2. 佛山科学技术学院生命科学与工程学院, 佛山 528225
2. School of Life and Engineering, Foshan University, Foshan 528225, China
羔羊断奶期间,由于外界环境变化、母子分离以及饲粮改变等,使羔羊产生应激反应,导致机体抗氧化能力和免疫功能下降[1-3],进而降低羔羊抵抗力,影响其生长发育。以往,可应用抗生素缓解羔羊断奶应激所产生的负面影响,但随着我国饲料“禁抗”法律的实施,对于寻找天然抗生素替代品已成为行业关注的重点[4]。岩藻多糖是于1913年从海藻中提取出的一种带负电荷和高度吸湿性的硫酸多糖[5-6],主要来源于海藻的细胞外基质,核心成分为岩藻糖,并具有中性糖和硫酸基的复杂分支。大量研究表明,岩藻多糖拥有广泛的药理活性和潜在的安全性,如抗癌、抗氧化、抗病毒、抗凝血、抗炎以及增加免疫活性[7-11]等。岩藻多糖作为饲料添加剂应用于动物生产中,能够提高断奶仔猪的抗氧化能力和免疫功能,并且对采食量、肠道形态及微生物菌群等方面也有促进作用[12-13]。我们前期研究结果表明,饲粮中添加岩藻多糖可提高羔羊生长性能、抗氧化能力和免疫功能,改善小肠形态结构[3]。但是,鲜有岩藻多糖对反刍动物肝脏功能和抗氧化能力以及脾脏抗氧化能力和免疫功能影响的报道。因此,本研究提出进一步假设,岩藻多糖对断奶羔羊肝脏和脾脏的免疫功能以及抗氧化能力均有积极影响,探究饲粮中添加岩藻多糖对断奶羔羊的器官指数、肝功能相关酶活性、肝脏和脾脏抗氧化指标以及脾脏免疫指标的影响,为岩藻多糖作为天然“替抗剂”改善幼龄反刍动物健康提供理论依据。
1 材料与方法 1.1 试验材料试验中使用的岩藻多糖由青岛某生物科技有限公司提供,呈黄色粉末状,具有海藻气味,纯度为98%,硫酸基含量为28.9%。
1.2 试验设计与饲养管理试验于2021年4月至2021年6月在广东省湛江市雷羊牧业有限公司进行。选用60只健康且体重相近的2月龄断奶川中黑山羊(去势公羔),随机分为4个组,每组15个重复,每个重复1只羊。对照组(CON组)饲喂基础饲粮,试验组(F1、F2和F3组)分别在基础饲粮中添加0.1%、0.3%和0.5%的岩藻多糖,岩藻多糖添加水平参考Yang等[3]。预试期7 d,正试期30 d。羔羊平均初始体重为(12.5±0.5) kg,均在2月龄时人工断奶。基础饲粮参照我国《肉羊饲养标准》(NY/T 861—2004)的营养要求配制,基础饲粮组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the basal diet (DM basis) |
在试验开始前打扫羊舍、消毒处理。先进行为期7 d的预试验,预试验期间对羔羊编号、驱虫、接种疫苗。每天08:30和17:30各饲喂1次,将每日饲喂的岩藻多糖均匀拌入精料中,先精后粗,自由饮水。饲养期间各组饲喂方式、试验环境及管理模式均相同。
1.3 样品采集 1.3.1 血清样品采集在试验第15天和第30天晨饲前,通过颈静脉采血,每只羊采血10 mL于促凝采血管中。采血管静置待有血清析出后,4 ℃、3 000 r/min离心10 min,将血清转移至2.0 mL无酶管中,-80 ℃保存,用于检测肝功能相关指标。
1.3.2肝脏和脾脏样品采集
在试验第31天,每组随机选择6只羊,采用颈动脉放血法屠宰,屠宰前禁食、禁水12 h,采集肝脏和脾脏组织于2.0 mL无酶离心管中,编号,转入液氮中速冻,之后转入-80 ℃保存,用于检测肝脏和脾脏抗氧化指标及脾脏免疫指标。
1.4 测定指标及方法 1.4.1 肝脏和脾脏指数试验结束后,取出肝脏和脾脏,吸掉其表面多余血液后分别称重,并按照以下公式计算肝脏指数与脾脏指数:
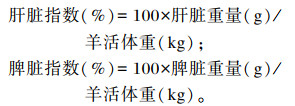
|
血清谷丙转氨酶(ALT)、谷草转氨酶(AST)、乳酸脱氢酶(LDH)活性均使用相应试剂盒(南京建成生物工程研究所)检测,操作时严格按照试剂盒说明书进行。
1.4.3 肝脏和脾脏抗氧化指标将肝脏和脾脏组织制成10%的匀浆,离心取上清。肝脏和脾脏丙二醛(MAD)、过氧化氢(H2O2)含量及总抗氧化能力(T-AOC)、总超氧化物歧化酶(T-SOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GSH-Px)活性均使用相应试剂盒(南京建成生物工程研究所)检测,操作时严格按照试剂盒说明书进行。
1.4.4 脾脏免疫指标脾脏肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)、白细胞介素-2(IL-2)、白细胞介素-6(IL-6)和白细胞介素-10(IL-10)含量均使用相应酶联免疫吸附测定(ELISA)试剂盒(江苏酶免实业有限公司)检测,操作时严格按照试剂盒说明书进行。
1.5 数据分析试验数据先用Excel 2019进行预处理,之后使用SPSS 26.0软件进行单因素方差分析(one-way ANOVA),采用Duncan氏法进行多重比较。结果用平均值和均值标准误(SEM)表示,P<0.05表示差异显著。
2 结果 2.1 饲粮中添加岩藻多糖对断奶羔羊肝脏和脾脏指数的影响如表 2所示,各组之间断奶羔羊的肝脏和脾脏指数均无显著差异(P>0.05)。
|
|
表 2 饲粮中添加岩藻多糖对断奶羔羊肝脏和脾脏指数的影响 Table 2 Effects of dietary fucoidan on liver and spleen indexes of weaned goat kids (n=6) |
