,鉴于植物精油化学成分复杂的特点,探明其中的主要活性成分对其应用于畜禽生产具有重要意义。
2.1.3 多酚类植物化合物多酚类植物化合物可以通过提高猪、禽肠道抗氧化能力、抗炎能力、免疫功能、消化和吸收能力及改善肠道菌群结构等促进其生产性能。饲粮中添加苦丁茶茶多酚(冬青多酚)能够通过改善肠道双糖酶活性,屏障功能和短链脂肪酸(SCFA)产生来缓解脂多糖(LPS)引发的肠道损伤,同时减少肠道炎症[56]。从石榴和诃子中提取的多酚成分通过下调TLRs/NF-κB信号通路,抑制APEC感染白来航鸡的炎症反应,降低感染鸡的死亡率[57]。从黑霉和蓝霉中提取的多酚物质在体外显著下调了鼠伤寒沙门氏菌T3SS调控的毒力因子基因表达,抑制了鼠伤寒沙门氏菌的致病性,在肉鸡体内显著减少盲肠沙门氏菌的定植数量[58],此外,多酚物质对肉鸡盲肠弯曲杆菌也有抑制作用[59]。
2.1.4 多糖类植物化合物多糖类植物化合物有黄芪多糖、白术多糖或牛膝多糖、香菇多糖、党参多糖和猴头菇多糖。饲粮中添加这些多糖类植物提取物可以通过调节猪、禽肠道菌群结构提高肠道免疫功能和屏障功能,促进猪、禽病原性肠道损伤的修复。饲粮中添加84 mg/kg香菇多糖可缓解断奶仔猪轮状病毒感染产生的腹泻,提高肠道形态结构和抗氧化能力,并回调肠道屏障和凋亡相关基因的表达[60]。黄芪多糖通过调控辅助性T细胞17(Th17)/调节性T细胞(Treg)平衡以及肠道菌群和代谢,缓解坏死性肠炎肉鸡的肠道炎症反应[61]。
2.2 微生态制剂目前,通过猪、禽病原性肠道损伤模型评估的微生态制剂主要包括鼠李糖乳杆菌、植物乳杆菌、丁酸梭菌、发酵乳杆菌和凝结芽孢杆菌等。鼠李糖乳杆菌LB1可减轻ETEC感染导致的腹泻、氧化应激、血液细胞组成和生化指标异常及死亡,其相关机制是提高仔猪的免疫力和抗氧化应激能力,并修复维持其肠道完整性,从而减轻ETEC造成的不利影响[62];植物乳杆菌可通过弱化或阻断p38丝裂原活化蛋白激酶(p38 MAPK)磷酸化和NF-κB信号通路减轻ETEC感染导致的炎症反应,从而表现出提高仔猪肠道上皮组织完整性的功效[63];丁酸梭菌可以抑制金黄色葡萄球菌和鼠伤寒沙门氏菌的增殖;进一步研究发现,丁酸梭菌可以通过上调上皮细胞生长因子受体(EGFR)的表达激活信号转导及转录激活蛋白3(STAT3)信号通路,促进肠道屏障相关基因的表达,同时抑制炎性细胞因子的表达,从而缓解肠道损伤[64]。在禽类生产中,乳酸杆菌属、肠球菌属、丁酸球菌属、梭菌属、酵母菌属、芽孢杆菌属部分益生菌有缓解肉鸡坏死性肠炎的作用,主要通过缓解产气荚膜梭菌感染肉鸡肠道炎症反应、产生抗菌物质、调节肠道菌群组成、提高免疫功能、维持肠道黏膜的完整性和改善肠道对营养物质的消化吸收功能来发挥作用[65]。乳酸杆菌、酵母菌属、肠球菌属、片球菌属、芽孢杆菌属部分益生菌对艾美耳球虫感染肉鸡肠道损伤具有缓解作用[30]。
益生元也具有增强猪、禽免疫力、抗炎及改善猪、禽肠道菌群平衡等功效。研究发现,褐(海)藻低聚糖可抑制大肠杆菌感染引起的炎性细胞因子的表达;该作用可能是由于这些低聚糖可以阻碍脂多糖与肠细胞的结合,从而抑制NF-κB的活性[66]。褐(海)藻低聚糖还可以缓解断奶仔猪感染大肠杆菌引起的肠黏膜损伤。原因包括以下2方面:一方面可以通过抑制线粒体依赖及肿瘤坏死因子受体1(TNFR1)依赖的细胞凋亡途径,减少肠细胞的死亡;另一方面通过增强细胞周期蛋白E-细胞周期蛋白依赖性蛋白激酶2(CDK2)复合物的形成,加速肠细胞的增殖[67]。低聚果糖和甘露低聚糖也可以抑制大肠杆菌感染引起的炎症反应,提高猪的肠道健康[68-69]。
2.3 功能性氨基酸色氨酸可以通过钙敏感受体(CaSR)/ras相关C3肉毒杆菌毒素底物1(Rac1)/磷脂酶-Cγ1(PLC-γ1)信号通路,缓解大肠杆菌感染引起的炎症反应及肠上皮细胞的损伤[70]。轮状病毒感染断奶仔猪时,饲粮中添加1%的亮氨酸可以通过激活哺乳动物雷帕霉素靶蛋白(mTOR)信号通路,增加空肠黏膜中黏蛋白和杯状细胞的数量,促进小肠的消化和吸收,从而缓解腹泻,提高仔猪的生长性能[71]。本实验室研究发现,饲粮中添加N-乙酰半胱氨酸(NAC)能够缓解PEDV感染仔猪诱导的肠黏膜损伤,改善PEDV感染仔猪肠道的吸收功能;而且还发现NAC在调控PEDV感染仔猪肠道微生物结构方面发挥积极效果[72-74]。在禽类生产中,饲粮中添加L-精氨酸可以缓解产气荚膜梭菌或鼠伤寒沙门氏菌感染肉鸡的肠道损伤和炎症反应,调控肠道菌群结构[75-76]。L-谷氨酰胺改善了坏死性肠炎肉鸡的生长性能和肠道形态[77]。在球虫疫苗感染肉鸡的模型中,精氨酸通过抑制TLR4,激活mTOR复合物1缓解了肠道黏膜损伤[78]。谷氨酰胺、L-精氨酸、L-苏氨酸混合添加促进了艾美耳球虫和大肠杆菌感染肉鸡肠道黏膜的再生和增殖,提高了感染肉鸡的饲料转化效率[79]。
2.4 其他营养物质除了上述的营养物质,中短链脂肪酸甘油酯、氧化锌、酸化剂、抗菌肽等也被报道对猪、禽肠道病原有抑制作用或改善肠道健康的作用,挖掘这些营养物质抗病原感染的作用机制对其应用具有重要的指导作用。本实验室研究发现,PEDV感染仔猪后,月桂酸单甘油酯可以显著缓解腹泻症状,提高肠道的形态结构,并具有抗炎的功效;蛋白组学结果表明,月桂酸单甘油酯可能通过干扰素信号通路促进机体恢复至稳态[11]。氧化锌能够抑制PEDV在小肠中的复制,提高小肠的抗氧化和抗炎水平,这可能与氧化锌影响回肠组织的嗜中性粒细胞脱颗粒过程有关[9]。营养物质复配可取得更好的抗肠道病原的效果,它们之间的协同或拮抗作用也是以后研究的重点之一。
已知的猪、禽病原性肠道损伤营养调控机制见图 2。
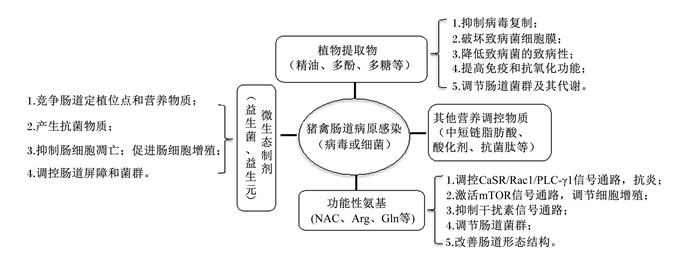
|
NAC:N-乙酰半胱氨酸N-acetylcysteine;Arg:精氨酸arginine;Gln:谷氨酰胺glutamine;CaSR/Rac1/PLC-γ1:钙敏感受体/ras相关C3肉毒杆菌毒素底物1/磷脂酶Cγ1 calcium-sensing receptors/ras-related C3 botulinum toxin substrate 1/phospholipase Cγ1。 图 2 猪、禽病原性肠道损伤营养调控机制(不代表所有营养物质均具有上述各种作用) Fig. 2 Nutritional regulation mechanism of pathogenic intestinal injury in pigs and poultry (not all nutrients have the above-mentioned effects) |
综上所述,在后抗生素时代,猪、禽病原性肠道损伤仍是困扰猪、禽健康养殖的一大难题。当前,已鉴定出多种能够改善猪、禽肠道健康的功能性营养物质,然而其有效成分以及修复和调控机制并不完全清楚;加之猪、禽病原性肠道损伤的机理没有被完全揭示,这些都阻碍了多靶标营养调控猪、禽肠道损伤技术的开发。因此,猪、禽病原性肠道损伤机理及其营养修复机制仍需进一步深入研究,为多靶标营养调控技术的开发奠定理论基础。
