2. 广西英路维特药物有限公司, 荔浦 546600
2. Guangxi Innovate Medical Technology Co., Ltd., Lipu 546600, China
犊牛生长发育和健康状况直接影响其生产性能,关乎养牛业的发展,但断奶前犊牛机体抵抗力弱,易受外界刺激而发生疾病[1]。因此,增强犊牛抵抗力,降低发病率,提高其生产性能,一直是养牛业追求的目标。抗生素可有效保障动物健康,但所造成的药物残留以及危害公众健康等问题不容忽视。在养殖业减抗、禁抗背景下,寻求天然、无抗、无残留、促生长的饲料添加剂具有重要意义[2]。多糖类、多酚类、皂苷类和氨基酸类[3]等天然植物提取物具有提高动物机体抗氧化活性[4]、促进肠道发育、增强机体免疫等作用[5],进而改善其生长性能,被广泛应用于动物生产[6-7]。研究表明,酵母多糖可促进犊牛的生长发育和营养物质的消化吸收[8];枸杞多糖可显著增强肉牛机体抗氧化能力,提高平均日增重[9];植物水解单宁可有效提高秦川牛对蛋白质吸收能力,增强免疫力,改善瘤胃微生物区系[10]。葛根多糖作为葛根天然成分,具有抗氧化[11]、抗菌[12]和调节免疫[13]等作用,可恢复结肠炎小鼠受损紧密连接蛋白表达水平和结构,恢复肠道屏障功能[14],还可上调腹泻小鼠肠道有益菌水平,改善菌群结构[15]。目前,葛根多糖在犊牛上的应用鲜有报导。因此,本研究旨在探究葛根多糖对健康犊牛基础临床、生理生化指标及血浆代谢物的影响,为其在犊牛生产中的应用提供理论基础。
1 材料与方法 1.1 试验材料本试验所用葛根多糖(纯度50.00%,平均分子质量1.09×105 u)由广西某药物有限公司提供。
1.2 试验动物与试验设计选择四川某规模化牧场临床检查健康,年龄[(6.20±3.65) d]和体重[(47.09±4.50) kg]相近的荷斯坦犊牛24头,随机分为对照组(C组)和试验组(E组),每组12头。参考相关文献[16]并经预试验(每天每头牛在牛奶中添加200、400、800 mg/kg葛根多糖,连续5 d,观察至21 d,期间犊牛腹泻率分别为1.19%、0、0)确定,E组每天每头牛在牛奶中添加400 mg/kg葛根多糖,C组不添加。连续饲喂5 d,并持续观察21 d。为确保全量摄入,先将葛根多糖溶于少量牛奶,待犊牛吃完后再饲喂剩余部分牛奶。
1.3 饲养管理所有犊牛单笼饲养,出生后称重,30~120 min内饲喂初乳4 L,3日龄提供自由饮水,7日龄提供干草和犊牛开食料;3周龄内,饲喂常乳2次/d,每次4 L。定期清理消毒,所有试验犊牛均无既往病史和用药史。犊牛开食料营养水平见表 1。
|
|
表 1 犊牛开食料营养水平(干物质基础) Table 1 Nutrient levels of calves starter (DM basis) |
每日饲喂前测定试验第1~7天各组犊牛直肠温度、心率、呼吸频率。
1.4.2 体重及腹泻率测定试验开始前、第7天、第14天和第21天对犊牛体重进行测定,统计腹泻犊牛数并计算腹泻率,计算公式如下:
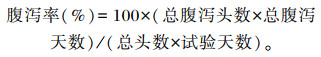
|
在试验第1天、第3天、第5天和第7天饲喂前分别采集各试验犊牛颈静脉血,添加乙二胺四乙酸(EDTA)抗凝,获得犊牛血浆样品;无抗凝血样室温静置1 h,3 000 r/min离心5 min,获得犊牛血清样品。部分第7天血浆样品置于-80 ℃保存,用于后续血浆代谢物检测与分析。
1.4.4 血常规及血清生化指标检测分别使用IDEXX Procyte Dx全自动五分类血细胞分析仪和斯马特全自动生化分析仪检测各组犊牛第1天、第3天、第5天和第7天血常规指标和血清生化指标。
1.4.5 血浆代谢物检测与分析将-80 ℃的血浆样品复融,吸取适量样本加入预冷甲醇/乙腈/水溶液(2 ∶ 2 ∶ 1,体积比),涡旋混匀,超声30 min(冰水浴),-20 ℃静置10 min,4 ℃、12 000 r/min离心15 min,吸取上清液,采用Agilent 1 290 Infinity LC超高效液相色谱仪进行物质分离,在AB 6 600 Triple TOF质谱仪下基于IDA采集模式进行一级、二级质谱数据的采集。采集条件如下:轰击能量为(35±15) ev,每50 ms采集15张二级谱图。电喷雾离子源(ESI)参数设置如下:雾化气压(GSI)、辅助气压及气帘气压分别为60、60、30 psi;温度为600 ℃,喷雾电压为5 500 V(正离子模式)和-5 500 V(负离子模式)。
使用ProteoWizard软件将原始质谱转成mzXML格式,XCMS保留时间矫正、峰识别、峰提取、峰积分、峰对齐等工作,并使用二级质谱数据库对峰进行物质鉴定。然后进行正交偏最小二乘法-判别分析(OPLS-DA),获得变量重要性投影(VIP)。差异代谢物筛选条件为变异倍数(FC)>1.5或FC<0.67,VIP>1和P <0.05。将筛选到的差异代谢物导入KEGG数据库,注释到差异代谢物相关的代谢通路,最后通过MetaboAnalyst 5.0在线数据库进行通路富集分析。