如表 3所示,试验第15天,与CON组相比,F1、F2和F3组断奶羔羊血清LDH活性分别显著降低了21.22%、19.60%和16.14%(P<0.05),F1、F2和F3组之间血清LDH活性差异不显著(P>0.05);各组之间血清ALT和AST活性差异不显著(P>0.05)。试验第30天,与CON组相比,F1、F2和F3组断奶羔羊血清AST活性分别显著降低了36.90%、44.94%和51.54%(P<0.05),F1、F2和F3组之间血清AST活性差异不显著(P>0.05);与CON组相比,F2和F3组断奶羔羊血清LDH活性分别显著降低了6.30%和20.89%(P<0.05)。
|
|
表 3 饲粮中添加岩藻多糖对断奶羔羊肝功能相关指标的影响 Table 3 Effects of dietary fucoidan on liver function related indexes of weaned goat kids (n=6) |
如表 4所示,与CON组相比,F1、F2和F3组断奶羔羊肝脏MDA含量分别显著降低了20.93%、38.95%和38.95%(P<0.05),且F1组肝脏MDA含量显著高于F2和F3组(P<0.05)。与CON组相比,F1、F2和F3组断奶羔羊肝脏H2O2含量分别显著降低了37.07%、41.97%和55.02%(P<0.05),且F1和F2组肝脏H2O2含量显著高于F3组(P<0.05)。与CON组相比,F2和F3组断奶羔羊肝脏GSH-Px活性分别显著提高了20.57%和13.80%(P<0.05),且F1组肝脏GSH-Px活性显著低于F2和F3组(P<0.05)。各组之间断奶羔羊肝脏CAT、T-SOD活性和T-AOC差异不显著(P>0.05)。
|
|
表 4 饲粮中添加岩藻多糖对断奶羔羊肝脏抗氧化指标的影响 Table 4 Effects of dietary fucoidan on liver antioxidant indexes of weaned goat kids (n=6) |
如表 5所示,与CON组相比,F1、F2和F3组断奶羔羊脾脏H2O2含量分别显著降低了10.63%、23.15%和23.09%(P<0.05),且F1组脾脏H2O2含量显著高于F2和F3组(P<0.05)。与CON组相比,F2和F3组断奶羔羊脾脏CAT活性分别显著提高了10.01%和21.05%(P<0.05),且F1、F2和F3组之间脾脏CAT活性差异显著(P<0.05)。与CON组相比,F2和F3组断奶羔羊脾脏T-SOD活性分别显著提高了10.32%和13.10%(P<0.05),且F2和F3组脾脏T-SOD活性显著高于F1组(P<0.05)。各组之间断奶羔羊肝脏MDA含量、GSH-Px活性以及T-AOC差异不显著(P>0.05)。
|
|
表 5 岩藻多糖对断奶羔羊脾脏抗氧化指标的影响 Table 5 Effects of dietary fucoidan on spleen antioxidant indexes of weaned goat kids (n=6) |
如表 6所示,与CON组相比,F1、F2和F3组断奶羔羊脾脏TNF-α含量分别显著降低了17.99%、41.93%和46.15%(P<0.05),且F1组脾脏TNF-α含量显著高于F2和F3组(P<0.05)。与CON组相比,F1、F2和F3组断奶羔羊脾脏IL-6含量分别显著降低了21.11%、41.34%、62.57%(P<0.05),F2和F3组脾脏IL-1β含量分别显著降低了22.24%和34.13%(P<0.05),F1、F2和F3组脾脏IL-2含量分别显著提高了78.96%、170.56%和271.50%(P<0.05),F1、F2和F3组脾脏IL-10含量分别显著提高了23.71%、47.69%、65.69%(P<0.05),且F1、F2和F3组之间脾脏IL-1β、IL-2、IL-6和IL-10含量差异显著(P<0.05)。
|
|
表 6 饲粮中添加岩藻多糖对断奶羔羊脾脏免疫指标的影响 Table 6 Effects of dietary fucoidan on spleen immune indexes of weaned goat kids (n=6) |
肝脏是外源性物质与药物代谢的主要器官,ALT、AST和LDH是分布在肝细胞质、线粒体中的重要酶类,其活性是评价肝功能的重要指标[14]。当肝细胞受损时,产生的自由基会破坏细胞膜和细胞器,使肝细胞肿胀甚至坏死,与此同时ALT、AST和LDH从细胞释放入血,引起血清ALT、AST和LDH活性增加[15],最终杀死有害细胞。Jeong等[16]和Zhang等[17]研究表明,多糖可有效抑制肝脏损伤所导致的血清ALT、AST和LDH活性的升高,维持肝细胞膜的稳定和加强肝脏组织损伤的修复,增强抗氧化作用,减轻病理损伤。而岩藻多糖目前虽然并未明确纳入我国饲料添加剂目录中,但其对动物除了有抗肿瘤[18-19]、抗糖尿病[20]、抗凝血[21]等药理作用外,也在肝脏的保护方面有积极影响[22]。研究发现,岩藻多糖可通过其抗氧化活性改善黄曲霉毒素B1引起的肝脏和肾脏功能损害,其抗氧化活性可有效降低尼罗罗非鱼(Oreochromis niloticus)血清ALT和AST活性[23]。本研究结果中,饲粮中添加岩藻多糖能有效降低血清AST和LDH活性,其中添加0.3%岩藻多糖在第30天时能显著降低血清AST活性,添加0.3%和0.5%岩藻多糖在第15天和第30天时均能显著降低血清LDH活性。这与Wang等[24]和Jeong等[16]的研究结果一致,表明岩藻多糖对断奶羔羊肝脏具有积极的保护作用,增强肝脏功能,有效防止肝脏损伤,稳定机体健康。
