肌肉是动物躯体的重要组成部分,仅骨骼肌就占到全身体重的40%左右[1]。骨骼肌在动物新陈代谢调控、运动调节、能量储存等方面至关重要。禽类的肌肉肌纤维发育特点与脊椎动物类似,由成肌细胞经过增殖、迁移、黏附、融合并最终形成肌纤维,肌纤维数量在胚胎期基本被确定了[2-3],而出生后肌纤维主要发生肌纤维的肥大和类型转化。胚胎期成肌细胞的增殖分化决定家禽出生后肌肉的生长潜力,而肌纤维密度和直径影响着禽肉产量和品质。因此,肉鸡肌纤维特性形成机制及调控研究可为肉鸡产肉性能和肉品质调控提供理论支撑。
1 肉鸡肌纤维特性及其与产肉性能的关系 1.1 肌纤维的结构骨骼肌由大量的肌纤维组成,肌细胞呈长柱状,末端呈圆锥形,其长短和粗细随肌肉的种类和生理状况而异,一般长1~4 mm,直径10~100 μm。肌细胞之间由肌内膜隔开,20~300条肌纤维聚集形成肌束,其外部被肌束膜所包裹,1层较厚的肌外膜将数条肌束集合在一起,形成肌肉。
肌纤维主要包括肌纤维膜、细胞核、肌原纤维和肌浆。肌纤维膜又称肌膜,是肌纤维表面1层不明显的肌膜。肌纤维属于多核细胞,每条肌纤维含有多个细胞核,最多可达几百个。细胞核一般为椭圆形,位于肌纤维的边缘,染色质呈小块状,核仁明显,有1~2个。肌浆内含有丰富的肌原纤维,大量线粒体、脂滴和糖原等。肌原纤维很细,每条肌原纤维上有明暗相间的横纹,分别称为明带(Ⅰ带)和暗带(A带)。Ⅰ带中央有1条暗线,称为Z线,相邻2个Z线间的肌原纤维,被称为肌节。肌节是肌原纤维结构和功能的基本单元,主要由粗肌丝、细肌丝和肌联蛋白构成,如图 1所示。
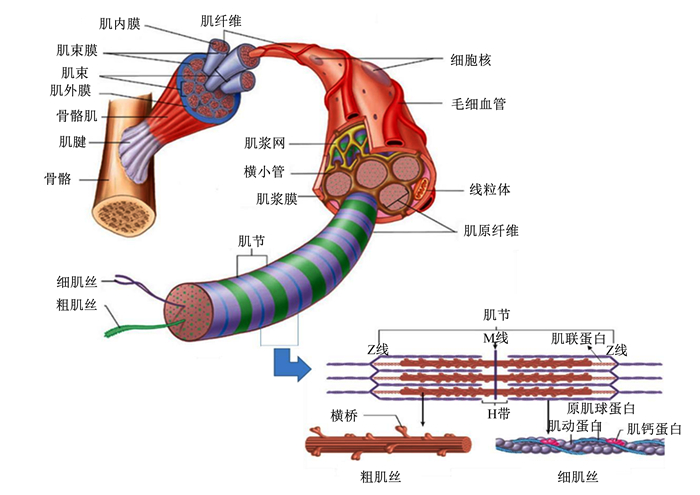
|
图 1 骨骼肌结构 Fig. 1 Skeletal muscle structure |
肌纤维依据其功能和组成可以分为多种类型。根据肌纤维所含酶的种类和活性特点可分为氧化型纤维(红肌纤维)、酵解型纤维(白肌纤维)以及中间型纤维。氧化型肌纤维含有更多的线粒体和细胞色素,浆膜中红色的肌球蛋白含量高;酵解型肌纤维细胞色素或肌球蛋白含量较少。红肌纤维通常含有较多的脂肪,有氧代谢酶如细胞色素氧化酶、琥珀酸脱氢酶活性很高,而糖原降解所必需的ATP酶和磷酸化酶活性很低。白肌纤维则含有较高的糖原,ATP酶和磷酸化酶活性很高,有氧代谢酶的活性很低。有些肌纤维同时具有有氧代谢和厌氧酵解代谢的能力,称为中间型肌纤维。近年来,基于肌球蛋白重链(MyHC)种类,将骨骼肌肌纤维分为慢速氧化型(Ⅰ型)、快速氧化型(Ⅱa型)、快速酵解型(Ⅱb型)与中间型(Ⅱx型)4种[4]。
1.3 肉鸡肌肉肌纤维特性与产肉性能的关系肌纤维结构和组成是决定肌肉品质的结构基础。肌纤维数量在胚胎期已经确定,出生后肌肉生长主要由于肌纤维肥大和肌纤维类型转化。肌纤维直径、密度和横截面积是描述肌肉特征的重要参数。研究表明,肌纤维的直径与肌肉嫩度呈正相关,Ⅰ型纤维与Ⅱb型纤维相比,直径较细,剪切力更低,表现出更好的嫩度特性。Ⅰ型纤维与肌内脂肪含量呈正相关,相较于Ⅱ型纤维具有更高的脂质与磷脂含量,肉质风味更佳[5]。陈宽维等[6]研究发现,白肌纤维直径大于红肌纤维和中间型肌纤维直径。固始鸡腿肌中的红肌纤维和中间型肌纤维的横截面积随周龄增加而增大,白肌纤维的含量逐渐增加,且公鸡比母鸡增加得快且明显[7]。Huo等[8]比较了雪山鸡和罗斯308鸡胸肌肌纤维特性,发现雪山鸡胸肌具有更高的肌纤维密度和更低的糖酵解潜力。肌纤维类型转化过程中,肌肉品质发生较大变化,红肌纤维比例高则肌红蛋白含量高,肉色鲜红,白肌纤维比例高时,肌肉系水力相对较低,肌肉中ATP酶活性与糖原含量高,屠宰后肌肉pH下降快[9]。
2 肉鸡肌纤维形成规律 2.1 胚胎期肌纤维的发育规律及分子机制胚胎期肌纤维的发育分为4个阶段:体节分化成生皮肌节,肌源性祖细胞(MPC)增殖、分化成成肌细胞,成肌细胞增殖、分化成肌管,肌管成熟为肌纤维。MPC来源于中胚层,由中胚层形成体节,体节分化为生骨节和生皮肌节[10-11],生皮肌节进一步分化为生皮节和生肌节,生肌节逐渐形成MPC。MPC在胚胎早期大量增殖后向躯体和四肢迁移,分化为单核成肌细胞。成肌细胞大量增殖、分化形成肌细胞,肌细胞经过迁移、相互黏附及融合形成多核的初级肌管,进一步融合为次级肌管,大量的肌管分化、融合为初级肌纤维,再融合形成次级肌纤维。未融合成肌纤维且位于肌纤维的肌膜和基底膜之间的细胞称为卫星细胞,在机体受到损伤时,卫星细胞由静息状态转变为活跃状态,参与骨骼肌的再生[12-13]。
