在人工授精技术普及的规模化生猪养殖过程中,卵泡发育质量及排卵率是决定母猪繁殖性能的主要因素之一。以高繁殖性能而著名的梅山母猪,其单个发情周期内最多可比商品杂交母猪多排11.3个成熟卵子[1],这表明商品母猪的排卵率仍有较大的提高潜力。母猪卵泡发育同时受营养物质和生殖激素的调控[2-3]。肠道微生物不仅与饲粮营养物质之间存在紧密互作,而且参与调控生殖激素的分泌与代谢[4-11]。因此,基于肠道微生物探究母猪卵泡发育质量的营养调控具有重要意义。
1 母猪卵泡发育 1.1 母猪卵泡发育生理过程母猪的高繁殖性能与其较短的产仔间隔、高排卵率、高产仔数和卵泡发育生理机制有关[2-3]。母猪卵泡发育分为2个阶段:60日龄前的对促性腺激素不敏感阶段和60日龄后的受下丘脑-垂体-性腺轴调控阶段[12]。母猪卵泡发育始于胎儿期,此时卵原细胞进行有丝分裂,随后进入减数第一次分裂的前期,并被扁平的前颗粒细胞包围,即原始卵泡。在此过程中,卵泡的生长和发育主要受旁分泌产生的生长因子调控,如生长分化因子9(growth differentiation factor 9, GDF9)和骨形成蛋白15(bone morphogenetic protein 15, BMP15)等[2]。60日龄后,促性腺激素开始影响母猪卵巢卵泡发育,同时卵巢对垂体促性腺激素的负反馈调节在60~100日龄之间逐步建立[12]。初情期后,母猪卵泡发育主要以卵泡波的方式出现(图 1)。然而,由卵泡中颗粒细胞凋亡导致的卵泡闭锁造成99%以上发育中的卵泡在排卵前退化[13]。
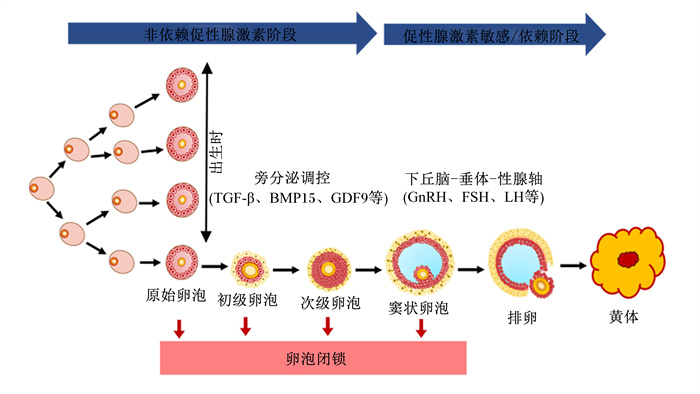
|
TGF-β:转化生长因子-β transforming growth factor-β;BMP15:骨形成蛋白15 bone morphogenetic protein 15;GDF9:生长分化因子9 growth differentiation factor 9;GnRH:促性腺激素释放激素gonadotropin-releasing hormone;FSH:促卵泡激素follicle-stimulating hormone;LH:促黄体激素luteinizing hormone。 图 1 母猪卵泡发育生理过程 Fig. 1 Physiological process of follicular development of sows |
哺乳动物卵巢颗粒细胞和卵母细胞主导卵泡发育,是决定卵泡发育质量和命运的关键因素[14]。颗粒细胞在卵泡发育中起着至关重要的作用,它们负责合成分泌卵泡生长和卵母细胞成熟所需的生长因子和类固醇[15]。卵母细胞则通过分泌卵母细胞分泌因子调控颗粒细胞增殖[3]。颗粒细胞和卵母细胞之间的物质交换通过缝隙连接进行,并允许相邻细胞共享1 kDa以内的小分子[3]。卵母细胞和颗粒细胞通过紧密互作影响卵泡的发育与成熟,它们之间一般存在以下2种作用机制:1)缝隙连接能够完成卵母细胞和卵丘颗粒细胞之间的小分子(离子、氨基酸和代谢产物等)的交换和转运;2)一种细胞类型产生的细胞因子和生长因子能够结合另一种细胞类型上存在的特定受体并且激活信号通路[3](图 2)。因此,营养调控母猪卵泡发育质量需同时关注卵母细胞和颗粒细胞。

|
cAMP:环磷酸腺苷cyclic adenosine monophosphate;cGMP:环磷酸鸟苷cyclic guanosine monophosphate;GDF9:生长分化因子9 growth differentiation factor 9;BMP15:骨形成蛋白15 bone morphogenetic protein 15。 图 2 母猪卵巢卵泡中颗粒细胞与卵母细胞之间互作示意图 Fig. 2 Schematic diagram of interaction between granulosa cells and oocytes in ovarian follicles of sows |