3.2 饲粮中添加岩藻多糖对断奶羔羊肝脏和脾脏抗氧化能力的影响在畜禽生产中,由于自然环境、外界因素以及体内物质代谢的影响,动物机体会产生大量自由基。然而,过多的自由基则会氧化机体正常细胞和组织,继而抑制各种抗氧化酶的活性,引起各种疾病的发生[25],导致畜禽生长性能下降和饲养成本的增加,不利于畜牧业的发展。有研究表明,摄入多糖能够提高机体清除自由基的能力,维持体内氧化还原的动态平衡[26-27]。本研究结果表明,饲粮中添加岩藻多糖能提高肝脏GSH-Px活性,降低肝脏MDA和H2O2含量,且肝脏CAT活性也呈上升趋势。相似的,杨晋[28]研究发现,饲粮中添加岩藻多糖可以提高仔猪血清SOD、GSH-Px和CAT活性,并降低血清MDA含量。此外,本研究结果同样与Yang等[3]、Zhao等[29]和Song等[30]的研究结果一致。此外,饲粮中添加岩藻多糖能提高脾脏CAT和T-SOD活性,降低脾脏H2O2含量,这与郭广振等[4]、姜秀梅等[31]和刘功成等[32]的研究结果一致。这些研究结果表明,岩藻多糖能够减轻机体脂质过氧化反应,增强超氧阴离子自由基的清除效率,提高脾脏细胞内H2O2的催化分解,防止过高含量的H2O2对机体组织造成损伤。但是,本研究表明岩藻多糖对于断奶羔羊肝脏和脾脏指数并无显著影响,这与郭广振等[4]研究结果不尽相同,可能与饲粮种类、岩藻多糖添加量和饲喂次数等因素不同有关。总的来说,饲粮中添加岩藻多糖能够提高断奶羔羊肝脏和脾脏的抗氧化能力。
3.3 饲粮中添加岩藻多糖对断奶羔羊脾脏免疫功能的影响脾脏为机体中最重要的免疫器官,在脾脏等防御器官受到刺激时会产生并分泌一系列细胞免疫因子,其主要参与机体的免疫调节和炎症应答[33]。炎症是免疫系统对潜在有害刺激的最初反应,各种外来抗原可诱导巨噬细胞与中性粒细胞向接触部位迁移,产生并释放如一氧化氮(NO)、TNF-α、IL-1β和IL-6等促炎细胞因子,这些细胞因子增强单核细胞、粒细胞、淋巴细胞和肥大细胞向损伤部位趋化,提高或激活炎性应答,增加了组织损伤的风险,从而促进炎症的延长[34-36]。而产生的IL-2和IL-10等抗炎因子会控制炎性反应,从而使机体免疫状态不受损害。因此,抑制细胞因子的产生或功能是控制炎症的关键机制。大量研究表明,过度的炎症会破坏机体健康的组织结构,而多糖具有提高脾脏免疫抗炎功能的作用,可激活各种免疫细胞以及分泌多种细胞因子,为天然的免疫调节剂[32, 37-38]。如Lee等[39]研究发现,岩藻多糖通过下调诱导型一氧化氮合酶(iNOS)、环氧合酶-2(COX-2)和促炎细胞因子TNF-α、IL-6和IL-1β的表达水平,显著抑制脂多糖(LPS)诱导的RAW 264.7巨噬细胞中的NO产生;Ni等[40]同样发现,岩藻多糖能抑制前列腺素E2(PGE2)以及促炎因子TNF-α、IL-1β和IL-6的合成,从而降低炎症反应,提高免疫能力。本试验结果表明,饲粮中添加岩藻多糖能降低羔羊脾脏中促炎因子TNF-α、IL-6与IL-1β含量,这与上述试验结果一致,并提高脾脏中抗炎因子IL-2与IL-10含量,这与Choi等[41]和路垚等[42]的研究结果一致。由此可见,岩藻多糖能够减少促炎因子的产生,进而减少炎性应答,降低组织受伤几率,还能提高抗炎因子的生成,从而调控机体的炎症反应,增强羔羊脾脏免疫功能,进而整体提高并改善羔羊的免疫功能。
4 结论饲粮中添加岩藻多糖能够有效改善断奶羔羊的肝功能,提高肝脏和脾脏抗氧化能力,增强脾脏免疫功能。在本试验条件下,饲粮中添加0.3%岩藻多糖为宜。
| [1] |
杨泓涛, 邓位喜, 杨玉能, 等. 黄芪多糖对舍饲黔北麻羊断奶羔羊生长性能、生化指标及免疫功能的影响[J]. 饲料研究, 2021, 44(9): 29-32. YANG H T, DENG W X, YANG Y N, et al. Effects of Astragalus polysaccharide on growth performance, biochemical indexes and immune function of weaned lambs of Qianbei Ma sheep[J]. Feed Research, 2021, 44(9): 29-32 (in Chinese). DOI:10.13557/j.cnki.issn1002-2813.2021.09.007 |
| [2] |
史晨迪, 赵晓雅, 田沛知, 等. 羔羊断奶应激期饲喂方式对其生长性能和血清生化指标的影响[J/OL]. 动物营养学报: 1-15[2022-03-30]. http://kns.cnki.net/kcms/detail/11.5461.S.20220317.1014.016.html SHI C D, ZHAO X Y, TIAN P Z, et al. Effects of feeding methods on growth performance and serum biochemical indices of lambs during weaning stress period[J/OL]. Chinese Journal of Animal Nutrition: 1-15[2022-03-30]. http://kns.cnki.net/kcms/detail/11.5461.S.20220317.1014.016.html. (in Chinese) |
| [3] |