胚胎期肌纤维的发育是一个复杂且精密的过程,MPC分化过程中各基因的时序表达是控制分化成为成肌细胞的关键[14]。Pax3是初期成肌过程的关键基因,生肌节轴下部位周围的旁泌因子激活Pax3基因的转录,表达Pax3的MPC维持增殖状态而不进入肌原性分化程序,直到到达靶位点后才激活肌肉特异性的转录因子生肌决定因子5(Myf5)和成肌分化蛋白(MyoD)启动生肌过程,这一过程主要通过Shh和Wnts信号传导通路激活[15-17]。Pax7在卫星细胞的激活、增殖和分化过程中扮演着至关重要的角色[18]。Pax7联合组蛋白甲基转移酶复合物刺激靶基因Myf5的转录活性,调节卫星细胞定向成肌分化[19]。miR-206和miR-486可以通过抑制Pax7的表达,使肌细胞退出增殖期,加速到达分化阶段[20]。Myf5和MyoD基因表达是MPC向成肌细胞分化的标志,而肌细胞生成素(Myogenin)和生肌决定因子6(Myf6)基因则在成肌细胞的融合和分化中起作用。成肌细胞分化程序的激活主要依赖于MyoD和其他肌肉分化因子如生肌调节因子4(MRF4)、肌细胞增强因子2(MEF2)等基因的表达及Notch信号通路[21]。研究表明,miR-148a-3p通过靶向动力蛋白轻链2(DYNLL2)促进肌凝蛋白重链(MYHC)蛋白的表达,促进成肌细胞的分化[22]。miR-16-5p靶向抑制SESN1基因,调控p53信号通路,从而调控鸡成肌细胞的增殖、分化和凋亡[23]。
2.2 出生后肌纤维的发育规律及分子机制出生后肌纤维的发育主要体现在肌纤维肥大及肌纤维类型的转化,目前关于肌纤维肥大分子机制的系统性研究相对较少。Li等[24]鉴定到半胱氨酸和甘氨酸富含蛋白3(CSRP3)、平滑肌蛋白2(LMOD2)、MUSTN1和慢型肌球蛋白结合蛋白C1(MYBPC1)对肌纤维组装和肥大有重要的正调控作用[25],而肌球蛋白轻链1(MYL1)和肌球蛋白轻链4(MYL4)通过抑制成肌细胞增殖对肌生成有负调控作用[26-27]。Zhang等[28]发现SMARCD3-OT1可以增强SMARCD3X4的表达,从而诱导肌肉肥大,同时SMARCD3-OT1可以促进慢收缩纤维转化为快收缩纤维。锚蛋白重复结构域蛋白2(ANKRD2)不仅参与肌发生、肌源性分化,且在鸡骨骼肌慢肌纤维和快肌纤维中选择性表达[29]。肌纤维发育涉及多条信号通路,主要包括Ca2+通路、丝裂原激活的蛋白激酶(MAPK)信号通路、叉头转录因子(FoxO)信号通路和胰岛素样生长因子1-磷脂酰肌醇-3-羟激酶-蛋白激酶(IGF1-PI3K/AKT)通路等。其中Ca2+信号通路中,活化的钙神经素会导致活化T细胞核因子(NFAT)去磷酸化,从而入核并协同转录因子肌细胞增强因子2(MEF2)结合至特定位点,启动慢肌基因的表达,促进慢肌表型的出现[30]。MAPK信号通路主要在肌肉受损伤时发挥作用,诱导卫星细胞由静息状态转变为活跃状态[31]。FoxO信号通路在出生后肌肉发育过程中调控相关遗传因子的表达[32]。最新研究发现,LncIRS1可以作为miR-15a、miR-15b-5p和miR-15c-5p的ceRNA来调控胰岛素受体底物1(IRS1)的表达,通过促进AKT的磷酸化调控肌细胞增殖和分化,影响肌肉质量[33]。
3 肉鸡肌纤维性状形成的调控方式 3.1 母体营养胚胎期是肌纤维发育的关键时期,胚胎期MPC的增殖和分化影响着肌纤维的数量,从而影响动物肌肉生长潜力。研究表明,母体营养可能作为改善子代生长和生产的一种方式[34-36]。Moraes等[37]研究了罗斯708肉种鸡产蛋期不同能量和蛋白质水平对子代体重的影响,结果表明母体饲粮营养对其子代体重的影响有性别依赖。Lesuisse等[38]研究表明,A系种鸡产蛋期间减少25%饲粮蛋白质水平能够提高子代生长速度并且改善饲料转化率,提高子代肉鸡的生产性能。