采食量及能量摄入水平通过调控生殖激素的分泌影响母猪卵泡发育,特别是后备母猪。后备母猪哺乳期限制采食量不仅显著降低了血浆胰岛素样生长因子1(insulin-like growth factor 1,IGF1)浓度和卵泡大小,而且降低了卵母细胞质量和卵泡液中IGF1和类固醇类激素(雌二醇和孕激素)浓度[16]。类似地,后备母猪哺乳期限制采食量(70%自由采食量)显著降低了血浆中促卵泡激素(follicle-stimulating hormone,FSH)、促黄体激素(luteinizing hormone,LH)浓度以及卵泡的数量、质量和大小[17]。排卵率较高的母猪在初情前和发情周期中均有较高的血浆FSH浓度[18],而后备母猪血浆中高浓度的IGF1与更大的卵泡和更短的断奶至发情间隔相关[19]。后备母猪在黄体期(第2次排卵后第1天至第13天)限制采食量显著降低了卵泡大小和血浆雌激素浓度,同时减少了排卵率和胚胎数[20]。以上研究结果表明,采食量与能量摄入水平可通过下丘脑-垂体轴调控FSH和LH浓度,并通过IGF1直接影响卵泡发育与成熟。
2.2 饲粮蛋白质和氨基酸对母猪卵泡发育质量的影响现代母猪的高繁殖性能促使其营养物质需要量显著增加,特别是优质饲粮蛋白质和氨基酸[21]。哺乳期饲粮蛋白质水平从16.0%降至14.3%,同时保证氨基酸平衡,可在泌乳高峰期提高饲料氮利用率,但对母猪的繁殖性能无显著影响[22]。饲粮中的蛋白质经消化后以氨基酸或肽的形式吸收代谢,必需氨基酸和功能性氨基酸影响母猪繁殖性能和卵泡发育质量[23-26]。赖氨酸作为猪玉米-豆粕型饲粮中的第一限制性氨基酸,初产哺乳母猪的低赖氨酸摄入量(0.4%)不仅损害了卵泡发育质量,也降低了排卵率[25]。然而,与中等赖氨酸摄入量(1.0%)相比,高赖氨酸摄入量(1.6%)对卵泡发育质量没有进一步的积极影响[25]。母猪妊娠期饲喂低蛋白质水平(7.5%)饲粮降低了仔猪出生时卵巢重量,促进了仔猪卵巢组织凋亡通路相关基因的表达[27]。
2.3 饲粮纤维对母猪卵泡发育质量的影响饲粮添加纤维(300 g/d)可减少后备母猪卵泡闭锁,促进卵泡成熟[4]。在后备母猪配种前(初情期前3周至第1个发情周期的第19天)饲喂高纤维(麸皮或羽扇豆纤维)饲粮可以促进后备母猪的卵泡发育,对血浆中LH浓度无显著影响,但这种效果依赖于纤维来源[28]。低纤维水平的商品配方饲料使梅山母猪产仔数显著降低,而提高饲粮纤维水平在增加产仔数的同时升高了粪便中短链脂肪酸(short-chain fatty acids,SCFAs)浓度[8]。在不同商品猪场调研表明,母猪粪便中SCFAs浓度与产仔数存在显著正相关性[29]。饲粮纤维可能通过其代谢产物SCFAs促进结肠血清素-褪黑素合成,进而改善高能量水平饲粮诱导的母猪卵泡闭锁,促进母猪卵泡发育与成熟[4]。
2.4 饲粮脂肪酸对母猪卵泡发育质量的影响脂肪酸不仅是细胞主要的产能底物,而且作为类固醇激素和信号分子的前体调控哺乳卵泡发育[30]。未成熟的猪卵母细胞含有约156 ng的脂质,远高于牛和羊的卵母细胞,后者通常分别为58和4 ng[31]。猪卵母细胞在体外成熟过程中可使用内源性甘油三酯作为能量来源[32]。低繁殖性能母猪卵泡液中的氨基酸、脂肪酸、嘌呤和嘧啶的代谢发生显著改变,其中月桂酸浓度可能与卵泡发育质量和卵细胞质量呈负相关[33]。在经产母猪的哺乳期饲粮中添加亚麻油酸或α-亚麻油酸不仅可显著缩短母猪产后返情间隔,而且具有提高随后胎次的产仔数的趋势[34]。多不饱和脂肪酸(polyunsaturated fatty acid,PUFA)是动物的必需营养物质[35],在产前和哺乳期的饲粮中添加0.3%鱼油(ω-3 PUFA)可以增加下一胎次的产仔数,且这种效果与能量摄入水平无关[36]。相反,另有一些研究报道了添加ω-3 PUFA对母猪繁殖性能的不利影响或无影响[37-38]。ω-3 PUFA来源的多样性可能与这种不确定的发现有关,如植物油、海产品油和鱼油[38-39]。考虑到胆固醇是母猪卵泡颗粒细胞合成类固醇类生殖激素的前体,PUFA可能通过影响胆固醇代谢调控卵巢相关激素的合成与分泌[40]。然而,目前缺少饲粮添加ω-3 PUFA对母猪卵泡发育影响的相关研究。