YANG W G, CHEN J Y, GUO G Z, et al. The effects of fucoidan dietary supplementation on growth performance, serum antioxidant capacity, immune function indices and intestinal morphology in weaned kids[J]. Animals, 2022, 12(5): 574. DOI:10.3390/ani12050574 |
| [4] |
郭广振, 杨伟光, 刘娟, 等. 岩藻多糖对断奶羔羊生长性能、器官指数及血清生化、抗氧化和免疫指标的影响[J/OL]. 动物营养学报: 1-10[2022-03-10]. http://kns.cnki.net/kcms/detail/11.5461.S.20220120.1539.038.html. GUO G Z, YANG W G, LIU J, et al. Effects of fucoidan on growth performance, organ indices and serum biochemical, antioxidant and immune indices of weaned lambs[J/OL]. Chinese Journal of Animal Nutrition: 1-10[2022-03-10]. http://kns.cnki.net/kcms/detail/11.5461.S.20220120.1539.038.html. (in Chinese) |
| [5] |
CUI K Y, TAI W J, SHAN X D, et al. Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens[J]. International Journal of Biological Macromolecules, 2018, 120(Pt.B): 1817-1822. |
| [6] |
ZHAO Y, ZHENG Y Z, WANG J, et al. Fucoidan extracted from Undaria pinnatifida: source for nutraceuticals/functional foods[J]. Marine Drugs, 2018, 16(9): 321. DOI:10.3390/md16090321 |
| [7] |
ZHANG Z Y, TERUYA K, ETO H, et al. Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells[J]. Bioscience, Biotechnology and Biochemistry, 2013, 77(2): 235-242. DOI:10.1271/bbb.120631 |
| [8] |
EL RASHED Z, LUPIDI G, GRASSELLI E, et al. Antioxidant and antisteatotic activities of fucoidan fractions from marine and terrestrial sources[J]. Molecules, 2021, 26(15): 4467. DOI:10.3390/molecules26154467 |
| [9] |
SUN T H, ZHANG X H, MIAO Y, et al. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from Laminaria japonica[J]. Journal of Ocean University of China, 2018, 17(3): 705-711. DOI:10.1007/s11802-018-3794-1 |
| [10] |
JIN W H, ZHANG Q B, WANG J, et al. A comparative study of the anticoagulant activities of eleven fucoidans[J]. Carbohydrate Polymers, 2013, 91(1): 1-6. DOI:10.1016/j.carbpol.2012.07.067 |
| [11] |
APOSTOLOVA E, LUKOVA P, BALDZHIEVA A, et al. Immunomodulatory and anti-inflammatory effects of fucoidan: a review[J]. Polymers, 2020, 12(10): 2338. DOI:10.3390/polym12102338 |
| [12] |
REILLY P, O'DOHERTY J V, PIERCE K M, et al. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig[J]. Animal, 2008, 2(10): 1465-1473. DOI:10.1017/S1751731108002711 |
| [13] |