Li等[39]在爱拔益加(AA)种鸡25周龄时对其进行能量限饲(11.70、9.36、8.19和5.85 MJ/kg DM),结果表明适量降低母体饲粮能量水平能够提高后代肌肉的抗氧化能力,但如果能量过低会降低子代胸肌和腿肌的总超氧化物歧化酶(T-SOD)和谷胱甘肽过氧化物酶(GSH-Px)活性。此外,Wu等[40]通过对产蛋期AA种鸡限饲(100%、80%、70%和50%饲喂水平)发现,随限饲程度提升胚蛋重量随之降低,子代胸肌纤维的直径和密度逐渐减小,肌肉生长抑制素(MSTN)基因表达显著降低。以上结果表明,母体饲粮营养水平的差异影响到母体对胚蛋的营养沉积,从而导致了子代肌纤维发育差异。
在鸡胚的发育过程当中,蛋内营养是供子代胚胎生长发育唯一的营养来源,种蛋种类与后代雏鸡出生重呈线性相关[41]。许多研究表明,在鸡胚内注射生长因子、氨基酸或其他营养素能够改善肌肉和肠道的发育。Gonzalez等[42]发现,在给发育中的鸡胚卵黄囊中注射250 mM的烟酰胺核苷,能够增加鸡胚孵化时的胸大肌重量和肌纤维密度。Xu等[43]试验结果表明,胚蛋注射1 mol/L烟酰胺核苷的雏鸡肌纤维密度高于注射250和500 mM处理组。而Ma等[44]在鸡胚孵化第7天于气室注射β-羟基-β-甲基丁酸(HMB)和孵化第18天羊膜注射HMB,结果表明孵化第7天气室注射HMB提高了4.34%孵化率,显著提高了雏鸡血浆生长激素、胰岛素和胰岛素样生长因子-1的水平,而HMB羊膜注射显著上调了MyoD和肌原蛋白的mRNA表达来激活卫星细胞,从而促进鸡胚肌肉的生长发育。
3.2 饲粮营养水平饲粮营养水平能够影响肉鸡的糖、蛋白质和脂质代谢等生理过程,因此很可能影响到肉鸡的肌肉肌纤维的生长[45]。而骨骼肌的生长很大程度上依赖于卫星细胞的肌肉特异性干细胞的增殖和分化。研究表明,在饲粮中添加维生素D3代谢物25-羟基胆钙化醇,可以促进孵化后肉鸡的骨骼肌生长[46]。Lackner等[47]研究饲粮不同水平的组氨酸和赖氨酸比例(0.44、0.54、0.64)和β-丙氨酸水平(0、0.5%)对肉品质的影响,发现高组氨酸和赖氨酸比例能够增加肉鸡血浆和骨骼肌中的肌肽浓度,而β-丙氨酸仅在35 d时增加血浆中的肌肽含量,组氨酸补充剂能够提高胸肌中的肌肽含量,但是对肉品质影响较小。Watanabe等[48]通过对罗斯308肉鸡进行赖氨酸限饲(90%和100%),结果表明,相较于100%赖氨酸组,90%赖氨酸组胸肌中游离谷氨酸含量高了51.8%,游离的谷氨酰胺、甘氨酸、缬氨酸、异亮氨酸、亮氨酸、络氨酸、苯丙氨酸、组氨酸和3-甲基组氨酸含量均显著提高,表明短期饲喂低赖氨酸饲粮可以增加鸡肌肉中游离谷氨酸和其他游离氨基酸的含量,一定程度改善肉质。De Carvalho等[49]研究表明,与饲喂无机猛相比,有机锰有效提升了肉鸡平均日增重和胸肌抗氧化能力。Ahsan等[50]通过间歇性降低饲粮中可消化赖氨酸含量或代谢能水平发现,饲喂85%可消化赖氨酸饲粮肉鸡肌变性和坏死程度、炎症反应、脂质沉积、CD3+和巨噬细胞浸润以及热休克蛋白70的表达均显著降低,有效降低了肉鸡木质化鸡胸肉发生率。
3.3 植物源功能成分近年来有研究发现,多种植物功能成分对肉鸡肌肉品质具有很好的调控作用[51]。研究发现,大豆异黄酮中的活性成分大豆苷元可增加葡萄糖转运载体4(GLUT4)的表达和葡萄糖摄取来提高骨骼肌中胰岛素的敏感性[52]。大豆异黄酮在结构和功能上皆类似于动物雌激素,能够协同生长激素、胰岛素等参与调控动物体肌肉蛋白质的合成[53]。Ogawa等[54]发现,饲粮中添加大豆苷元可以通过雌激素受体β(ERβ)来降低泛素特异性蛋白酶19(USP19)的表达和蛋白质的降解,从而抑制骨骼肌的萎缩。Yoshino等[55]的研究表明,大豆苷元可以通过沉默信息调节因子2相关酶1(SIRT1)相关途径调节转录网络,进而调节肌肉细胞中的线粒体生物发生。非黄酮类多酚化合物白藜芦醇也被报道具有诱导酵解型肌纤维向氧化型肌纤维转化,从而改善肌肉品质的作用,其调控作用可能是通过抑制FoxO1的表达来实现的[56]。研究发现,饲粮中添加白藜芦醇延缓了肉鸡胸肌pH的下降,降低了滴水损失以及肌肉中的乳酸含量,改善了肌肉颜色[57]。Wang等[45]于母鸡饲粮中添加5%的植物甾醇酯可提高子代肌纤维密度和肌细胞生成素表达,在雌性子代中还可激活胆汁酸受体促进MyoD的表达。