1.5 数据统计分析采用SPSS 27.0统计软件进行单因素方差分析和LSD法多重比较,统计结果用平均值±标准差表示,以P <0.05表示差异显著。
2 结果与分析 2.1 葛根多糖对健康犊牛基础临床指标的影响由表 2可知,试验第1~7天,C组与E组之间犊牛体温、心率和呼吸频率均无显著差异(P>0.05)。
|
|
表 2 葛根多糖对健康犊牛基础临床指标的影响 Table 2 Effects of Pueraria polysaccharides on basic clinical indices of healthy calves |
由表 3可知,试验第1~7天、第8~14天、第15~21天,C组与E组之间犊牛体重无显著差异(P>0.05),但各时间段E组犊牛体重均高于C组。这说明饲喂葛根多糖对犊牛后续生长具有一定的促进作用。
|
|
表 3 葛根多糖对健康犊牛体重的影响 Table 3 Effects of Pueraria polysaccharides on body weight of healthy calves |
由表 4可知,试验第1~7天,C组和E组犊牛无腹泻。试验第8~21天,C组有3头犊牛腹泻,腹泻率为5.36%;E组犊牛无腹泻,腹泻率为0。这说明葛根多糖具有预防犊牛腹泻的作用。
|
|
表 4 葛根多糖对健康犊牛腹泻率的影响 Table 4 Effects of Pueraria polysaccharides on diarrhea rate of healthy calves |
由表 5可知,C组与E组之间犊牛血常规指标均无显著差异(P>0.05)。这说明添加400 mg/kg葛根多糖对犊牛血常规指标无明显影响。
|
|
表 5 葛根多糖对健康犊牛血常规指标的影响 Table 5 Effects of Pueraria polysaccharides on blood routine indices of healthy calves |
由表 6可知,C组与E组之间犊牛血清生化指标均无显著差异(P>0.05)。这说明添加400 mg/kg葛根多糖对犊牛血清生化指标无明显影响。
|
|
表 6 葛根多糖对健康犊牛血清生化指标的影响 Table 6 Effects of Pueraria polysaccharides on serum biochemical indices of healthy calves |
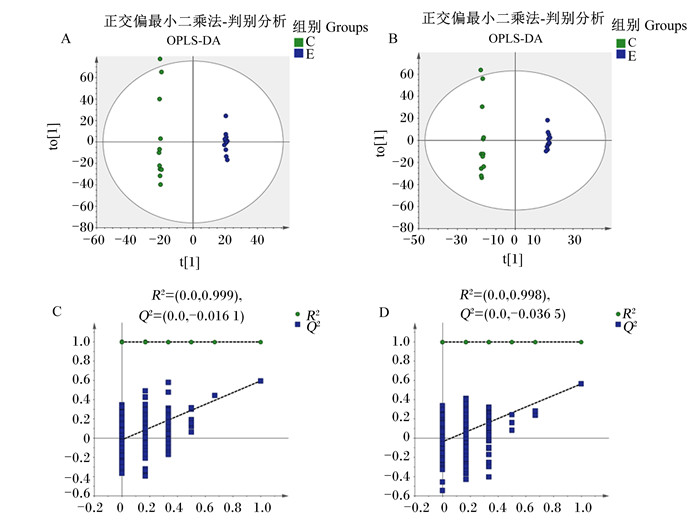
使用有监督的模式识别方法OPLS-DA对数据模型可靠性进行分析。如图 1-A所示,在正离子模式下R2Y=0.775;如图 1-B所示,在负离子模式下R2Y=0.90,表明模型建立较好,所获得的数据可用于进一步分析。采用置换检验对模型进行过拟合检验,图 1-C、图 1-D显示了在正负离子模式下,模型的R2和Q2均逐渐下降,说明原模型不存在过拟合现象,模型稳定性良好。
|
A、C为正离子模式下所得结果,B、D为负离子模式下所得结果。 A and C were the result from ESI+, and B and D were the result from ESI-. 图 1 OPLS-DA得分图 Fig. 1 OPLS-DA scores graphs |
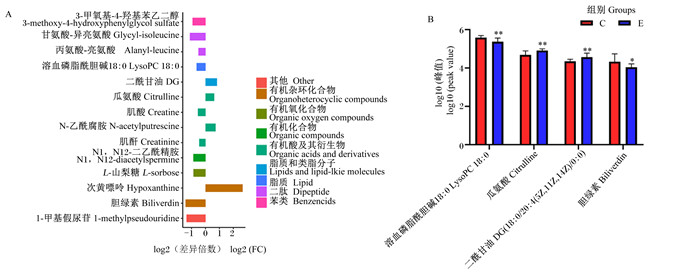
在正、负离子模式下,共检测到14种差异代谢物。由表 7可知,血浆溶血磷脂酰胆碱18 ∶ 0、胆绿素等10种代谢物丰度显著下调(P <0.05),血浆二酰甘油、瓜氨酸、N-乙酰腐胺和次黄嘌呤4种代谢物丰度显著上调(P <0.05)。对血浆差异代谢物进行分类,发现共涉及苯类、二肽、脂质、脂质和类脂分子、有机酸及其衍生物、有机化合物、有机氧化合物、有机杂环化合物和未分类代谢物(图 2-A)。进一步筛选出葛根多糖影响健康犊牛的关键血浆代谢物,即二酰甘油、次黄嘌呤、瓜氨酸和溶血磷脂酰胆碱18 ∶ 0(图 2-B)。
|
|
表 7 2组之间血浆差异代谢物 Table 7 Plasma differential metabolites between two groups |
|
*代表差异显著(P<0.05)。 * mean significant difference (P <0.05). 图 2 2组血浆差异代谢物FC值柱状图(A)和关键代谢物的log10峰值图(B) Fig. 2 FC value histogram of plasma differential metabolites (A) and key metabolite peaks figure of log10 (B) between 2 groups |