WALSH A M, SWEENEY T, O'SHEA C J, et al. Effects of supplementing dietary laminarin and fucoidan on intestinal morphology and the immune gene expression in the weaned pig[J]. Journal of Animal Science, 2012, 90(Suppl 4): 284-286. |
| [14] |
黄庆勇. 蛹虫草多糖对亚急性酒精损伤小鼠血清肝功能指标的影响[J]. 北华大学学报(自然科学版), 2018, 19(1): 45-48. HUANG Q Y. Effects of Cordyceps militaris polysaccharides on serum liver function indexes of subacute alcohol-induced liver injury in mice[J]. Journal of Beihua University(Natural Science), 2018, 19(1): 45-48 (in Chinese). |
| [15] |
张弛, 刘瑛, 李华珠, 等. 血清谷丙转氨酶在正常范围内对非酒精性脂肪性肝病的预测[J]. 世界华人消化杂志, 2011, 19(8): 841-844. ZHANG C, LIU Y, LI H Z, et al. Predictive significance of normal serum alanine aminotransferase in patients with nonalcoholic fatty liver disease[J]. World Chinese Journal of Digestology, 2011, 19(8): 841-844 (in Chinese). |
| [16] |
JEONG S C, KIM S M, JEONG Y T, et al. Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower[J]. Chinese Medicine, 2013, 8(1): 7. DOI:10.1186/1749-8546-8-7 |
| [17] |
ZHANG Y, LI H T, HU T, et al. Metabonomic profiling in study hepatoprotective effect of polysaccharides from Flammulina velutipes on carbon tetrachloride-induced acute liver injury rats using GC-MS[J]. International Journal of Biological Macromolecules, 2018, 110: 285-293. DOI:10.1016/j.ijbiomac.2017.12.149 |
| [18] |
LI X L, HE Y L, ZENG P J, et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China[J]. Journal of Cellular and Molecular Medicine, 2019, 23(1): 4-20. DOI:10.1111/jcmm.13564 |
| [19] |
LIU L Q, LI M Z, YU M L, et al. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota[J]. International Journal of Biological Macromolecules, 2019, 121: 743-751. DOI:10.1016/j.ijbiomac.2018.10.083 |
| [20] |
WANG D Y, ZHAO X M, LIU Y L. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats[J]. International Journal of Biological Macromolecules, 2017, 102: 396-404. DOI:10.1016/j.ijbiomac.2017.04.056 |
| [21] |
LIU X, HAO J J, HE X X, et al. A rhamnan-type sulfated polysaccharide with novel structure from Monostroma angicava Kjellm (Chlorophyta) and its bioactivity[J]. Carbohydrate Polymers, 2017, 173: 732-748. DOI:10.1016/j.carbpol.2017.06.031 |
| [22] |
YUAN Y, CHE L H, QI C, et al. Protective effects of polysaccharides on hepatic injury: a review[J]. International Journal of Biological Macromolecules, 2019, 141: 822-830. DOI:10.1016/j.ijbiomac.2019.09.002 |
| [23] |
