家禽免疫系统的功能受年龄、饲粮营养物质、饲料和能量摄入量以及遗传和环境等因素的影响。作为提高生产水平的策略之一,营养免疫调节被表述为营养物质的靶向补充,并调控特定发育阶段的免疫功能。评估最适免疫反应的关键:一是衡量感染状态下机体对病原体的反应[1];二是基于不同日龄阶段评估机体免疫机能的差别[2]。基于以上关键因素,结合不同生理阶段和状态下的营养目标与免疫特性,本文按种禽、关键时期(胚期和商品代饲养期)和疾病状态3个主要层面进行了阐述。针对不同层面,选择何种营养物质进行适度的免疫表型修饰是目前系统探索的热点。此外,免疫调节不仅受到营养物质介导表观遗传修饰的直接作用,而且受到采食关联性肠道菌群的间接影响。因此,本文对各层面的营养物质需求和免疫调节效应进行梳理,同时分析表观遗传修饰和肠道微生物在免疫营养中发挥的作用,旨在为行业提供参考。
1 免疫营养的关键层面基于级联放大效应,优化种禽营养状态具有广泛调控子代表型的潜力[3]。如子代通过胎盘、种蛋沉积等途径获得母源营养物质,在这一窗口期干预母体营养,可有效调控子代表型。禽类出壳后免疫系统尚未发育成熟,种禽营养调控可针对性地提高子代出雏早期的免疫力[4]。
由于孵化后期卵黄囊能量供给不足及幼雏出壳后开食延迟,孵化后期和出雏早期的营养状态对于生长发育愈发重要。近年来,胚蛋给养技术逐步实现了在孵化关键阶段(主要集中在后期),针对性改善出雏后的表型[5]。而出雏后的商品代是以生产性能为导向的品种改良,集约化养殖虽使得生长速度大幅提高,但免疫器官发育相对不足[2]。针对生产前期的免疫功能低下,营养性或功能性饲料添加剂的使用是十分必要的。
病毒和细菌性疾病对当今家禽业仍然构成威胁,此外环境(卫生条件差、压力和生态失调)等因素使家禽处于高代谢需求状态,易引发疾病。因此必须采取预防和控制措施,而营养是有效改善手段之一。利用营养手段提高禽类免疫力来抵抗病原体感染等疾病有很大的发挥空间[6]。以上层面是家禽免疫营养的研究侧重点,精准营养在区分生产目标的同时,靶向免疫调控也应是具体化和个性化的调控方案。
2 免疫营养的主要调控策略 2.1 免疫调节目标“营养程序化”理论指出,发育关键时期的营养状况将对机体的组织器官功能产生长期影响。近40年来,家禽胚蛋给养不仅使其对机体的影响贯穿发育始末,同时增加了胚期影响力[7]。孵化后期和出雏早期是免疫器官发育的关键期;而商品代饲养期的营养调控强调提高生产性能,减少免疫耗能,因此胚蛋给养可为其提供基础支持,2个环节的核心是针对免疫系统早期发育的调控。
对于种禽营养,间接的“营养程序化”重视的是传代效应。如饲粮添加20 mg/kg的大豆苷元或30%的有机微量矿物质促进种母鸡抗体转移和子代肉仔鸡体液免疫应答[4, 8]。而目前父源传代营养调控更多集中于提高父系繁殖性能及子代生产性能上[9]。传代效应的发挥可能更多是在营养表观遗传学的基础上进行的[10],通过营养传代表观遗传调控基因表达并在代际间传递并放大。
在疾病状态下配制调节免疫力的饲粮时,必须明晰免疫系统影响疾病状态下机体营养重分配的机制。如在代谢性疾病中,高度激活的免疫系统启动了机体防护,改变了营养代谢反应,使免疫耗能增加,生产潜力降低[11]。为确保该状态的营养需求,免疫细胞可提升赖氨酸、精氨酸和葡萄糖等营养物质转运水平以及脂肪氧化相关的酶活性[12-13]。免疫代谢作为先天性和适应性免疫调节的主要机制,通过改变全身代谢状态影响免疫细胞代谢,导致免疫反应的改变[6, 11]。图 1简要概述了在感染期间营养免疫和免疫代谢之间的互作效应,这共同决定了疾病的结果或个体的健康[1, 6, 11, 14]。
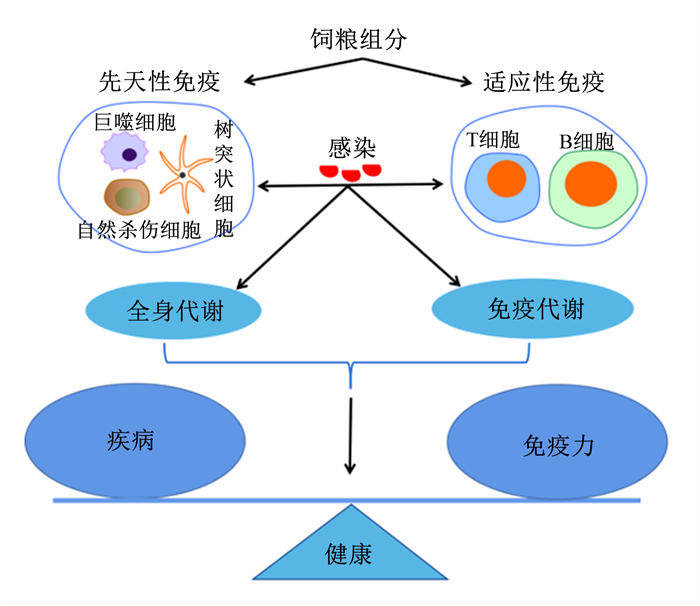
|
图 1 感染期间营养免疫和免疫代谢之间的互作效应 Fig. 1 Interaction between nutritional immunity and immune metabolism during infection[1, 6, 11, 14] |