致谢:
感谢武汉轻工大学动物科学与营养工程学院易丹教授对文稿所提的宝贵意见。
| [1] |
ZHENG L, DUARTE M E, SEVAROLLI LOFTUS A, et al. Intestinal health of pigs upon weaning: challenges and nutritional intervention[J]. Frontiers in Veterinary Science, 2021, 8: 628258. DOI:10.3389/fvets.2021.628258 |
| [2] |
WICKRAMASURIYA S S, PARK I, LEE K, et al. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry[J]. Vaccines, 2022, 10(2): 172. DOI:10.3390/vaccines10020172 |
| [3] |
LEE I K, KYE Y C, KIM G, et al. Stress, nutrition, and intestinal immune responses in pigs-a review[J]. Asian-Australasian Journal of Animal Sciences, 2016, 29(8): 1075-1082. DOI:10.5713/ajas.16.0118 |
| [4] |
徐子伟. 仔猪肠道损伤修复营养调控及其机制和应用[J]. 动物营养学报, 2014, 26(10): 3033-3045. XU Z W. Mechanism and application for nutritional regulations of intestine damage repair in piglets[J]. Chinese Journal of Animal Nutrition, 2014, 26(10): 3033-3045 (in Chinese). DOI:10.3969/j.issn.1006-267x.2014.10.015 |
| [5] |
PATRA A K, AMASHEH S, ASCHENBACH J R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds-a comprehensive review[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(20): 3237-3266. DOI:10.1080/10408398.2018.1486284 |
| [6] |
XING Y Y, GU X Y, RUAN G J, et al. Probiotics for the treatment of gastric diseases[J]. Nutrition and Cancer, 2022, 20: 1-7. |
| [7] |
WU G Y, BAZER F W, DAI Z L, et al. Amino acid nutrition in animals: protein synthesis and beyond[J]. Annual Review of Animal Biosciences, 2014, 2: 387-417. DOI:10.1146/annurev-animal-022513-114113 |
| [8] |
HOU Y Q, WU G Y. Nutritionally nonessential amino acids: a misnomer in nutritional sciences[J]. Advances in Nutrition, 2017, 8(1): 137-139. DOI:10.3945/an.116.012971 |
| [9] |
ZHANG Q, WU T, LI S Y, et al. Protective effect of zinc oxide and its association with neutrophil degranulation in piglets infected with porcine epidemic diarrhea virus[J]. Oxidative Medicine and Cellular Longevity, 2021, 2021: 3055810. |
| [10] |
LIU Y L, CHEN F, ODLE J, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge[J]. The Journal of Nutrition, 2012, 142(11): 2017-2024. DOI:10.3945/jn.112.164947 |
| [11] |
ZHANG Q, YI D, JI C Z, et al. Monolaurin confers a protective effect against porcine epidemic diarrhea virus infection in piglets by regulating the interferon pathway[J]. Frontiers in Immunology, 2021, 12: 797476. |
| [12] |
YANG P, MA Y X. Recent advances of vitamin D in immune, reproduction, performance for pig: a review[J]. Animal Health Research Reviews, 2021, 22(1): 85-95. DOI:10.1017/S1466252321000049 |
| [13] |
LIU Q, WANG H Y. Porcine enteric coronaviruses: an updated overview of the pathogenesis, prevalence, and diagnosis[J]. Veterinary Research Communications, 2021, 45(2/3): 75-86. |
| [14] |
DOERKSEN T, CHRISTENSEN T, LU A, et al. Assessment of porcine Rotavirus-associated virome variations in pigs with enteric disease[J]. Veterinary Microbiology, 2022, 270: 109447. DOI:10.1016/j.vetmic.2022.109447 |