ABDEL-DAIM M M, DAWOOD M A O, ALEYA L, et al. Effects of fucoidan on the hematic indicators and antioxidative responses of Nile tilapia (Oreochromis niloticus) fed diets contaminated with aflatoxin B1[J]. Environmental Science and Pollution Research, 2020, 27(11): 12579-12586. DOI:10.1007/s11356-020-07854-w |
| [24] |
WANG Y Q, WEI J G, TU M J, et al. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation[J]. International Journal of Molecular Sciences, 2018, 19(12): 4050. DOI:10.3390/ijms19124050 |
| [25] |
MU S, YANG W J, HUANG G L. Antioxidant activities and mechanisms of polysaccharides[J]. Chemical Biology & Drug Design, 2021, 97(3): 628-632. |
| [26] |
TAN Z, LIU A M, LUO M, et al. Geniposide inhibits alpha-naphthylisothiocyanate-induced intrahepatic cholestasis: the downregulation of STAT3 and NF-κB signaling plays an important role[J]. The American Journal of Chinese Medicine, 2016, 44(4): 721-736. DOI:10.1142/S0192415X16500397 |
| [27] |
HUANG H L, CHEN F, LONG R, et al. The antioxidant activities in vivo of bitter gourd polysaccharide[J]. International Journal of Biological Macromolecules, 2020, 145: 141-144. DOI:10.1016/j.ijbiomac.2019.12.165 |
| [28] |
杨晋. 海藻多糖替代抗生素对断奶仔猪生长性能和肠道屏障功能的影响[D]. 硕士学位论文. 南昌: 江西农业大学, 2019: 22-34. YANG J. Effects of seaweed polysaccharide substitute for antibiotics on growth performance and intestinal barrier function of weaned piglets[D]. Master's Thesis. Nanchang: Jiangxi Agricultural University, 2019: 22-34. (in Chinese) |
| [29] |
ZHAO H J, ZHANG J J, LIU X C, et al. The antioxidant activities of alkalic-extractable polysaccharides from Coprinus comatus on alcohol-induced liver injury in mice[J]. Scientific Reports, 2018, 8(1): 11695. DOI:10.1038/s41598-018-30104-6 |
| [30] |
SONG X L, SHEN Q, LIU M, et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases[J]. International Journal of Biological Macromolecules, 2018, 114: 979-988. DOI:10.1016/j.ijbiomac.2018.04.001 |
| [31] |
姜秀梅, 徐小磊, 殷一民, 等. 金针菇多糖对衰老小鼠脾脏抗氧化能力的影响[J]. 北华大学学报(自然科学版), 2016, 17(4): 488-491. JIANG X M, XU X L, YIN Y M, et al. Effect of polysaccharides from Flammulina velutipes on the antioxidant capacity of spleen in aging mice[J]. Journal of Beihua University(Natural Science), 2016, 17(4): 488-491 (in Chinese). |
| [32] |
刘功成, 王作伟, 李亭亭, 等. 重楼叶多糖改善D-半乳糖衰老模型小鼠脾脏免疫功能和抗氧化能力[J]. 食品工业科技, 2015, 36(16): 366-369, 383. LIU G C, WANG Z W, LI T T, et al. Effect of polysaccharides from the leaves of Paris polyphylla on immune function and antioxidant capacities of mouse spleen tissue in a D-galactose-induced aging mouse model[J]. Science and Technology of Food Industry, 2015, 36(16): 366-369, 383 (in Chinese). |