2.5 饲粮营养物质调控母猪卵泡发育质量的可能途径饲粮营养物质主要通过影响中间代谢产物和激素的分泌对哺乳动物卵泡发育过程进行调控[41-43]。机体循环系统中的IGF1和瘦素分别通过与下丘脑和垂体中的相应受体结合而间接影响卵泡发育[44-45]。在分解代谢状态下,较低的IGF1和瘦素浓度可以减少FSH和LH的释放[46-47]。IGF1可以与FSH协同刺激颗粒细胞增殖和类固醇生成[48]。体外试验中,瘦素可调节颗粒细胞分泌雌二醇[49],但瘦素在体内对卵泡发育作用仍待阐明。在能量负平衡期间,增强的脂肪代谢导致代谢中间产物非酯化脂肪酸浓度升高,而升高的非酯化脂肪酸可以体外抑制颗粒细胞增殖并诱发凋亡[50]。与低质量卵泡相比,高质量卵泡的卵泡液中葡萄糖浓度较低,但乳酸和支链氨基酸浓度较高[51]。与此类似,卵泡液中饱和脂肪酸/不饱和脂肪酸的比例与卵母细胞质量呈负相关[52]。因此,营养物质中间代谢产物或激素是营养调控母猪卵泡发育质量的途径。
3 肠道微生物对母猪卵泡发育质量的调控作用 3.1 肠道微生物对母猪卵泡发育质量的影响近年来,饲喂低纤维素水平的饲粮导致以高产仔数著名的梅山母猪产仔数显著下降[8]。然而,在一个繁殖周期内将饲粮中纤维水平由2.5%增加到7.5%,梅山母猪的产仔数显著提高,这种提高与肠道微生物的改变显著相关[8]。同一品种的高产仔数母猪与低产仔数母猪的肠道微生物也有明显的差异,而且粪便中SCFAs浓度与产仔数呈显著正相关[29, 53]。值得注意的是,高产梅山母猪的粪便微生物移植可促进受体长×大母猪的卵泡发育与成熟,减少卵泡闭锁[53-54]。因此,肠道微生物可能通过其代谢产物影响母猪卵泡发育与成熟。
3.2 肠道微生物对母猪卵泡发育相关生殖激素的影响肠道微生物作为宿主的第2基因组,具有内分泌器官的功能,参与调控生殖激素的分泌和代谢,特别是类固醇类生殖激素孕激素和雌二醇[10-11]。肠道微生物通过合成并分泌水解酶β-葡萄糖醛酸酶和β-葡萄糖苷酶调控雌激素的代谢[55]。我国地方高产梅山母猪的血浆雌二醇浓度显著高于杂交白种猪[56],而梅山母猪的肠道微生物移植可升高杂交白种猪的血浆雌二醇浓度[53-54]。因此,肠道微生物可能通过影响卵泡发育相关生殖激素调控母猪卵泡发育质量。
3.3 肠道微生物在饲粮营养物质调控母猪卵泡发育质量中的作用肠道微生物参与宿主对摄取的营养物质的消化、吸收与代谢[4, 10, 57]。后备母猪饲粮中纤维素的肠道微生物代谢产物SCFAs可能通过增加卵泡液中5-羟色胺和褪黑素浓度缓解高能量饲粮诱导的卵泡闭锁[4]。SCFAs不仅可作为信号分子以剂量依赖方式激活G蛋白偶联受体(G-protein-coupled receptors,GPR)43、GPR41和GPCR109A等[58],而且可通过表观遗传修饰调控基因表达[59-60]。猪颗粒细胞体外培养试验表明,0.05 mmol/L丁酸促进孕激素分泌;5和10 mmol/L的丁酸抑制孕激素分泌,却以剂量依赖方式促进雌二醇分泌[61]。丁酸只有在达到10 mmol/L时才能促进猪卵巢颗粒细胞GPR43和GPR41基因的表达[61]。基于人和小鼠的相关数据,目前普遍认为猪卵泡内生理浓度的SCFAs无法达到激活GPR的阈值[4, 62]。然而,考虑到种属差异性和SCFAs作用的累加效应,生理状态下母猪卵泡液中SCFAs浓度是否可激活GPR及对卵母细胞和颗粒细胞的生理效应都亟待解答[61-62]。