3.4 肠道微生物近年来研究发现,肠道微生物对于肌肉生长发育和肌纤维类型转化具有重要作用。研究发现,无菌小鼠骨骼肌重量和肌肉力量均显著低于野生型小鼠,而无菌小鼠移植野生型小鼠的肠道微生物后肌肉重量和力量均明显改善[58]。肠道微生物对肌纤维性状的影响可能主要与其代谢物有关[59]。肠道中微生物发酵后产生的短链脂肪酸已被广泛证实可以调控肌肉的代谢和功能[60]。丁酸可以有效抑制肌萎缩相关基因如肌肉特异性指环蛋白1(MuRF1)和肌萎缩蛋白Fbox-1(Atrogin-1)的表达[61-62],而丙酸可以作为糖异生的底物激活糖异生反应,通过影响糖代谢参与机体的能量代谢,从而影响肌肉的生长发育[63]。Chang等[64]发现饲粮中添加丁酸钠后上调了腓肠肌中MyHC Ⅰ、MyHC Ⅱa、肌红蛋白和肌钙蛋白等氧化型肌纤维相关蛋白的表达,从而促进骨骼肌中氧化型肌纤维的形成。Song等[65]试验发现,鸡胚处于肠道微生物群失调并暴露于脂多糖(LPS)中时肌肉纤维的直径、肢体的发育和体细胞的生长均受到抑制,LPS通过诱导产生的活性氧促成了视黄酸信号扰动,从而影响体节形成和分化。Lei等[66]发现,肉鸡肠道微生物群能够影响肌肉持水能力,通过粪菌移植改变肉鸡的肠道菌群后,肌肉的持水能力也相应改变,并且经16S rDNA测序筛选到了与肌肉滴水损失、肌纤维直径相关的细菌属Lachnoclostridium spp.。Bai等[67]的研究表明,在肉鸡饲粮中添加枯草芽孢杆菌可以有效改善肉鸡肉质,包括增加胸肌pH、改善肉色、降低蒸煮损失及滴水损失。
4 小结肌纤维的组织学特性是决定肉品质的结构基础,众多研究发现胚胎期成肌细胞的分化增殖和出生后肌纤维类型的转化受Ca2+信号通路、Pax3/7和MoyD转录因子等多重调控,而母体效应、饲粮营养水平、植物活性成分和肠道微生物等均可调控肉鸡肌纤维特性,从而影响鸡肉品质。但是关于肌纤维发育过程中的具体分子机制和调控通路仍有待进一步挖掘,而关于营养和环境等因素影响肌纤维特性和肉品质的具体靶点和途径同样需要进一步探索,来推动肉鸡肉品质调控方案的完善。
| [1] |
HINDI S M, KUMAR A. TRAF6 regulates satellite stem cell self-renewal and function during regenerative myogenesis[J]. Journal of Clinical Investigation, 2016, 126(1): 151-168. |
| [2] |
HAUSMAN G J, POULOS S P. A method to establish co-cultures of myotubes and preadipocytes from collagenase digested neonatal pig semitendinosus muscles[J]. Journal of Animal Science, 2005, 83(5): 1010-1016. DOI:10.2527/2005.8351010x |
| [3] |
SWATLAND H J. Muscle growth in the fetal and neonatal pig[J]. Journal of Animal Science, 1973, 37(2): 536-545. DOI:10.2527/jas1973.372536x |
| [4] |
PETTE D, STARON R S. Myosin isoforms, muscle fiber types, and transitions[J]. Microscopy Research and Technique, 2000, 50(6): 500-509. DOI:10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7 |
| [5] |