| [15] |
ANNAMALAI T, SAIF L J, LU Z Y, et al. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs[J]. Veterinary Immunology and Immunopathology, 2015, 168(3/4): 193-202. |
| [16] |
LI Y C, WU Q X, HUANG L L, et al. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine[J]. Nature Communications, 2018, 9(1): 3811. DOI:10.1038/s41467-018-06056-w |
| [17] |
CURRY S M, SCHWARTZ K J, YOON K J, et al. Effects of porcine epidemic diarrhea virus infection on nursery pig intestinal function and barrier integrity[J]. Veterinary Microbiology, 2017, 211: 58-66. DOI:10.1016/j.vetmic.2017.09.021 |
| [18] |
JUNG K, MIYAZAKI A, SAIF L J. Immunohistochemical detection of the vomiting-inducing monoamine neurotransmitter serotonin and enterochromaffin cells in the intestines of conventional or gnotobiotic (Gn) pigs infected with porcine epidemic diarrhea virus (PEDV) and serum cytokine responses of Gn pigs to acute PEDV infection[J]. Research in Veterinary Science, 2018, 119: 99-108. DOI:10.1016/j.rvsc.2018.06.009 |
| [19] |
JUNG K, SAIF L J, WANG Q H. Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control[J]. Virus Research, 2020, 286: 198045. DOI:10.1016/j.virusres.2020.198045 |
| [20] |
OUYANG K, SHYU D L, DHAKAL S, et al. Evaluation of humoral immune status in porcine epidemic diarrhea virus (PEDV) infected sows under field conditions[J]. Veterinary Research, 2015, 46: 140. DOI:10.1186/s13567-015-0285-x |
| [21] |
ANNAMALAI T, LIN C M, GAO X, et al. Cross protective immune responses in nursing piglets infected with a US spike-insertion deletion porcine epidemic diarrhea virus strain and challenged with an original US PEDV strain[J]. Veterinary Research, 2017, 48(1): 61. DOI:10.1186/s13567-017-0469-7 |
| [22] |
GIMENEZ-LIROLA L G, ZHANG J Q, CARRILLO-AVILA J A, et al. Reactivity of porcine epidemic diarrhea virus structural proteins to antibodies against porcine enteric coronaviruses: diagnostic implications[J]. Journal of Clinical Microbiology, 2017, 55(5): 1426-1436. DOI:10.1128/JCM.02507-16 |
| [23] |
KOONPAEW S, TEERAVECHYAN S, FRANTZ P N, et al. PEDV and PDCoV pathogenesis: the interplay between host innate immune responses and porcine enteric coronaviruses[J]. Frontiers in Veterinary Science, 2019, 6: 34. DOI:10.3389/fvets.2019.00034 |
| [24] |
DUBREUIL J D. Enterotoxigenic Escherichia coli and probiotics in swine: what the bleep do we know?[J]. Bioscience of Microbiota, Food and Health, 2017, 36(3): 75-90. DOI:10.12938/bmfh.16-030 |
| [25] |
MIRHOSEINI A, AMANI J, NAZARIAN S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it[J]. Microbial Pathogenesis, 2018, 117: 162-169. DOI:10.1016/j.micpath.2018.02.032 |
| [26] |
WANG H X, ZHONG Z F, LUO Y, et al. Heat-stable enterotoxins of enterotoxigenic Escherichia coli and their impact on host immunity[J]. Toxins, 2019, 11(1): 24. DOI:10.3390/toxins11010024 |
| [27] |