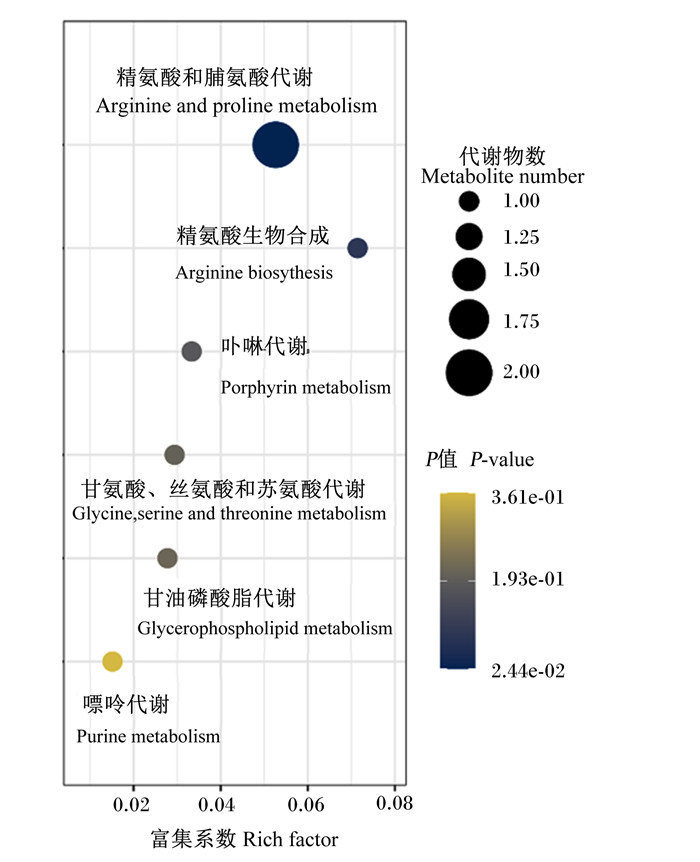
利用Metaboanalyst通路富集分析功能,对血浆差异代谢物进行通路富集分析。如图 3所示,上述差异代谢物共涉及精氨酸和脯氨酸代谢,精氨酸生物合成,卟啉代谢,甘氨酸、苏氨酸和丝氨酸代谢,甘油磷酸脂代谢和嘌呤代谢6条代谢通路。与饲喂葛根多糖的健康犊牛血浆代谢物改变最为相关的是精氨酸和脯氨酸代谢(P <0.05)。
|
x轴为富集系数,y轴为P值,圆圈代表代谢通路,圆圈颜色深浅表示代谢物通路中代谢物显著性变化,圆圈大小与代谢物数相对应。 The x axis is the rich factor, the y axis is P-value. The circle represents the metabolic pathway, the color depth of the circle indicates a significant change in metabolic pathway, and the circle size corresponds metabolites number. 图 3 血浆差异代谢物KEGG通路分析 Fig. 3 KEGG pathway analysis of plasma differential metabolites |
体温、心率和呼吸频率等临床指标常用于评估机体健康[17],血常规和血清生化指标同样也被普遍用于评价动物健康状况,其中血常规指标是衡量机体生理健康状态和疾病严重程度的重要依据[18],丙氨酸氨基转移酶(ALT)、碱性磷酸酶(ALP)均是反映肝脏功能的可靠指标[19],肌酐(CREA)和尿素氮(UN)是反映蛋白质代谢和肾功能的重要指标[20],球蛋白包含大量炎症反应蛋白,与机体炎症状态密切相关[21]。本试验中,试验组犊牛连续5 d饲喂葛根多糖,体温、心率、呼吸频率较对照组犊牛无显著变化。结合血常规和血清生化指标检测结果发现,葛根多糖同样也没有对试验犊牛产生显著影响。由此可见,每天每头牛在牛奶中添加400 mg/kg葛根多糖对健康犊牛无明显临床副作用。
3.2 葛根多糖对健康犊牛血浆代谢物的影响多糖通过调节机体代谢发挥抗氧化,降低炎症反应等作用[22-24]。本研究中,健康犊牛饲喂葛根多糖后血浆二酰甘油、瓜氨酸和次黄嘌呤等丰度显著升高。研究表明,二酰甘油可抑制胆汁酸的分泌,并参与氧化应激下肠道损伤的修复,用于治疗腹泻[25]。此外,二酰甘油还是多种信号分子的有效激活剂,是维持细胞间信息传递的关键物质[26]。二酰甘油参与了犊牛机体多种信号调节,其丰度的增加有利于健康犊牛生长发育。瓜氨酸是一种非必需氨基酸,在精氨酸琥珀酸裂解酶催化下转化为精氨酸[27]。本试验发现,牛奶中添加葛根多糖上调了瓜氨酸参与的精氨酸生物合成通路,而精氨酸作为新生犊牛生长过程中的一种条件性必需氨基酸[28],除在蛋白质合成中起作用外,还是一氧化氮(NO)和多胺合成的常见底物,从而满足肠道细胞增殖的关键调控作用[29],且研究表明精氨酸具有促进犊牛肠道发育的作用[30]。虽然在检测到的差异代谢物中并没有精氨酸,但饲喂葛根多糖后上调了瓜氨酸的水平及其参与的精氨酸生物合成通路。并且,在试验第8~21天,对照组犊牛有3头发生了腹泻,而试验组犊牛在试验期间未发生腹泻。由此可推断,葛根多糖可能通过促进健康犊牛肠道发育,从而预防了犊牛腹泻。而次黄嘌呤可调节肠道上皮细胞能量代谢,改善肠道屏障功能并促进伤口愈合[31]。通过以上结果表明,葛根多糖具有提高机体抗氧化能力、促进肠道发育的作用,从而防止犊牛腹泻。
此外,健康犊牛饲喂葛根多糖后溶血磷脂酰胆碱18 ∶ 0等差异代谢物下调。溶血磷脂酰胆碱是由细胞膜衍生的磷脂酰胆碱产生,具有调节免疫功能的作用[32]。体现为溶血磷脂酰胆碱促进单核细胞趋化增强,并激活巨噬细胞[33]等。相关研究表明,发生围产期疾病的奶牛血浆代谢物中常出现溶血磷脂酰胆碱水平升高[34]。溶血磷脂酰胆碱18 ∶ 0水平显著下调提示葛根多糖在调节健康犊牛免疫反应方面发挥作用。在哺乳动物中,胆绿素通过胆绿素还原酶还原为胆红素[35]。胆红素是一种抗氧化剂,具有清除活性氧作用,以保护细胞免受氧化损伤[36]。饲喂葛根多糖后胆绿素显著下调,推测可能是葛根多糖促进了胆绿素还原为胆红素,以增强健康犊牛机体的抗氧化能力。肌酸是一种含氮的有机酸,在肝脏、肾脏中由精氨酸、甲硫氨酸和甘氨酸合成[37]。肌酸通过肌酸-磷酸肌酸系统,在肌酸激酶的催化下转化为磷酸肌酸,为机体功能[38]。肌酸水平下调,表明葛根多糖可促进犊牛生长,增加犊牛机体的能量需求,从而加速肌酸代谢。而肌酐由肌肉组织中的肌酸及磷酸肌酸代谢而来[39],随肌酸减少同样出现了下调趋势。本试验中,犊牛体重虽无显著差异,但葛根多糖对健康犊牛体重升高的趋势高于对照组,推测可能是由于饲喂葛根多糖的时间较短所致。后续可通过增加饲喂天数来探究葛根多糖对健康犊牛生产性能的影响。