目前,在胚蛋给养模式下进行初探,营养供给方案主要包括:1)糖类,包括黄芪多糖和棉子糖等,如在胚期第12天通过气室注射4.5 mg的棉子糖(0.2 mL)能改善肉鸡回肠黏膜形态,提高T细胞和B细胞表达水平[15];2)氨基酸,包括苏氨酸、精氨酸和丝氨酸等,如在胚期第14天通过卵黄囊注射20或30 mg苏氨酸(0.5 mL)可增强雏鸡对绵羊红细胞的体液免疫应答[16];3)维生素,包括维生素C、维生素E和叶酸等,如在胚期第11天的卵黄囊内注射3 mg维生素C(0.1 mL)可提高肉雏鸡21日龄脾脏白细胞介素-4(interleukin-4,IL-4)的表达,降低白细胞介素-1β(interleukin-1β,IL-1β)的表达[5];4)矿物元素,包括硒、碘和铁等,如胚期第18天在羊膜腔注射10或20 μg的硒(0.1 mL)能够提高肉鸡针对产气荚膜梭菌和坏死性肠炎的血清抗体水平[17]。但胚蛋注射的位点、时间点以及功能性营养物质种类和剂量的选择尚未形成系统标准化方案。
针对商品代肉禽,营养供给主要关注:1)多糖类,包括中药复方多糖、香菇多糖及酵母细胞壁多糖等;2)油脂类,如饲粮添加50或300 mg/kg包膜肉桂油可促进肉鸡非炎症状态下的体液和黏膜免疫机能[18];3)氨基酸,如苏氨酸、L-茶氨酸和L-精氨酸等;4)矿物质,如硒缺乏抑制了肉鸡免疫应答的多效机制[19]。不够,虽然选择较多,但部分营养物质没有证明其在疾病状态下的适度免疫激活效应[20]。因此下面进一步梳理疾病状态下的免疫营养(表 1)[21-36]。
|
|
表 1 家禽疾病状态下免疫营养研究示例 Table 1 Examples of immunonutrition studies in poultry under disease states |
此外,营养物质的添加需要整体解决方案,包括性质、类型、组合方式和剂量等;同时,必须充分考虑各环节的免疫调控侧重点,包括免疫系统发育、免疫细胞底物、调节免疫细胞活性、减少免疫病理、调节激素活性、影响肠道生理和在感染期间限制病原体对营养的利用等[37]。
3 表观遗传调控免疫营养表观遗传是在DNA碱基序列不变的条件下引起基因表达发生可逆和可遗传的改变, 主要的修饰形式为DNA甲基化、微小RNA(microRNA,miRNA)修饰和组蛋白修饰。表观遗传信息的重编程发生于2个阶段:1)从原始生殖细胞到配子细胞产生;2)从卵子受精到胚胎发育[38](图 2)。二者分别为家禽父母传代和胚期表观遗传的理论基础。2个阶段表现出环境的可塑性,为营养物质介导表观遗传调控奠定基础[10]。图 2列举了通过不同表观遗传机制影响免疫系统的主要营养物质[10, 39-40]。
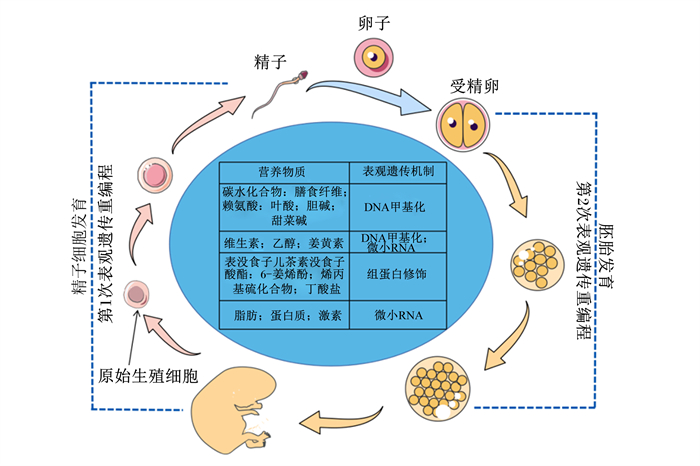
|
图 2 表观遗传重编程及通过表观遗传机制影响免疫系统的主要营养物质 Fig. 2 Epigenetic reprogramming and major nutrients affecting immune system through epigenetic mechanisms[10, 39-40] |
母源营养可以通过表观遗传调控后代基因表达,比如种母鸡高锌饲粮(300 mg/kg)可能通过改变子代锌指蛋白A20(zinc finger protein A20,A20)的DNA甲基化和组蛋白乙酰化修饰,抑制核因子-κB(nuclear factor kappa-B,NF-κB)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)和IL-1β的转录,通过A20-NF-κB通路增强后代的免疫力[41]。种公鸡饲粮添加1%黄芪多糖,一是显著影响睾丸免疫进程相关miRNA的表达[42],二是调控细胞因子信号传导抑制蛋白-1(suppressor of cytokine signaling-1,SOCS-1)启动子甲基化以及髓样分化因子88(myeloid differentiation factor 88,MyD88)和β干扰素TIR结构域衔接蛋白(TIR-domain containing adaptor inducing interferon-β,TRIF)的组蛋白修饰,诱导子代肉鸡空肠黏膜的内毒素耐受样免疫反应[3]。