WU T, LV Y, LI X N, et al. Establishment of a recombinant Escherichia coli-induced piglet diarrhea model[J]. Frontiers in Bioscience, 2018, 23(8): 1517-1534. DOI:10.2741/4658 |
| [28] |
ZHANG Q, ZHANG L J, LYU Y, et al. Dietary supplementation of Lactobacillus zeae regulated the gut microbiome in piglets infected with enterotoxigenic Escherichia coli[J]. Czech Journal of Animal Science, 2022, 67(1): 27-38. DOI:10.17221/136/2021-CJAS |
| [29] |
SONG X K, GAO Y L, XU L X, et al. Partial protection against four species of chicken coccidia induced by multivalent subunit vaccine[J]. Veterinary Parasitology, 2015, 212(3/4): 80-85. |
| [30] |
MADLALA T, OKPEKU M, ADELEKE M A. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review[J]. Parasite, 2021, 28: 48. DOI:10.1051/parasite/2021047 |
| [31] |
DAHIYA J P, WILKIE D C, VAN KESSEL A G, et al. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era[J]. Animal Feed Science and Technology, 2006, 129(1/2): 60-88. |
| [32] |
EMAMI N K, DALLOUL R A. Centennial review: recent developments in host-pathogen interactions during necrotic enteritis in poultry[J]. Poultry Science, 2021, 100(9): 101330. DOI:10.1016/j.psj.2021.101330 |
| [33] |
PRESCOTT J F, PARREIRA V R, MEHDIZADEH GOHARI I, et al. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review[J]. Avian Pathology, 2016, 45(3): 288-294. DOI:10.1080/03079457.2016.1139688 |
| [34] |
SHOJADOOST B, VINCE A R, PRESCOTT J F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review[J]. Veterinary Research, 2012, 43(1): 74. DOI:10.1186/1297-9716-43-74 |
| [35] |
WADE B, KEYBURN A. The true cost of necrotic enteritis[J]. World Poultry, 2015, 31: 16-17. |
| [36] |
DZIVA F, STEVENS M P. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts[J]. Avian Pathology, 2008, 37(4): 355-366. DOI:10.1080/03079450802216652 |
| [37] |
KATHAYAT D, LOKESH D, RANJIT S, et al. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies[J]. Pathogens, 2021, 10(4): 467. DOI:10.3390/pathogens10040467 |
| [38] |
MELLATA M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends[J]. Foodborne Pathogens and Disease, 2013, 10(11): 916-932. DOI:10.1089/fpd.2013.1533 |
| [39] |
FOLEY S L, JOHNSON T J, RICKE S C, et al. Salmonella pathogenicity and host adaptation in chicken-associated serovars[J]. Microbiology and Molecular Biology Reviews, 2013, 77(4): 582-607. DOI:10.1128/MMBR.00015-13 |
| [40] |
AWAD W A, HESS C, HESS M. Re-thinking the chicken-Campylobacter jejuni interaction: a review[J]. Avian Pathology, 2018, 47(4): 352-363. DOI:10.1080/03079457.2018.1475724 |
| [41] |
易丹, 侯永清. 天然植物活性成分与猪肠道健康的研究进展[J]. 动物营养学报, 2020, 32(10): 4518-4524. YI D, HOU Y Q. Research progress on natural plant bioactive compounds and swine intestinal health[J]. Chinese Journal of Animal Nutrition, 2020, 32(10): 4518-4524 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.10.005 |
| [42] |