WENG K Q, HUO W R, LI Y, et al. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers[J]. Poultry Science, 2022, 101(1): 101537. DOI:10.1016/j.psj.2021.101537 |
| [6] |
陈宽维, 李慧芳, 张学余, 等. 肉鸡肌纤维与肉质关系研究[J]. 中国畜牧杂志, 2002, 38(6): 6-7. CHEN K W, LI H F, ZHANG X Y, et al. Study on the relation between muscle fiber and meat quality in broilers[J]. Chinese Journal of Animal Science, 2002, 38(6): 6-7 (in Chinese). DOI:10.3969/j.issn.0258-7033.2002.06.002 |
| [7] |
魏法山, 康相涛, 李国喜, 等. 固始鸡生长过程中不同类型肌纤维面积比的变化[J]. 西北农林科技大学学报(自然科学版), 2006, 34(2): 7-11. WEI F S, KANG X T, LI G X, et al. Study on the area proportion of different muscle fibers during the growing period of Gushi chicks[J]. Journal of Northwest A & F University (Natural Science Edition), 2006, 34(2): 7-11 (in Chinese). DOI:10.3321/j.issn:1671-9387.2006.02.002 |
| [8] |
HUO W R, WENG K Q, LI Y, et al. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers[J]. Poultry Science, 2022, 101(3): 101649. DOI:10.1016/j.psj.2021.101649 |
| [9] |
IMMONEN K, PUOLANNE E. Variation of residual glycogen-glucose concentration at ultimate pH values below 5.75[J]. Meat Science, 2000, 55(3): 276-283. |
| [10] |
BOWER N I, DE LA SERRANA D G, COLE N J, et al. Stac3 is required for myotube formation and myogenic differentiation in vertebrate skeletal muscle[J]. The Journal of Biological Chemistry, 2012, 287(52): 43936-43949. DOI:10.1074/jbc.M112.361311 |
| [11] |
WEBB S E, MILLER A L. Visualization of Ca2+ signaling during embryonic skeletal muscle formation in vertebrates[J]. Cold Spring Harbor Perspectives in Biology, 2011, 3(2): a004325. |
| [12] |
SIEBER B, CORONAS-SERNA J M, MARTIN S G. A focus on yeast mating: from pheromone signaling to cell-cell fusion[J/OL]. Seminars in Cell & Developmental Biology. (2022-02-08)[2022-08-21]. https://pubmed.ncbi.nlm.nih.gov/35148940/.