3.2 胚期肉鸡胚胎心脏、肝脏和肌肉组织的甲基化水平会随胚龄增加而上升,分为3个时期:2~4胚龄、5~13胚龄和14~19胚龄。基于DNA甲基化在不同胚龄的差异性,在胚期第11天在卵黄囊内注射0.1 mL的叶酸(100~150 μg)可调控肉鸡出壳后脾脏IL-4的组蛋白3上的第4位赖氨酸的甲基化(methylation of lysine 4 on histone 3,H3K4)以及白细胞介素-6(interleukin-6,IL-6)的组蛋白3上的第9位赖氨酸的甲基化(methylation of lysine 9 on histone 3,H3K9)水平,提高IL-4基因表达[43]。在胚期第11天通过卵黄囊注射3 mg维生素C(0.1 mL)提高肉鸡脾脏IL-4和DNA甲基转移酶3A(DNA methyltransferase 3A,DNMT3A)的表达,并降低干扰素-γ(interferon-γ,IFN-γ)的表达[5]。
3.3 疾病状态下疾病状态下目前需要主要关注:1)常量营养素,如怀孕大鼠采食蛋白质限制性饮食会持续改变后代肝脏中特定胞嘧啶的甲基化[44]。2)微量营养素,如叶酸、B族维生素等均参与DNA甲基化调控,硒和镁则是甲基化相关酶的辅助因子。3)生物活性物质,如姜黄素衍生的合成类似物通过miRNA控制炎症反应,并抑制血管内皮生长因子和IL-6的产生[45];大豆多酚和染料木黄酮可提高DNA甲基化和降低组蛋白H3上的乙酰化,使IL-6启动子处发生基因沉默[46]。目前,家禽营养表观遗传学机制并不明确,但快速发展的分子遗传、细胞生物学技术以及功能基因组学为进一步解析奠定了基础。
4 肠道微生物与免疫营养的互作作为黏膜免疫系统的基本器官,肠道实现了营养吸收和病原体防御的结合,共生微生物发挥关键调节作用。免疫系统能够调节共生微生物的生态位,而营养物质介导微生物所发生的变化通过改变免疫细胞的信号传导途径和免疫细胞的发育以及受体的识别/感知,对宿主免疫产生直接或间接的影响[47]。图 3展示了胃肠道中营养物质、免疫系统和共生微生物间的双向互作[47-52]。本节将简要阐述益生菌及其代谢产物在家禽免疫营养中的作用。
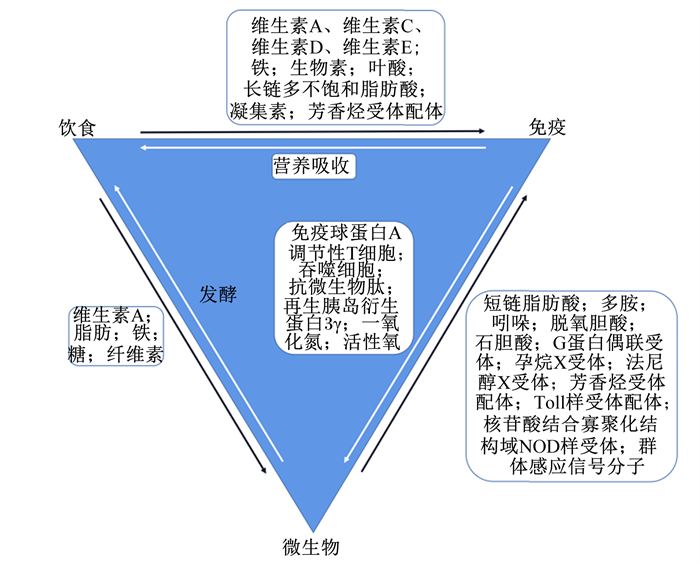
|
图 3 营养物质、免疫系统和共生微生物之间的互作 Fig. 3 Interactions between nutrients, immune system or symbiotic microbiota[47-52] |
研究发现,在胚期第18日龄通过羊膜联合注射乳杆菌、屎肠球菌和双歧杆菌,可降低出壳肉雏鸡回肠和盲肠扁桃体中IL-4、INF-γ和Toll样受体2(Toll-like receptor 2,TLR2)等基因的表达[53]。枯草芽孢杆菌(1.6×109 CFU/g)、杆菌肽锌(20 mg/kg)和硫酸黏菌素(4 mg/kg)的混合添加可促进蛋鸡空肠分泌型免疫球蛋白A(secreted immunoglobulin A,sIgA)的分泌[54]。此外,芽孢杆菌的群体信号分子γ-聚谷氨酸促进调节性T细胞(regulatory T cells,Tregs)的选择性分化并抑制辅助性T细胞17(T helper 17,Th17)的分化[55]。种母鸡饲粮中添加β-胡萝卜素、姜黄素、大蒜素和丁酸钠,可减轻抗生素介导的微生物紊乱所引起的空肠炎症[56]。嗜酸乳杆菌、罗伊氏乳杆菌和唾液乳杆菌单独或联合使用可以增强鸡巨噬细胞对禽流感病毒的抗病毒活性[57]。
如图 3所示,微生物的营养代谢产物主要包括短链脂肪酸(short-chain fatty acids,SCFAs)、次级胆汁酸和多胺等。SCFAs促进Tregs分化,激活G蛋白偶联受体(G protein-coupled receptors,GPCRs),并能调节巨噬细胞和树突状细胞的免疫效应[52]。饲粮添加400 mg/kg的包被丁酸钠可增加肉仔鸡胸腺与法氏囊指数,提高血液中免疫球蛋白M(immunoglobulin M, IgM)和免疫球蛋白G(immunoglobulin G, IgG)含量[58];此外,丁酸还可通过抑制脂多糖诱导的吞噬作用以促进免疫稳态的恢复[59]。其他代谢物共同激活核受体如芳香烃受体(aryl hydrocarbon receptor,AhR),通过表达于免疫细胞以感知代谢产物的存在。相关研究表明,脱氧胆酸和石胆酸通过其核受体法尼醇X受体(farnesoid X receptor,FXR)和孕烷X受体(pregnane X receptor,PXR)调节免疫系统,在PXR敲除小鼠中葡聚糖硫酸钠诱导的结肠炎更为严重[60]。