李鹏, 高铭坤, 呙于明. 植物提取物在家禽和猪生产中的应用研究进展[J/OL]. 中国畜牧杂志: 1-15[2022-07-25]. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZGXM20220318002&DbName=CAPJ2022.DOI:10.19556/j.0258-7033.20211019-08. LI P, GAO M K, GUO Y M. Advances in biological functions of plant extracts and its application in poultry and pig production[J/OL]. Chinese Journal of Animal Science: 1-15[2022-07-25]. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZGXM20220318002&DbName=CAPJ2022.DOI:10.19556/j.0258-7033.20211019-08. (in Chinese) |
| [43] |
HUANG C Y, WANG Y B, HE X, et al. The involvement of NF-κB/P38 pathways in Scutellaria baicalensis extracts attenuating of Escherichia coli K88-induced acute intestinal injury in weaned piglets[J]. British Journal of Nutrition, 2019, 122(2): 152-161. DOI:10.1017/S0007114519000928 |
| [44] |
WU M J, ZHANG Q, YI D, et al. Quantitative proteomic analysis reveals antiviral and anti-inflammatory effects of puerarin in piglets infected with porcine epidemic diarrhea virus[J]. Frontiers in Immunology, 2020, 11: 169. DOI:10.3389/fimmu.2020.00169 |
| [45] |
WU M J, YI D, ZHANG Q, et al. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus[J]. Scientific Reports, 2021, 11(1): 6552. DOI:10.1038/s41598-021-85880-5 |
| [46] |
LI Z H, CAO H, CHENG Y F, et al. Inhibition of porcine epidemic diarrhea virus replication and viral 3C-like protease by quercetin[J]. International Journal of Molecular Sciences, 2020, 21(21): 8095. DOI:10.3390/ijms21218095 |
| [47] |
AL-MNASER A, DAKHEEL M, ALKANDARI F, et al. Polyphenolic phytochemicals as natural feed additives to control bacterial pathogens in the chicken gut[J]. Archives of Microbiology, 2022, 204(5): 253. DOI:10.1007/s00203-022-02862-5 |
| [48] |
DU E C, WANG W W, GAN L P, et al. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens[J]. Journal of Animal Science and Biotechnology, 2016, 7: 19. DOI:10.1186/s40104-016-0079-7 |
| [49] |
YIN D F, DU E C, YUAN J M, et al. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens[J]. Scientific Reports, 2017, 7(1): 7334. DOI:10.1038/s41598-017-07420-4 |
| [50] |
BETANCOURT L, HUME M, RODRÍGUEZ F, et al. Effects of Colombian oregano essential oil (Lippia origanoides Kunth) and Eimeria species on broiler production and cecal microbiota[J]. Poultry Science, 2019, 98(10): 4777-4786. DOI:10.3382/ps/pez193 |
| [51] |
UPADHAYA S D, CHO S H, CHUNG T K, et al. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima[J]. Poultry Science, 2019, 98(7): 2919-2926. DOI:10.3382/ps/pez040 |
| [52] |
CHANG L Y, DI K Q, XU J, et al. Effect of natural garlic essential oil on chickens with artificially infected Eimeria tenella[J]. Veterinary Parasitology, 2021, 300: 109614. DOI:10.1016/j.vetpar.2021.109614 |
| [53] |
KELLY C, GUNDOGDU O, PIRCALABIORU G, et al. The in vitro and in vivo effect of carvacrol in preventing campylobacter infection, colonization and in improving productivity of chicken broilers[J]. Foodborne Pathogens and Disease, 2017, 14(6): 341-349. DOI:10.1089/fpd.2016.2265 |
| [54] |