考虑到肠道微生物可通过代谢产物SCFAs诱导IGF1的分泌[63],饲粮添加纤维对母猪繁殖性能的改善作用可能与IGF1相关。早期研究发现,IGF1可在体外促进母猪卵巢颗粒细胞增殖和分化,同时促进卵泡发育和卵母细胞成熟[64-65]。高繁殖性能梅山母猪的肠道微生物移植可促进受体长×大母猪的卵泡发育,增加粪便中SCFAs浓度,升高血浆雌二醇和IGF1浓度,同时降低血浆孕激素浓度[53-54]。SCFAs也可通过GPR41促进脂肪细胞分泌瘦素[66-67]。瘦素主要由白色脂肪组织合成分泌,不仅调节能量平衡,而且影响雌性动物繁殖功能[68]。瘦素能够直接促进卵巢颗粒细胞中的芳香化酶活性[69],梅山猪的血浆中较高的瘦素浓度或许可以解释其颗粒细胞中的芳香化酶活性显著高于杂交白猪[70]。在母猪饲粮中增加纤维水平可缓解高脂肪水平饲粮诱发的卵泡闭锁,这种效果至少部分通过肠道微生物-SCFAs-5-羟色胺-褪黑激素轴实现[4]。综上所述,母猪肠-卵巢轴的可能调控途径如图 3所示。

|
SCFAs:短链脂肪酸short-chain fatty acids;GPR43:G蛋白偶联受体43 G protein-coupled receptors 43;GPR41:G蛋白偶联受体41 G protein-coupled receptors 41;FSH:促卵泡激素follicle-stimulating hormone;LH:促黄体激素luteinizing hormone;GnRH:促性腺激素释放激素gonadotropin-releasing hormone;Kisspeptin:吻素;AVPV:前脑室周围核anteroventral periventricular nucleus;ARC:下丘脑弓状核arcuate nucleus。 图 3 肠道微生物调控母猪卵泡发育潜在途径 Fig. 3 Potential pathways of gut microbiota regulating follicle development of sows |
卵泡发育质量是决定母猪繁殖性能的关键因素之一。饲粮中主要营养物质包括能量、蛋白质、纤维和脂肪酸,均可直接或间接影响母猪繁殖性能。母猪肠道微生物直接参与饲粮营养物质的消化、吸收和代谢,其组成和功能与母猪的繁殖性能显著相关,可能介导饲粮营养物质对母猪繁殖性能的调控作用。然而,现有研究主要探究饲粮营养物质及肠道微生物与母猪产仔数等繁殖性能指标的相关性,缺少因果关系及其中分子机制的相关研究。在建立饲粮营养物质与母猪繁殖性能相关性的基础上,进一步解析营养物质调控卵泡发育质量等具体繁殖相关生理过程的分子机制,对于开发相应的精准营养调控策略具有重要意义。此外,肠道微生物与饲粮营养物质存在密切互作关系,通过添加功能性营养物质定向调控肠道微生物组成和代谢模式,有望提高母猪卵泡发育质量。
| [1] |
CHRISTENSON R K. Ovulation rate and embryonic survival in Chinese Meishan and white crossbred pigs[J]. Journal of Animal Science, 1993, 71(11): 3060-3066. DOI:10.2527/1993.71113060x |
| [2] |
KNOX R V. Follicle development in pigs: state of the art[J/OL]. Molecular Reproduction & Development: 1-11[2022-07-15]. https://doi.org/10.1002/mrd.23576.DOI: 10.1002/mrd.23576.