过往研究中,免疫营养调控所面临的明显问题是忽略了肠道微生物组的作用,因此诸如营养物质直接影响免疫反应的作用机制很可能需要重新评估[61]。此外,阐明营养-共生菌-免疫三者如何在分子水平发生,以及如何将营养介导的微生物的改变转化为适度的免疫调节效应,将是未来研究的关键。
5 小结家禽免疫营养追求实现营养供求与免疫稳态间的平衡,并通过精准营养调控,改善肠道微生态健康,促进适时免疫。但仍需要重视以下问题:1)每种营养物质在维持免疫稳态中都发挥作用,摄入不足和过量会造成负面影响,且尝试以剂量依赖的添加方式不仅成本昂贵,有时还会导致疾病易感性增加;2)不同生理阶段和状态下的免疫机能存在差别,只有实现适度的免疫激活效应,才可以利用“营养一体化”理论来指导免疫调节。此外,还要综合考量表观遗传调控和肠道微生物对其的影响,尤其是营养-共生菌-免疫间的互作机制还需要进一步明晰。在未来的研究中,免疫营养还需要在非营养成分、营养工程、基因组学、免疫学及遗传学等方面共同进行探索。
| [1] |
KOGUT M H. Impact of nutrition on the innate immune response to infection in poultry[J]. Journal of Applied Poultry Research, 2009, 18(1): 111-124. DOI:10.3382/japr.2008-00081 |
| [2] |
ALKIE T N, YITBAREK A, HODGINS D C, et al. Development of innate immunity in chicken embryos and newly hatched chicks: a disease control perspective[J]. Avian Pathology, 2019, 48(4): 288-310. DOI:10.1080/03079457.2019.1607966 |
| [3] |
LI Y L, LEI X Y, GUO W, et al. Transgenerational endotoxin tolerance-like effect caused by paternal dietary Astragalus polysaccharides in broilers' jejunum[J]. International Journal of Biological Macromolecules, 2018, 111: 769-779. DOI:10.1016/j.ijbiomac.2018.01.095 |
| [4] |
FAN H, LV Z P, GAN L P, et al. Transcriptomics-related mechanisms of supplementing laying broiler breeder hens with dietary daidzein to improve the immune function and growth performance of offspring[J]. Journal of Agricultural and Food Chemistry, 2018, 66(8): 2049-2060. DOI:10.1021/acs.jafc.7b06069 |
| [5] |
ZHU Y F, LI S Z, DUAN Y L, et al. Effects of in ovo feeding of vitamin C on post-hatch performance, immune status and DNA methylation-related gene expression in broiler chickens[J]. The British Journal of Nutrition, 2020, 124(9): 903-911. DOI:10.1017/S000711452000210X |
| [6] |
KARLSSON E A, BECK M A, MACIVER N J. Editorial: nutritional aspects of immunity and immunometabolism in health and disease[J]. Frontiers in Immunology, 2020, 11: 595115. DOI:10.3389/fimmu.2020.595115 |