ALLAOUA M, BONNAFÉ E, ETIENNE P, et al. A carvacrol-based product reduces Campylobacter jejuni load and alters microbiota composition in the caeca of chickens[J]. Journal of Applied Microbiology, 2022, 132(6): 4501-4516. DOI:10.1111/jam.15521 |
| [55] |
WOJNICKI S J, MORRIS A, SMITH B N, et al. Immunomodulatory effects of whole yeast cells and capsicum in weanling pigs challenged with pathogenic Escherichia coli[J]. Journal of Animal Science, 2019, 97(4): 1784-1795. DOI:10.1093/jas/skz063 |
| [56] |
XU X, HUA H W, WANG L M, et al. Holly polyphenols alleviate intestinal inflammation and alter microbiota composition in lipopolysaccharide-challenged pigs[J]. British Journal of Nutrition, 2020, 123(8): 881-891. DOI:10.1017/S0007114520000082 |
| [57] |
ZHONG X L, SHI Y R, CHEN J J, et al. Polyphenol extracts from Punica granatum and Terminalia chebula are anti-inflammatory and increase the survival rate of chickens challenged with Escherichia coli[J]. Biological & Pharmaceutical Bulletin, 2014, 37(10): 1575-1582. |
| [58] |
SALAHEEN S, JAISWAL E, JOO J, et al. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella Typhimurium[J]. International Journal of Food Microbiology, 2016, 237: 128-135. DOI:10.1016/j.ijfoodmicro.2016.08.027 |
| [59] |
SIMA F, STRATAKOS A C, WARD P, et al. A novel natural antimicrobial can reduce the in vitro and in vivo pathogenicity of T6SS positive Campylobacter jejuni and Campylobacter coli chicken isolates[J]. Frontiers in Microbiology, 2018, 9: 2139. DOI:10.3389/fmicb.2018.02139 |
| [60] |
MAO X B, HU H Y, XIAO X C, et al. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model[J]. Food & Function, 2019, 10(4): 2094-2101. |
| [61] |
SONG B C, LI P, YAN S J, et al. Effects of dietary astragalus polysaccharide supplementation on the Th17/Treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis[J]. Frontiers in Immunology, 2022, 13: 781934. DOI:10.3389/fimmu.2022.781934 |
| [62] |
WU T, SHI Y T, ZHANG Y Y, et al. Lactobacillus rhamnosus LB1 alleviates enterotoxigenic Escherichia coli-induced adverse effects in piglets by improving host immune response and anti-oxidation stress and restoring intestinal integrity[J]. Frontiers in Cellular and Infection Microbiology, 2021, 11: 724401. DOI:10.3389/fcimb.2021.724401 |
| [63] |
李海花, 梁东梅, 乔家运. 植物乳杆菌抑制产肠毒素型大肠杆菌诱发猪肠上皮细胞炎症反应的分子机制[J]. 动物营养学报, 2021, 33(7): 4018-4029. LI H H, LIANG D M, QIAO J Y. Molecular mechanism of Lactobacillus plantarum inhibiting inflammatory response induced by enterotoxigenic Escherichia coli in porcine intestinal epithelial cells[J]. Chinese Journal of Animal Nutrition, 2021, 33(7): 4018-4029 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.07.043 |
| [64] |
MA M P, ZHAO Z T, LIANG Q Y, et al. Overexpression of pEGF improved the gut protective function of Clostridium butyricum partly through STAT3 signal pathway[J]. Applied Microbiology and Biotechnology, 2021, 105(14): 5973-5991. |
| [65] |