|
| [13] |
ZHANG L, LI Q Q, WU J J, et al. Analysis of SARS-CoV-2 variants B.1.617:host tropism, proteolytic activation, cell-cell fusion, and neutralization sensitivity[J]. Emerging Microbes & Infections, 2022, 11(1): 1024-1036. |
| [14] |
聂庆华, 徐海平, 张敏. 非编码RNAs调控家禽骨骼肌生长发育的表观遗传机制研究进展[J]. 华南农业大学学报, 2019, 40(5): 111-118. NIE Q H, XU H P, ZHANG M. Research progress of epigenetic mechanism of noncoding RNAs regulating avian skeletal muscle development[J]. Journal of South China Agricultural University, 2019, 40(5): 111-118 (in Chinese). |
| [15] |
TREMBLAY P, DIETRICH S, MERICSKAY M, et al. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors[J]. Developmental Biology, 1998, 203(1): 49-61. DOI:10.1006/dbio.1998.9041 |
| [16] |
DASTON G, LAMAR E, OLIVIER M, et al. Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse[J]. Development (Cambridge, England), 1996, 122(3): 1017-1027. DOI:10.1242/dev.122.3.1017 |
| [17] |
MAROTO M, RESHEF R, MVNSTERBERG A E, et al. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue[J]. Cell, 1997, 89(1): 139-148. DOI:10.1016/S0092-8674(00)80190-7 |
| [18] |
OUSTANINA S, HAUSE G, BRAUN T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification[J]. The EMBO Journal, 2004, 23(16): 3430-3439. DOI:10.1038/sj.emboj.7600346 |
| [19] |
MCKINNELL I W, ISHIBASHI J, LE GRAND F, et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex[J]. Nature Cell Biology, 2008, 10(1): 77-84. DOI:10.1038/ncb1671 |
| [20] |
DEY B K, GAGAN J, DUTTA A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7[J]. Molecular and Cellular Biology, 2011, 31(1): 203-214. DOI:10.1128/MCB.01009-10 |
| [21] |
张江丽. 胚胎发育中骨骼肌组织的形成及调控[J]. 安徽农业科学, 2007, 35(21): 6447-6448, 6566. ZHANG J L. Morphogenesis and regulation of skeletal muscle tissue in the embryo development[J]. Journal of Anhui Agricultural Sciences, 2007, 35(21): 6447-6448, 6566 (in Chinese). DOI:10.3969/j.issn.0517-6611.2007.21.057 |
| [22] |
LI Y F, YUAN P T, FAN S X, et al. Weighted gene co-expression network indicates that the DYNLL2 is an important regulator of chicken breast muscle development and is regulated by miR-148a-3p[J]. BMC Genomics, 2022, 23(1): 258. DOI:10.1186/s12864-022-08522-8 |
| [23] |
CAI B L, MA M T, CHEN B, et al. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation[J]. Cell Death & Disease, 2018, 9(3): 367. |
| [24] |
LI D F, PAN Z X, ZHANG K, et al. Identification of the differentially expressed genes of muscle growth and intramuscular fat metabolism in the development stage of yellow broilers[J]. Genes, 2020, 11(3): 244. DOI:10.3390/genes11030244 |
| [25] |
WANG Z X, LIANG W S, LI X X, et al. Characterization and expression of MUSTN1 gene from different duck breeds[J]. Animal Biotechnology, 2022, 33(4): 723-730. DOI:10.1080/10495398.2020.1828905 |
| [26] |
HAN S S, CUI C, WANG Y, et al. Knockdown of CSRP3 inhibits differentiation of chicken satellite cells by promoting TGF-β/Smad3 signaling[J]. Gene, 2019, 707: 36-43. DOI:10.1016/j.gene.2019.03.064 |
| [27] |
ZHANG S Z, XIE H Q, XU Y, et al. An experimental study of the role of myosin light chain in myogenesis in vitro[J]. Chinese Journal of Reparative and Reconstructive Surgery, 2008, 22(6): 753-758. |
| [28] |
ZHANG J, CAI B L, MA M T, et al. LncRNA SMARCD3-OT1 promotes muscle hypertrophy and fast-twitch fiber transformation via enhancing SMARCD3X4 expression[J]. International Journal of Molecular Sciences, 2022, 23(9): 4510. DOI:10.3390/ijms23094510 |
| [29] |
STAMENKOVIC N, JASNIC J, NOVKOVIC M, et al. Cloning and expression profiling of muscle regulator ANKRD2 in domestic chicken Gallus gallus[J]. Histochemistry and Cell Biology, 2020, 154(4): 383-396. DOI:10.1007/s00418-020-01899-1 |
| [30] |
CHIN E R, OLSON E N, RICHARDSON J A, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type[J]. Genes & Development, 1998, 12(16): 2499-2509. |
| [31] |
HE H R, YIN H D, YU X K, et al. PDLIM5 affects chicken skeletal muscle satellite cell proliferation and differentiation via the p38-MAPK pathway[J]. Animals: an Open Access Journal From MDPI, 2021, 11(4): 1016. |
| [32] |
YOSHIDA T, DELAFONTAINE P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy[J]. Cells, 2020, 9(9): 1970. DOI:10.3390/cells9091970 |
| [33] |
LI Z H, CAI B L, ABDALLA B A, et al. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway[J]. Journal of Cachexia Sarcopenia and Muscle, 2019, 10(2): 391-410. DOI:10.1002/jcsm.12374 |