| [7] |
李德生, 娄玉杰. 胚蛋给养对家禽早期营养及免疫应答影响研究进展[J]. 中国畜牧杂志, 2016, 52(7): 86-91. LI D S, LOU Y J. Research progress of in ovo feeding on early nutrition and immune response in poultry[J]. Chinese Journal of Animal Science, 2016, 52(7): 86-91 (in Chinese). DOI:10.3969/j.issn.0258-7033.2016.07.018 |
| [8] |
OVIEDO-RONDÓN E O, LEANDRO N M, ALI R, et al. Broiler breeder feeding programs and trace minerals on maternal antibody transfer and broiler humoral immune response1[J]. Journal of Applied Poultry Research, 2013, 22(3): 499-510. DOI:10.3382/japr.2012-00708 |
| [9] |
TRIQUES G E, DE CRISTO A B, CANEVESE M, et al. Effect of antioxidant supplementation in diets of roosters during the post-peak phase on reproduction and production characteristics of offspring[J]. Ciência Animal Brasileira, 2019, 20: 1-13. |
| [10] |
PAPARO L, DI COSTANZO M, DI SCALA C, et al. The influence of early life nutrition on epigenetic regulatory mechanisms of the immune system[J]. Nutrients, 2014, 6(11): 4706-4719. DOI:10.3390/nu6114706 |
| [11] |
MAKOWSKI L, CHAIB M, RATHMELL J C. Immunometabolism: from basic mechanisms to translation[J]. Immunological Reviews, 2020, 295(1): 5-14. DOI:10.1111/imr.12858 |
| [12] |
HUMPHREY B D, RUDRAPPA S G. Increased glucose availability activates chicken thymocyte metabolism and survival[J]. The Journal of Nutrition, 2008, 138(6): 1153-1157. DOI:10.1093/jn/138.6.1153 |
| [13] |
RUDRAPPA S G, HUMPHREY B D. Energy metabolism in developing chicken lymphocytes is altered during the embryonic to posthatch transition[J]. The Journal of Nutrition, 2007, 137(2): 427-432. DOI:10.1093/jn/137.2.427 |
| [14] |
HENDRIKS W H, VERSTEGEN M W A, BABINSZKY L. Poultry and pig nutrition: challenges of the 21st century[M]. Wageningen: Wageningen Academic Publishers, 2019: 428.