|
| [3] |
COSTERMANS N G J. Physiological and molecular aspects of ovarian follicular developmental competence in sows[D]. Ph. D. Thesis. Wageningen: Wageningen University & Research, 2020.
|
| [4] |
ZHUO Y, CAO M, GONG Y C, et al. Gut microbial metabolism of dietary fibre protects against high energy feeding induced ovarian follicular atresia in a pig model[J]. British Journal of Nutrition, 2021, 125(1): 38-49. DOI:10.1017/S0007114520002378 |
| [5] |
GRAHAM M E, HERBERT W G, SONG S D, et al. Gut and vaginal microbiomes on steroids: implications for women's health[J]. Trends in Endocrinology & Metabolism, 2021, 32(8): 554-565. |
| [6] |
HAN Q X, WANG J, LI W P, et al. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome[J]. Microbiome, 2021, 9(1): 101. DOI:10.1186/s40168-021-01046-5 |
| [7] |
SILVA M S B, GIACOBINI P. Don't trust your gut: when gut microbiota disrupt fertility[J]. Cell Metabolism, 2019, 30(4): 616-618. DOI:10.1016/j.cmet.2019.09.005 |
| [8] |
JIANG X Y, LU N S, XUE Y, et al. Crude fiber modulates the fecal microbiome and steroid hormones in pregnant Meishan sows[J]. General and Comparative Endocrinology, 2019, 277: 141-147. DOI:10.1016/j.ygcen.2019.04.006 |
| [9] |
QI X Y, YUN C Y, PANG Y L, et al. The impact of the gut microbiota on the reproductive and metabolic endocrine system[J]. Gut Microbes, 2021, 13(1): 1894070. DOI:10.1080/19490976.2021.1894070 |
| [10] |
HUSSAIN T, MURTAZA G, KALHORO D H, et al. Relationship between gut microbiota and host-metabolism: emphasis on hormones related to reproductive function[J]. Animal Nutrition, 2021, 7(1): 1-10. DOI:10.1016/j.aninu.2020.11.005 |
| [11] |
NURIEL-OHAYON M, NEUMAN H, ZIV O, et al. Progesterone increases Bifidobacterium relative abundance during late pregnancy[J]. Cell Reports, 2019, 27(3): 730-736.e3. DOI:10.1016/j.celrep.2019.03.075 |
| [12] |
KNOX R V. Physiology and endocrinology symposium: factors influencing follicle development in gilts and sows and management strategies used to regulate growth for control of estrus and ovulation[J]. Journal of Animal Science, 2019, 97(4): 1433-1445. DOI:10.1093/jas/skz036 |
| [13] |
UYAR A, TORREALDAY S, SELI E. Cumulus and granulosa cell markers of oocyte and embryo quality[J]. Fertility and Sterility, 2013, 99(4): 979-997. DOI:10.1016/j.fertnstert.2013.01.129 |
| [14] |
EPPIG J J. Reproduction: oocytes call, granulosa cells connect[J]. Current Biology, 2018, 28(8): R354-R356. DOI:10.1016/j.cub.2018.03.005 |
| [15] |
MATSUDA F, INOUE N, MANABE N, et al. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells[J]. The Journal of Reproduction and Development, 2012, 58(1): 44-50. DOI:10.1262/jrd.2011-012 |
| [16] |
COSTERMANS N G J, TEERDS K J, MIDDELKOOP A, et al. Consequences of negative energy balance on follicular development and oocyte quality in primiparous sows[J]. Biology of Reproduction, 2020, 102(2): 388-398. DOI:10.1093/biolre/ioz175 |
| [17] |
KAUFFOLD J, GOTTSCHALK J, SCHNEIDER F, et al. Effects of feeding level during lactation on FSH and LH secretion patterns, and follicular development in primiparous sows[J]. Reproduction in Domestic Animals, 2008, 43(2): 234-238. DOI:10.1111/j.1439-0531.2007.00880.x |
| [18] |
KNOX R V, VATZIAS G, NABER C H, et al. Plasma gonadotropins and ovarian hormones during the estrous cycle in high compared to low ovulation rate gilts[J]. Journal of Animal Science, 2003, 81(1): 249-260. DOI:10.2527/2003.811249x |
| [19] |
HAN T, BJÖRKMAN S, SOEDE N M, et al. IGF-1 concentration patterns and their relationship with follicle development after weaning in young sows fed different pre-mating diets[J]. Animal, 2020, 14(7): 1493-1501. DOI:10.1017/S1751731120000063 |