4 结论葛根多糖对健康犊牛无明显临床副作用,可通过调节犊牛机体二酰甘油、瓜氨酸、溶血磷脂酰胆碱18 ∶ 0和胆绿素等代谢,促进肠道发育,增强机体抗氧化能力,降低机体炎性反应,从而减少犊牛腹泻,促进犊牛生长。
| [1] |
郭文杰, 刘书杰, 冯宇哲, 等. 哺乳期补饲开食料对牦牛犊牛生长性能、腹泻频率和发病频率的影响[J]. 动物营养学报, 2022, 34(1): 422-431. GUO W J, LIU S J, FENG Y Z, et al. Effects of starter feed on growth performance, diarrhea frequency and incidence frequency of yak calves during lactation[J]. Chinese Journal of Animal Nutrition, 2022, 34(1): 422-431 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.01.040 |
| [2] |
胡亦清, 郭贝贝, 杨明睿, 等. 植物多糖和葛根素对舍饲山羊生产性能、血清生化指标和肉品质的影响[J]. 动物营养学报, 2020, 32(12): 5788-5794. HU Y Q, GUO B B, YANG M R, et al. Effects of plant polysaccharide and puerarin on growth performance, serum biochemical indices and meat quality of captive goats[J]. Chinese Journal of Animal Nutrition, 2020, 32(12): 5788-5794 (in Chinese). |
| [3] |
OZASLAN M, OGUZKAN S B. Use of plant extracts in alternative medicine[J]. Pakistan Journal of Biological Sciences, 2018, 21(1): 1-7. |
| [4] |
KUMAR S, KUMARI R, MISHRA S. Pharmacological properties and their medicinal uses of Cinnamomum: a review[J]. Journal of Pharmacy and Pharmacology, 2019, 71(12): 1735-1761. DOI:10.1111/jphp.13173 |
| [5] |
TINGIRIKARI J M R. Microbiota-accessible pectic poly- and oligosaccharides in gut health[J]. Food & Function, 2018, 9(10): 5059-5073. |
| [6] |
郭贝贝, 胡亦清, 杨明睿, 等. 日粮中添加益生菌和植物多糖对圈养山羊血清抗氧化指标及肉品质的影响[J]. 江西农业大学学报, 2020, 42(2): 291-297. GUO B B, HU Y Q, YANG M R, et al. Effects of dietary probiotics and plant polysaccharides on serum antioxidant indexes and meat quality in captive goats[J]. Acta Agriculturae Universitatis Jiangxiensis, 2020, 42(2): 291-297 (in Chinese). DOI:10.13836/j.jjau.2020034 |
| [7] |
翟金凤, 郭东新, 田河. 中草药饲料添加剂在畜牧生产中的研究与应用[J]. 草业科学, 2013, 30(7): 1131-1134. ZHAI J F, GUO D X, TIAN H, et al. Research and application of Chinese herbal feed additives in animal production[J]. Pratacultural Science, 2013, 30(7): 1131-1134 (in Chinese). |
| [8] |