|
| [15] |
BERROCOSO J D, KIDA R, SINGH A K, et al. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken[J]. Poultry Science, 2017, 96(6): 1573-1580. DOI:10.3382/ps/pew430 |
| [16] |
KADAM M M, BHANJA S K, MANDAL A B, et al. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens[J]. British Poultry Science, 2008, 49(6): 736-741. DOI:10.1080/00071660802469333 |
| [17] |
LEE S H, LILLEHOJ H S, JANG S I, et al. Effects of in ovo injection with Selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens[J]. Poultry Science, 2014, 93(5): 1113-1121. DOI:10.3382/ps.2013-03770 |
| [18] |
GUO S S, CHENG Q, LI Y H, et al. Effects of dietary coated-oleum cinnamomi supplementation on the immunity and intestinal integrity of broiler chickens[J]. Animal Science Journal, 2018, 89(11): 1581-1590. DOI:10.1111/asj.13094 |
| [19] |
PAN T R, LIU T Q, TAN S R, et al. Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency[J]. Biological Trace Element Research, 2018, 182(2): 364-372. DOI:10.1007/s12011-017-1110-3 |
| [20] |
KOGUT M H. Issues and Consequences of using nutrition to modulate the avian immune response[J]. Journal of Applied Poultry Research, 2017, 26(4): 605-612. DOI:10.3382/japr/pfx028 |
| [21] |
LIU L, SHEN J, ZHAO C, et al. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide[J]. International Journal of Biological Macromolecules, 2015, 72: 624-632. DOI:10.1016/j.ijbiomac.2014.08.057 |
| [22] |
LI R, SONG Z H, ZHAO J F, et al. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers[J]. Animal Nutrition, 2018, 4(3): 265-272. DOI:10.1016/j.aninu.2018.05.002 |
| [23] |
BI Y, NAN X M, ZHENG S S, et al. Effects of dietary threonine and immune stress on growth performance, carcass trait, serum immune parameters, and intestinal MUC2 and NF-κB gene expression in Pekin ducks from hatch to 21 days[J]. Poultry Science, 2018, 97(1): 177-187. DOI:10.3382/ps/pex283 |
| [24] |
CHEN Z, ZHOU Y W, LIANG C, et al. Effects of γ-aminobutyric acid on the tissue structure, antioxidant activity, cell apoptosis, and cytokine contents of bursa of Fabricius in chicks under heat stress[J]. Archives Animal Breeding, 2016, 59(1): 97-105. DOI:10.5194/aab-59-97-2016 |
| [25] |
HU H, DAI S F, LI J Q, et al. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway[J]. Poultry Science, 2020, 99(3): 1454-1461. DOI:10.1016/j.psj.2019.11.001 |
| [26] |
HUANG Y L, LUO Q H, XIAO F, et al. Research note: responses of growth performance, immune traits, and small intestinal morphology to dietary supplementation of Chromium propionate in heat-stressed broilers[J]. Poultry Science, 2020, 99(10): 5070-5073. DOI:10.1016/j.psj.2020.07.005 |
| [27] |
MIN Y N, NIU Z Y, SUN T T, et al. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene[J]. Poultry Science, 2018, 97(4): 1238-1244. DOI:10.3382/ps/pex417 |
| [28] |
CHEN Y P, ZHANG H, CHENG Y F, et al. Dietary L-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age[J]. The British Journal of Nutrition, 2018, 119(11): 1254-1262. DOI:10.1017/S0007114518000740 |
| [29] |
XIAO M, MI Y L, LIU L J, et al. Taurine regulates mucosal barrier function to alleviate lipopolysaccharide-induced duodenal inflammation in chicken[J]. Amino Acids, 2018, 50(11): 1637-1646. DOI:10.1007/s00726-018-2631-6 |
| [30] |