| [34] |
LONG N M, TOUSLEY C B, UNDERWOOD K R, et al. Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle[J]. Journal of Animal Science, 2012, 90(1): 197-206. DOI:10.2527/jas.2011-4237 |
| [35] |
GARDNER D S, BELL R C, SYMONDS M E. Fetal mechanisms that lead to later hypertension[J]. Current Drug Targets, 2007, 8(8): 894-905. DOI:10.2174/138945007781386901 |
| [36] |
LONG N M, NIJLAND M J, NATHANIELSZ P W, et al. The effect of early to mid-gestational nutrient restriction on female offspring fertility and hypothalamic-pituitary-adrenal axis response to stress[J]. Journal of Animal Science, 2010, 88(6): 2029-2037. DOI:10.2527/jas.2009-2568 |
| [37] |
MORAES T G V, PISHNAMAZI A, WENGER I I, et al. Energy and protein dilution in broiler breeder pullet diets reduced offspring body weight and yield[J]. Poultry Science, 2019, 98(6): 2555-2561. DOI:10.3382/ps/pey603 |
| [38] |
LESUISSE J, SCHALLIER S, LI C, et al. Multigenerational effects of a reduced balanced protein diet during the rearing and laying period of broiler breeders. 2. Zootechnical performance of the F1 broiler offspring[J]. Poultry Science, 2018, 97(5): 1666-1676. DOI:10.3382/ps/pey014 |
| [39] |
LI F, YANG X, YANG Y, et al. Maternal energy restriction by low starch or fat affects carcase trait, meat quality and muscle antioxidative properties in Arbor Acres broilers[J]. Italian Journal of Animal Science, 2019, 18(1): 1419-1430. DOI:10.1080/1828051X.2019.1679044 |
| [40] |
WU H Z, SUN H, MA C Z, et al. Effects of maternal dietary energy restriction on breast muscle fibre development in the offspring of broiler breeders[J]. Animal Bioscience, 2021, 34(11): 1829-1838. DOI:10.5713/ab.20.0712 |
| [41] |
WIDOWSKI T M, COOLEY L, HENDRIKSEN S, et al. Maternal age and maternal environment affect egg composition, yolk testosterone, offspring growth and behaviour in laying hens[J]. Scientific Reports, 2022, 12(1): 1828. DOI:10.1038/s41598-022-05491-6 |
| [42] |
GONZALEZ J M, JACKSON A R. In ovo feeding of nicotinamide riboside affects broiler pectoralis major muscle development[J]. Translational Animal Science, 2020, 4(3): txaa126. DOI:10.1093/tas/txaa126 |
| [43] |
XU X X, JACKSON A R, GONZALEZ J M. The effects of in ovo nicotinamide riboside dose on broiler myogenesis[J]. Poultry Science, 2021, 100(3): 100926. DOI:10.1016/j.psj.2020.12.024 |
| [44] |
MA Y B, ZHANG F D, WANG J, et al. Effect of in ovo feeding of β-hydroxy-β-methylbutyrate on hatchability, muscle growth and performance in prenatal and posthatch broilers[J]. Journal of the Science of Food and Agriculture, 2020, 100(2): 755-763. DOI:10.1002/jsfa.10080 |
| [45] |
WANG L S, ZUO X D, ZHAO W J, et al. Effect of maternal dietary supplementation with phytosterol esters on muscle development of broiler offspring[J]. Acta Biochimica Polonica, 2020, 67(1): 135-141. |
| [46] |
AVILA L P, LEIVA S F, ABASCAL-PONCIANO G A, et al. Effect of combined maternal and post-hatch dietary 25-hydroxycholecalciferol supplementation on broiler chicken pectoralis major muscle growth characteristics and satellite cell mitotic activity[J]. Journal of Animal Science, 2022, 100(8): skac192. DOI:10.1093/jas/skac192 |
| [47] |
LACKNER J, ALBRECHT A, MITTLER M, et al. Effect of feeding histidine and β-alanine on carnosine concentration, growth performance, and meat quality of broiler chickens[J]. Poultry Science, 2021, 100(11): 101393. DOI:10.1016/j.psj.2021.101393 |
| [48] |
WATANABE G, KOBAYASHI H, SHIBATA M, et al. Reduction in dietary lysine increases muscle free amino acids through changes in protein metabolism in chickens[J]. Poultry Science, 2020, 99(6): 3102-3110. DOI:10.1016/j.psj.2019.11.025 |
| [49] |
DE CARVALHO B R, DA CRUZ FERREIRA JÚ NIOR H, DA SILVA VIANA G, et al. In-feed organic and inorganic manganese supplementation on broiler performance and physiological responses[J]. Animal Bioscience, 2021, 34(11): 1811-1821. DOI:10.5713/ab.20.0797 |
| [50] |
AHSAN U, ĪPEK E, ÖZDEMIR Ö S, et al. Intermittent dilution of dietary digestible lysine lowers the incidence of white striping by suppressing the growth, lipid synthesis, and muscle damage in broiler chickens[J/OL]. Journal of the Science of Food and Agriculture. (2000-07-21)[2022-08-21]. https://pubmed.ncbi.nlm.nih.gov/35861039/.