董金金, 高艳霞, 李妍, 等. 酵母多糖对哺乳犊牛生长性能、消化代谢和血清生化指标的影响[J]. 动物营养学报, 2018, 30(11): 4650-4659. DONG J J, GAO Y X, LI Y, et al. Effects of yeast polysaccaride on growth performance, digestion metabolism and serum biochemical indexes of sucking calves[J]. Chinese Journal of Animal Nutrition, 2018, 30(11): 4650-4659 (in Chinese). DOI:10.3969/j.issn.1006-267x.2018.11.042 |
| [9] |
马吉锋, 王建东, 于洋, 等. 饲喂枸杞多糖对架子牛免疫球蛋白水平和细胞因子分泌的影响[J]. 畜牧与兽医, 2019, 51(3): 123-125. MA J F, WANG J D, YU Y, et al. Effects of Lycium barbarum polysaccharide on the immunoglobulin level and secretion of cytokines in feeder cattle[J]. Animal Husbandry & Veterinary Medicine, 2019, 51(3): 123-125 (in Chinese). |
| [10] |
常玮学, 杨英超, 夏志军, 等. 不同添加量的植物水解单宁对秦川肉牛血清生化指标和瘤胃消化指标、瘤胃微生物区系的影响[J]. 动物营养学报, 2022, 34(3): 1642-1654. CHANG W X, YANG Y C, XIA Z J, et al. Effects of different addition amounts of plant hydrolyzed tannins on serum biochemical indexes, rumen digestion indexes and rumen microflora of Qinchuan beef cattle[J]. Chinese Journal of Animal Nutrition, 2022, 34(3): 1642-1654 (in Chinese). DOI:10.3969/j.issn.1006-267x.2022.03.027 |
| [11] |
FENG L, YIN J Y, NIE S P, et al. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia[J]. International Journal of Biological Macromolecules, 2016, 91: 946-953. DOI:10.1016/j.ijbiomac.2016.05.030 |
| [12] |
CHUKEATIROTE E. Antimicrobial property and antioxidant composition of crude extracts of Pueraria mirifica, Butea superba and Mucuna macrocarpa[J]. Maejo International Journal of Science and Technology, 2009, 3(1): 212-221. |
| [13] |
DONG Z, ZHANG M M, LI H X, et al. Structural characterization and immunomodulatory activity of a novel polysaccharide from Pueraria lobata (Willd.) Ohwi root[J]. International Journal of Biological Macromolecules, 2020, 154: 1556-1564. DOI:10.1016/j.ijbiomac.2019.11.040 |
| [14] |
CHOI S, WOO J K, JANG Y S, et al. Fermented Pueraria lobata extract ameliorates dextran sulfate sodium-induced colitis by reducing pro-inflammatory cytokines and recovering intestinal barrier function[J]. Laboratory Animal Research, 2016, 32(3): 151-159. DOI:10.5625/lar.2016.32.3.151 |
| [15] |