| [33] |
孔海军, 王凤华, 孔令生, 等. 维药阿里红多糖对运动疲劳小鼠Th1/Th2平衡及Foxp3 Scurfin蛋白表达调控研究[J]. 食品与药品, 2017, 19(5): 305-309. KONG H J, WANG F H, KONG L S, et al. Effects of polysaccharide from Fomes officinalis Ames on Th1/Th2 balance and Foxp3 scurfin expression in exercise-induced physical fatigue mice[J]. Food and Drug, 2017, 19(5): 305-309 (in Chinese). DOI:10.3969/j.issn.1672-979X.2017.05.001 |
| [34] |
MURALIDHARAN S, MANDREKAR P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation[J]. Journal of Leukocyte Biology, 2013, 94(6): 1167-1184. DOI:10.1189/jlb.0313153 |
| [35] |
KENNEDY M A. A brief review of the basics of immunology: the innate and adaptive response[J]. Veterinary Clinics of North America: Small Animal Practice, 2010, 40(3): 369-379. DOI:10.1016/j.cvsm.2010.01.003 |
| [36] |
KYUNG J, KIM D, PARK D, et al. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract[J]. Laboratory Animal Research, 2012, 28(2): 91-97. DOI:10.5625/lar.2012.28.2.91 |
| [37] |
周新. 大球盖菇多糖对过度运动大鼠脾脏免疫机能及p-p38MAPK蛋白表达的影响[J]. 扬州大学学报(农业与生命科学版), 2019, 40(6): 71-75. ZHOU X. Effects of polysaccharides from Stropharia rugloso-annulata on spleen immune function and p-p38MARK protein expression in overexercising rats[J]. Journal of Yangzhou University(Agricultural and Life Science), 2019, 40(6): 71-75 (in Chinese). |
| [38] |
张英楠, 刘洪章, 杨树宝. 刺五加多糖对免疫抑制雏鸡脾脏中T淋巴细胞分布及免疫功能的影响[J]. 中国家禽, 2021, 43(10): 32-38. ZHANG Y N, LIU H Z, YANG S B. Effects of Acanthopanax senticosus polysaccharide on distribution and immune function of T lymphocytes in spleen of immunosuppressed chickens[J]. China Poultry, 2021, 43(10): 32-38 (in Chinese). |
| [39] |
LEE S H, KO C I, AHN G, et al. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava[J]. Carbohydrate Polymers, 2012, 89(2): 599-606. |
| [40] |
NI L Y, WANG L, FU X T, et al. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica[J]. International Journal of Biological Macromolecules, 2020, 156: 717-729. |
| [41] |
CHOI J I, RAGHAVENDRAN H R B, SUNG N Y, et al. Effect of fucoidan on aspirin-induced stomach ulceration in rats[J]. Chemico-Biological Interactions, 2010, 183(1): 249-254. |
| [42] |
路垚, 杨琳燕, 马吉飞. 磷酸化姬松茸多糖对小鼠脾脏细胞因子mRNA表达的影响[J]. 黑龙江畜牧兽医, 2017(5): 234-237. |