|
| [51] |
LUCA S V, MACOVEI I, BUJOR A, et al. Bioactivity of dietary polyphenols: the role of metabolites[J]. Critical Reviews in Food Science and Nutrition, 2020, 60(4): 626-659. DOI:10.1080/10408398.2018.1546669 |
| [52] |
NOH D, LIM Y, LEE H, et al. Soybean-hop alleviates estrogen deficiency-related bone loss and metabolic dysfunction in ovariectomized rats fed a high-fat diet[J]. Molecules, 2018, 23(5): 1205. DOI:10.3390/molecules23051205 |
| [53] |
DOMÍNGUEZ-LÓPEZ I, YAGO-ARAGÓN M, SALAS-HUETOS A, et al. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review[J]. Nutrients, 2020, 12(8): 2456. DOI:10.3390/nu12082456 |
| [54] |
OGAWA M, KITANO T, KAWATA N, et al. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice[J]. The Journal of Nutritional Biochemistry, 2017, 49: 63-70. DOI:10.1016/j.jnutbio.2017.07.017 |
| [55] |
YOSHINO M, NAKA A, SAKAMOTO Y, et al. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells[J]. The Journal of Nutritional Biochemistry, 2015, 26(11): 1193-1199. DOI:10.1016/j.jnutbio.2015.05.010 |
| [56] |
WEN W X, CHEN X L, HUANG Z Q, et al. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway[J]. The Journal of Nutritional Biochemistry, 2020, 77: 108297. DOI:10.1016/j.jnutbio.2019.108297 |
| [57] |
ZHANG C, YANG L, ZHAO X H, et al. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers[J]. Journal of the Science of Food and Agriculture, 2018, 98(3): 1216-1221. DOI:10.1002/jsfa.8576 |
| [58] |
LAHIRI S, KIM H, GARCIA-PEREZ I, et al. The gut microbiota influences skeletal muscle mass and function in mice[J]. Science Translational Medicine, 2019, 11(502): eaan5662. DOI:10.1126/scitranslmed.aan5662 |
| [59] |
ZHAO L, HUANG Y F, LU L, et al. Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats[J]. Microbiome, 2018, 6(1): 107. DOI:10.1186/s40168-018-0492-6 |
| [60] |
CANFORA E E, MEEX R C R, VENEMA K, et al. Gut microbial metabolites in obesity, NAFLD and T2DM[J]. Nature Reviews Endocrinology, 2019, 15(5): 261-273. DOI:10.1038/s41574-019-0156-z |
| [61] |
LV W Q, LIN X, SHEN H, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women[J]. Journal of Cachexia Sarcopenia and Muscle, 2021, 12(6): 1860-1870. DOI:10.1002/jcsm.12788 |
| [62] |
WALSH M E, BHATTACHARYA A, SATARANATARAJAN K, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging[J]. Aging Cell, 2015, 14(6): 957-970. DOI:10.1111/acel.12387 |
| [63] |
HAN J H, KIM I S, JUNG S H, et al. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41[J]. PLoS One, 2014, 9(4): e95268. DOI:10.1371/journal.pone.0095268 |
| [64] |
CHANG S, CHEN X L, HUANG Z Q, et al. Dietary sodium butyrate supplementation promotes oxidative fiber formation in mice[J]. Animal Biotechnology, 2018, 29(3): 212-215. DOI:10.1080/10495398.2017.1358734 |
| [65] |
SONG J H, WANG C J, LONG D L, et al. Dysbacteriosis-induced LPS elevation disturbs the development of muscle progenitor cells by interfering with retinoic acid signaling[J]. The FASEB Journal, 2020, 34(5): 6837-6853. DOI:10.1096/fj.201902965R |
| [66] |
LEI J Q, DONG Y Y, HOU Q H, et al. Intestinal microbiota regulate certain meat quality parameters in chicken[J]. Frontiers in Nutrition, 2022, 9: 747705. DOI:10.3389/fnut.2022.747705 |
| [67] |
BAI K W, HUANG Q, ZHANG J F, et al. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens[J]. Poultry Science, 2017, 96(1): 74-82. DOI:10.3382/ps/pew246 |