| [20] |
CHEN T Y, STOTT P, ATHORN R Z, et al. Undernutrition during early follicle development has irreversible effects on ovulation rate and embryos[J]. Reproduction, Fertility, and Development, 2012, 24(6): 886-892. DOI:10.1071/RD11292 |
| [21] |
TOKACH M D, MENEGAT M B, GOURLEY K M, et al. Review: nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements[J]. Animal, 2019, 13(12): 2967-2977. DOI:10.1017/S1751731119001253 |
| [22] |
HUBER L, DE LANGE C F M, KROGH U, et al. Impact of feeding reduced crude protein diets to lactating sows on nitrogen utilization[J]. Journal of Animal Science, 2015, 93(11): 5254-5264. DOI:10.2527/jas.2015-9382 |
| [23] |
DING S J, AZAD M A K, FANG J, et al. Impact of sulfur-containing amino acids on the plasma metabolomics and intestinal microflora of the sow in late pregnancy[J]. Food & Function, 2019, 10(9): 5910-5921. |
| [24] |
BEEBE L F S, VASSILIEV I, MCILFATRICK S, et al. Adding essential amino acids at a low concentration improves the development of in vitro fertilized porcine embryos[J]. The Journal of Reproduction and Development, 2009, 55(4): 373-377. DOI:10.1262/jrd.20176 |
| [25] |
YANG H, FOXCROFT G R, PETTIGREW J E, et al. Impact of dietary lysine intake during lactation on follicular development and oocyte maturation after weaning in primiparous sows[J]. Journal of Animal Science, 2000, 78(4): 993-1000. DOI:10.2527/2000.784993x |
| [26] |
HUSSAIN T, TAN B, MURTAZA G, et al. Role of dietary amino acids and nutrient sensing system in pregnancy associated disorders[J]. Frontiers in Pharmacology, 2020, 11: 586979. DOI:10.3389/fphar.2020.586979 |
| [27] |
SUI S Y, JIA Y M, HE B, et al. Maternal low-protein diet alters ovarian expression of folliculogenic and steroidogenic genes and their regulatory microRNAs in neonatal piglets[J]. Asian-Australasian Journal of Animal Sciences, 2014, 27(12): 1695-1704. DOI:10.5713/ajas.2014.14335 |
| [28] |
WEAVER A C, KELLY J M, KIND K L, et al. Oocyte maturation and embryo survival in nulliparous female pigs (gilts) is improved by feeding a lupin-based high-fibre diet[J]. Reproduction, Fertility, and Development, 2013, 25(8): 1216-1223. DOI:10.1071/RD12329 |
| [29] |
URYU H, TSUKAHARA T, ISHIKAWA H, et al. Comparison of productivity and fecal microbiotas of sows in commercial farms[J]. Microorganisms, 2020, 8(10): 1469. DOI:10.3390/microorganisms8101469 |
| [30] |
KHAN R, JIANG X H, HAMEED U, et al. Role of lipid metabolism and signaling in mammalian oocyte maturation, quality, and acquisition of competence[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 639704. DOI:10.3389/fcell.2021.639704 |
| [31] |
MCEVOY T G, COULL G D, BROADBENT P J, et al. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida[J]. Journal of Reproduction and Fertility, 2000, 118(1): 163-170. DOI:10.1530/jrf.0.1180163 |
| [32] |
STURMEY R G, LEESE H J. Energy metabolism in pig oocytes and early embryos[J]. Reproduction, 2003, 126(2): 197-204. DOI:10.1530/rep.0.1260197 |
| [33] |
CHEN M X, ZHANG B, CAI S, et al. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows[J]. Journal of Proteomics, 2019, 200: 134-143. DOI:10.1016/j.jprot.2019.03.015 |
| [34] |
ROSERO D S, BOYD R D, MCCULLEY M, et al. Essential fatty acid supplementation during lactation is required to maximize the subsequent reproductive performance of the modern sow[J]. Animal Reproduction Science, 2016, 168: 151-163. DOI:10.1016/j.anireprosci.2016.03.010 |
| [35] |
KURLAK L O, STEPHENSON T J. Plausible explanations for effects of long chain polyunsaturated fatty acids (LCPUFA) on neonates[J]. Archives of Disease in Childhood: Fetal and Neonatal Edition, 1999, 80(2): F148-F154. DOI:10.1136/fn.80.2.F148 |