CHEN R, LIU B, WANG X Y, et al. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice[J]. International Journal of Biological Macromolecules, 2020, 158: 740-749. DOI:10.1016/j.ijbiomac.2020.04.201 |
| [16] |
LI Q, LIU W J, FENG Y L, et al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota[J]. International Journal of Biological Macromolecules, 2022, 209(Pt A): 858-870. |
| [17] |
PEARSON J M, PAJOR E A, CAMPBELL J R, et al. Clinical impacts of administering a nonsteroidal anti-inflammatory drug to beef calves after assisted calving on pain and inflammation, passive immunity, health, and growth[J]. Journal of Animal Science, 2019, 97(5): 1996-2008. DOI:10.1093/jas/skz094 |
| [18] |
BARGER A M. The complete blood cell count: a powerful diagnostic tool[J]. The Veterinary Clinics of North America: Small Animal Practice, 2003, 33(6): 1207-1222. DOI:10.1016/S0195-5616(03)00100-1 |
| [19] |
JEMIL I, NASRI R, ABDELHEDI O, et al. Beneficial effects of fermented sardinelle protein hydrolysates on hypercaloric diet induced hyperglycemia, oxidative stress and deterioration of kidney function in wistar rats[J]. Journal of Food Science and Technology, 2017, 54(2): 313-325. DOI:10.1007/s13197-016-2464-9 |
| [20] |
MARONO S, LOPONTE R, LOMBARDI P, et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age[J]. Poultry Science, 2017, 96(6): 1783-1790. DOI:10.3382/ps/pew461 |
| [21] |
ZHANG L X, CHEN L, XU A M. A simple model established by blood markers predicting overall survival after radical resection of pancreatic ductal adenocarcinoma[J]. Frontiers in Oncology, 2020, 10: 583. DOI:10.3389/fonc.2020.00583 |
| [22] |
CHU L L, YANG L C, LIN L Z, et al. Chemical composition, antioxidant activities of polysaccharide from pine needle (Pinus massoniana) and hypolipidemic effect in high-fat diet-induced mice[J]. International Journal of Biological Macromolecules, 2019, 125: 445-452. DOI:10.1016/j.ijbiomac.2018.12.082 |
| [23] |
QIAO Z J, DU X X, ZHUANG W Y, et al. Schisandra chinensis acidic polysaccharide improves the insulin resistance in type 2 diabetic rats by inhibiting inflammation[J]. Journal of Medicinal Food, 2020, 23(4): 358-366. DOI:10.1089/jmf.2019.4469 |