DU E C, WANG W W, GAN L P, et al. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens[J]. Journal of Animal Science and Biotechnology, 2016, 7(1): 19. DOI:10.1186/s40104-016-0079-7 |
| [31] |
ZHAO X N, YANG J, WANG L L, et al. Protection mechanism of Clostridium butyricum against Salmonella enteritidis infection in broilers[J]. Frontiers in Microbiology, 2017, 8: 1523. DOI:10.3389/fmicb.2017.01523 |
| [32] |
SUN J J, ZHANG C, ZHANG B K. Research note: effects of organic Zinc on broiler intestinal permeability and integrity in clostridium perfringens-challenged condition[J]. Poultry Science, 2020, 99(12): 6653-6656. DOI:10.1016/j.psj.2020.09.032 |
| [33] |
OKOROAFOR O N, OGUNNIRAN T M, IKENNA-EZEH N H, et al. Effects of dietary supplementation of vitamins E and C on oxidative stress induced by a Nigerian velogenic strain of the Newcastle disease virus (KUDU 113) in the brain and bursa of Fabricius of broiler chickens[J]. Veterinary World, 2021, 14(9): 2452-2461. |
| [34] |
MING K, HE M, SU L L, et al. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication[J]. Poultry Science, 2020, 99(4): 2146-2156. DOI:10.1016/j.psj.2019.11.060 |
| [35] |
AKHTAR T, ARA G, ALI N, et al. Effects of dietary supplementation of mannan-oligosaccharide on virus shedding in avian influenza (H9N2) challenged broilers[J]. Iranian Journal of Veterinary Research, 2016, 17(4): 268-272. |
| [36] |
SHOJADOOST B, KULKARNI R R, YITBAREK A, et al. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2[J]. Veterinary Immunology and Immunopathology, 2019, 207: 62-68. DOI:10.1016/j.vetimm.2018.12.002 |
| [37] |
KLASING K C. Nutritional modulation of resistance to infectious diseases[J]. Poultry Science, 1998, 77(8): 1119-1125. DOI:10.1093/ps/77.8.1119 |
| [38] |
HUGHES V. Epigenetics: the sins of the father[J]. Nature, 2014, 507(7490): 22-24. DOI:10.1038/507022a |
| [39] |
CLAYCOMBE K J, BRISSETTE C A, GHRIBI O. Epigenetics of inflammation, maternal infection, and nutrition[J]. The Journal of Nutrition, 2015, 145(5): 1109S-1115S. DOI:10.3945/jn.114.194639 |
| [40] |
VAN DER HEIJDEN C D C C, NOZ M P, JOOSTEN L A B, et al. Epigenetics and trained immunity[J]. Antioxidants & Redox Signaling, 2018, 29(11): 1023-1040. |
| [41] |
李昌武. 肉种鸡锌营养对子代肉鸡免疫机能的影响及其分子机制[D]. 博士学位论文. 北京: 中国农业大学, 2015. LI C W. Effects of dietary zinc nutrition of the broiler breeders on the immunity function of their offsprings and the related molecular mechanism[D]. Ph. D. Thesis. Beijing: China Agricultural University, 2015. (in Chinese) |
| [42] |
任小春, 李玉龙, 武圣儒, 等. 黄芪多糖对种公鸡不同组织miR-16表达的影响及其功能预测分析[J]. 动物营养学报, 2016, 28(6): 1887-1898. REN X C, LI Y L, WU S R, et al. Effects and bioinformatic analysis of Astragalus polysaccharide on miR-16 expression in different tissue of breeder cocks[J]. Chinese Journal of Animal Nutrition, 2016, 28(6): 1887-1898 (in Chinese). DOI:10.3969/j.issn.1006-267x.2016.06.032 |
| [43] |
LI S Z, ZHI L H, LIU Y L, et al. Effect of in ovo feeding of folic acid on the folate metabolism, immune function and epigenetic modification of immune effector molecules of broiler[J]. The British Journal of Nutrition, 2016, 115(3): 411-421. DOI:10.1017/S0007114515004511 |
| [44] |
LILLYCROP K A, PHILLIPS E S, TORRENS C, et al. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring[J]. The British Journal of Nutrition, 2008, 100(2): 278-282. DOI:10.1017/S0007114507894438 |