KULKARNI R R, GAGHAN C, GORRELL K, et al. Probiotics as alternatives to antibiotics for the prevention and control of necrotic enteritis in chickens[J]. Pathogens, 2022, 11(6): 692. DOI:10.3390/pathogens11060692 |
| [66] |
WAN J, ZHANG J, XU Q S, et al. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury[J]. Carbohydrate Polymers, 2021, 270: 118316. DOI:10.1016/j.carbpol.2021.118316 |
| [67] |
WAN J, ZHANG J, CHEN D W, et al. Alginate oligosaccharide alleviates enterotoxigenic Escherichia coli-induced intestinal mucosal disruption in weaned pigs[J]. Food & Function, 2018, 9(12): 6401-6413. |
| [68] |
LIU L, CHEN D W, YU B, et al. Fructooligosaccharides improve growth performance and intestinal epithelium function in weaned pigs exposed to enterotoxigenic Escherichia coli[J]. Food & Function, 2020, 11(11): 9599-9612. |
| [69] |
YU E, CHEN D W, YU B, et al. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge[J]. British Journal of Nutrition, 2021, 126(7): 993-1002. DOI:10.1017/S0007114520004948 |
| [70] |
LIU G M, GU K, WANG F, et al. Tryptophan ameliorates barrier integrity and alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 through the CaSR/Rac1/PLC-γ1 signaling pathway in porcine intestinal epithelial cells[J]. Frontiers in Immunology, 2021, 12: 748497. DOI:10.3389/fimmu.2021.748497 |
| [71] |
MAO X B, LIU M H, TANG J, et al. Dietary leucine supplementation improves the mucin production in the jejunal mucosa of the weaned pigs challenged by porcine rotavirus[J]. PLoS One, 2015, 10(9): e0137380. DOI:10.1371/journal.pone.0137380 |
| [72] |
WANG L, ZHOU J, HOU Y Q, et al. N-acetylcysteine supplementation alleviates intestinal injury in piglets infected by porcine epidemic diarrhea virus[J]. Amino Acids, 2017, 49(12): 1931-1943. DOI:10.1007/s00726-017-2397-2 |
| [73] |
WU T, LYU Y, LI X N, et al. Impact of N-acetylcysteine on the gut microbiota in the piglets infected with porcine epidemic diarrhea virus[J]. Frontiers in Veterinary Science, 2020, 7: 582338. |
| [74] |
YI D, HOU Y Q, XIAO H, et al. N-acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways[J]. Amino Acids, 2017, 49(12): 1915-1929. DOI:10.1007/s00726-017-2389-2 |
| [75] |
ZHANG B B, LV Z P, LI Z, et al. Dietary L-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens[J]. Frontiers in Microbiology, 2018, 9: 1716. DOI:10.3389/fmicb.2018.01716 |
| [76] |
ZHANG B B, LI G, SHAHID M S, et al. Dietary L-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium[J]. Poultry Science, 2020, 99(4): 1862-1874. |
| [77] |
XUE G D, BAREKATAIN R, WU S B, et al. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge[J]. Poultry Science, 2018, 97(4): 1334-1341. |
| [78] |
TAN J Z, APPLEGATE T J, LIU S S, et al. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens[J]. British Journal of Nutrition, 2014, 112(7): 1098-1109. |
| [79] |
GOTTARDO E T, PROKOSKI K, HORN D, et al. Regeneration of the intestinal mucosa in Eimeria and E. coli challenged broilers supplemented with amino acids[J]. Poultry Science, 2016, 95(5): 1056-1065. |