| [36] |
SMITS R J, LUXFORD B G, MITCHELL M, et al. Sow litter size is increased in the subsequent parity when lactating sows are fed diets containing n-3 fatty acids from fish oil[J]. Journal of Animal Science, 2011, 89(9): 2731-2738. DOI:10.2527/jas.2010-3593 |
| [37] |
MATEO R D, CARROLL J A, HYUN Y, et al. Effect of dietary supplementation of n-3 fatty acids and elevated concentrations of dietary protein on the performance of sows[J]. Journal of Animal Science, 2009, 87(3): 948-959. DOI:10.2527/jas.2008-0964 |
| [38] |
SMIT M N, PATTERSON J L, WEBEL S K, et al. Responses to n-3 fatty acid (LCPUFA) supplementation of gestating gilts, and lactating and weaned sows[J]. Animal, 2013, 7(5): 784-792. DOI:10.1017/S1751731112002236 |
| [39] |
ROSSI R, PASTORELLI G, CANNATA S, et al. Recent advances in the use of fatty acids as supplements in pig diets: a review[J]. Animal Feed Science and Technology, 2010, 162(1/2): 1-11. |
| [40] |
OTTE M V, MOREIRA F, BIANCHI I, et al. Effects of supplying omega-3 polyunsaturated fatty acids to gilts after weaning on metabolism and ovarian gene expression[J]. Journal of Animal Science, 2019, 97(1): 374-384. DOI:10.1093/jas/sky419 |
| [41] |
BARBE A, BONGRANI A, MELLOUK N, et al. Mechanisms of adiponectin action in fertility: an overview from gametogenesis to gestation in humans and animal models in normal and pathological conditions[J]. International Journal of Molecular Sciences, 2019, 20(7): 1526. DOI:10.3390/ijms20071526 |
| [42] |
MOREIRA F, CHEUICHE Z M G, RIZZOTO G, et al. Metabolic and reproductive parameters in prepubertal gilts after omega-3 supplementation in the diet[J]. Animal Reproduction Science, 2016, 170: 178-183. DOI:10.1016/j.anireprosci.2016.05.008 |
| [43] |
TIAN M, CHEN J M, LIU J X, et al. Dietary fiber and microbiota interaction regulates sow metabolism and reproductive performance[J]. Animal Nutrition, 2020, 6(4): 397-403. DOI:10.1016/j.aninu.2020.10.001 |
| [44] |
COSTERMANS N G J, SOEDE N M, VAN TRICHT F, et al. Follicular fluid steroid profile in sows: relationship to follicle size and oocyte quality[J]. Biology of Reproduction, 2020, 102(3): 740-749. DOI:10.1093/biolre/ioz217 |
| [45] |
KORDOWITZKI P, KRAJNIK K, SKOWRONSKA A, et al. Pleiotropic effects of IGF1 on the oocyte[J]. Cells, 2022, 11(10): 1610. DOI:10.3390/cells11101610 |
| [46] |
ODLE A K, AKHTER N, SYED M M, et al. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence[J]. Frontiers in Endocrinology, 2018, 8: 367. DOI:10.3389/fendo.2017.00367 |
| [47] |
WOLFE A, DIVALL S, WU S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1)[J]. Frontiers in Neuroendocrinology, 2014, 35(4): 558-572. DOI:10.1016/j.yfrne.2014.05.007 |
| [48] |
LIU J, KOENIGSFELD A T, CANTLEY T C, et al. Growth and the initiation of steroidogenesis in porcine follicles are associated with unique patterns of gene expression for individual components of the ovarian insulin-like growth factor system[J]. Biology of Reproduction, 2000, 63(3): 942-952. DOI:10.1095/biolreprod63.3.942 |
| [49] |
RUIZ-CORTÉS Z T, MARTEL-KENNES Y, GÉVRY N Y, et al. Biphasic effects of leptin in porcine granulosa cells[J]. Biology of Reproduction, 2003, 68(3): 789-796. DOI:10.1095/biolreprod.102.010702 |
| [50] |
BJERRE-HARPØTH V, FRIGGENS NC, THORUP V M, et al. Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation[J]. Journal of Dairy Science, 2012, 95(5): 2362-2380. DOI:10.3168/jds.2011-4419 |
| [51] |
WALLACE M, COTTELL E, GIBNEY M J, et al. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome[J]. Fertility and Sterility, 2012, 97(5): 1078-1084.e8. DOI:10.1016/j.fertnstert.2012.01.122 |