| [45] |
BAO B, AHMAD A, KONG D J, et al. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF[J]. PLoS One, 2012, 7(8): e43726. DOI:10.1371/journal.pone.0043726 |
| [46] |
SZARC VEL SZIC K, NDLOVU M N, HAEGEMAN G, et al. Nature or nurture: let food be your epigenetic medicine in chronic inflammatory disorders[J]. Biochemical Pharmacology, 2010, 80(12): 1816-1832. DOI:10.1016/j.bcp.2010.07.029 |
| [47] |
ALEXANDER M, TURNBAUGH P J. Deconstructing mechanisms of diet-microbiome-immune interactions[J]. Immunity, 2020, 53(2): 264-276. DOI:10.1016/j.immuni.2020.07.015 |
| [48] |
KLASING K C. Nutrition and the immune system[J]. British Poultry Science, 2007, 48(5): 525-537. DOI:10.1080/00071660701671336 |
| [49] |
BELKAID Y, HAND T W. Role of the microbiota in immunity and inflammation[J]. Cell, 2014, 157(1): 121-141. DOI:10.1016/j.cell.2014.03.011 |
| [50] |
LIN L, ZHANG J Q. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases[J]. BMC Immunology, 2017, 18(1): 2. DOI:10.1186/s12865-016-0187-3 |
| [51] |
SATYARAJ E. Emerging paradigms in immunonutrition[J]. Topics in Companion Animal Medicine, 2011, 26(1): 25-32. DOI:10.1053/j.tcam.2011.01.004 |
| [52] |
KIM C H. Immune regulation by microbiome metabolites[J]. Immunology, 2018, 154(2): 220-229. DOI:10.1111/imm.12930 |
| [53] |
PENDER C M, KIM S, POTTER T D, et al. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks[J]. Poultry Science, 2017, 96(5): 1052-1062. DOI:10.3382/ps/pew381 |
| [54] |
LI X Y, WU S R, LI X Y, et al. Simultaneous supplementation of Bacillus subtilis and antibiotic growth promoters by stages improved intestinal function of pullets by altering gut microbiota[J]. Frontiers in Microbiology, 2018, 9: 2328. DOI:10.3389/fmicb.2018.02328 |
| [55] |
LEE K, HWANG S, PAIK D J, et al. Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis[J]. Clinical and Experimental Immunology, 2012, 170(1): 66-76. DOI:10.1111/j.1365-2249.2012.04637.x |
| [56] |
GONG H Z, WU M, LANG W Y, et al. Effects of laying breeder hens dietary β-carotene, curcumin, allicin, and sodium butyrate supplementation on the growth performance, immunity, and jejunum morphology of their offspring chicks[J]. Poultry Science, 2020, 99(1): 151-162. DOI:10.3382/ps/pez584 |
| [57] |
SHOJADOOST B, KULKARNI R R, BRISBIN J T, et al. Interactions between Lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus[J]. Research in Veterinary Science, 2019, 125: 441-450. DOI:10.1016/j.rvsc.2017.10.007 |
| [58] |
LUO D, LI J L, XING T, et al. Combined effects of xylo-oligosaccharides and coated sodium butyrate on growth performance, immune function, and intestinal physical barrier function of broilers[J]. Animal Science Journal, 2021, 92(1): e13545. |
| [59] |
ZHOU Z Y, PACKIALAKSHMI B, MAKKAR S K, et al. Effect of butyrate on immune response of a chicken macrophage cell line[J]. Veterinary Immunology and Immunopathology, 2014, 162(1/2): 24-32. |
| [60] |
SHAH Y M, MA X C, MORIMURA K, et al. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2007, 292(4): G1114-G1122. DOI:10.1152/ajpgi.00528.2006 |
| [61] |
WILS-PLOTZ E L, KLASING K C. Effects of immunomodulatory nutrients on growth performance and immune-related gene expression in layer chicks challenged with lipopolysaccharide[J]. Poultry Science, 2017, 96(3): 548-555. DOI:10.3382/ps/pew376 |