| [52] |
O'GORMAN A, WALLACE M, COTTELL E, et al. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence[J]. Reproduction, 2013, 146(4): 389-395. DOI:10.1530/REP-13-0184 |
| [53] |
徐保阳. 肠道菌群干预对母猪卵巢和子宫发育的作用及其机制[D]. 博士学位论文. 武汉: 华中农业大学, 2021. XU B Y. Effect and mechanism of gut microbiota intervention on the development of sow's ovary and uterus[D]. Ph. D. Thesis. Wuhan: Huazhong Agricultural University, 2021. (in Chinese) |
| [54] |
XU B Y, QIN W X, YAN Y Q, et al. Gut microbiota contributes to the development of endometrial glands in gilts during the ovary-dependent period[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 57. DOI:10.1186/s40104-021-00578-y |
| [55] |
KWA M, PLOTTEL C S, BLASER M J, et al. The intestinal microbiome and estrogen receptor-positive female breast cancer[J]. Journal of the National Cancer Institute, 2016, 108(8): djw029. |
| [56] |
HUNTER M G. Comparison of ovarian function and embryo development in Meishan and large white hybrid pigs[J]. Reproduction in Domestic Animals, 1994, 29(4): 343-345. DOI:10.1111/j.1439-0531.1994.tb00571.x |
| [57] |
LEEMING E R, LOUCA P, GIBSON R, et al. The complexities of the diet-microbiome relationship: advances and perspectives[J]. Genome Medicine, 2021, 13(1): 10. DOI:10.1186/s13073-020-00813-7 |
| [58] |
ZHOU H, YU B, CHEN H, et al. Carbohydrates effects on nutrition and health functions in pigs[J]. Animal Science Journal, 2021, 92(1): e13557. |
| [59] |
VAN DER HEE B, WELLS J M. Microbial regulation of host physiology by short-chain fatty acids[J]. Trends in Microbiology, 2021, 29(8): 700-712. DOI:10.1016/j.tim.2021.02.001 |
| [60] |
YE Q H, ZENG X F, WANG S, et al. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells[J]. The FASEB Journal, 2021, 35(2): e21316. |
| [61] |
LU N S, LI M J, LEI H L, et al. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2017, 172: 89-97. DOI:10.1016/j.jsbmb.2017.06.004 |
| [62] |
KOH A, DE VADDER F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. DOI:10.1016/j.cell.2016.05.041 |
| [63] |
YAN J, HERZOG J W, TSANG K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(47): E7554-E7563. |
| [64] |
SPICER L J, ALPIZAR E, ECHTERNKAMP S E. Effects of insulin, insulin-like growth factor Ⅰ, and gonadotropins on bovine granulosa cell proliferation, progesterone production, estradiol production, and(or) insulin-like growth factor Ⅰ production in vitro[J]. Journal of Animal Science, 1993, 71(5): 1232-1241. DOI:10.2527/1993.7151232x |
| [65] |
MAZERBOURG S, BONDY C A, ZHOU J, et al. The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles?A comparative species study[J]. Reproduction in Domestic Animals, 2003, 38(4): 247-258. DOI:10.1046/j.1439-0531.2003.00440.x |
| [66] |
ZAIBI MS, STOCKER CJ, O'DOWD J, et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids[J]. FEBS letters, 2010, 584(11): 2381-2386. DOI:10.1016/j.febslet.2010.04.027 |
| [67] |
XIONG Y M, MIYAMOTO N, SHIBATA K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(4): 1045-1050. DOI:10.1073/pnas.2637002100 |
| [68] |
CATTEAU A, CAILLON H, BARRIÈRE P, et al. Leptin and its potential interest in assisted reproduction cycles[J]. Human Reproduction Update, 2016, 22(3): 320-341. DOI:10.1093/humupd/dmv057 |
| [69] |
KITAWAKI J, KUSUKI I, KOSHIBA H, et al. Leptin directly stimulates aromatase activity in human luteinized granulosa cells[J]. Molecular Human Reproduction, 1999, 5(8): 708-713. DOI:10.1093/molehr/5.8.708 |
| [70] |
HUNTER M G, BIGGS C, FAILLACE L S. Endocrine and follicular studies in Meishan pigs[J]. Journal of Reproduction and Fertility, 1993, 48: 261-270. |