| [24] |
丁孟汝, 王国栋, 袁平川, 等. 多糖调控糖脂代谢的作用及其机制研究进展[J]. 南方医科大学学报, 2021, 41(3): 471-475. DING M R, WANG G D, YUAN P C, et al. Research progress in the role and mechanism of polysaccharides in regulating glucose and lipid metabolism[J]. Journal of Southern Medical University, 2021, 41(3): 471-475 (in Chinese). |
| [25] |
SONG J, LI J, MOUROT J M, et al. Diacylglycerol kinase regulation of protein kinase D during oxidative stress-induced intestinal cell injury[J]. Biochemical and Biophysical Research Communications, 2008, 375(2): 200-204. DOI:10.1016/j.bbrc.2008.07.155 |
| [26] |
GOTO K, TANAKA T, NAKANO T, et al. DGKζ under stress conditions: "to be nuclear or cytoplasmic, that is the question"[J]. Advances in Biological Regulation, 2014, 54: 242-253. DOI:10.1016/j.jbior.2013.08.007 |
| [27] |
GONZALES J U, RAYMOND A, ASHLEY J, et al. Does L-citrulline supplementation improve exercise blood flow in older adults?[J]. Experimental Physiology, 2017, 102(12): 1661-1671. DOI:10.1113/EP086587 |
| [28] |
WU G Y, BAZER F W, DAVIS T A, et al. Arginine metabolism and nutrition in growth, health and disease[J]. Amino Acids, 2009, 37(1): 153-168. DOI:10.1007/s00726-008-0210-y |
| [29] |
TAN B, YIN Y L, KONG X F, et al. L-arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells[J]. Amino Acids, 2010, 38(4): 1227-1235. DOI:10.1007/s00726-009-0334-8 |
| [30] |
VAN KEULEN P, KHAN M A, DIJKSTRA J, et al. Effect of arginine or glutamine supplementation and milk feeding allowance on small intestine development in calves[J]. Journal of Dairy Science, 2020, 103(5): 4754-4764. DOI:10.3168/jds.2019-17529 |
| [31] |
LEE J S, WANG R X, ALEXEEV E E, et al. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function[J]. The Journal of Biological Chemistry, 2018, 293(16): 6039-6051. DOI:10.1074/jbc.RA117.000269 |
| [32] |
SILLIMAN C C, ELZI D J, AMBRUSO D R, et al. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium[J]. Journal of Leukocyte Biology, 2003, 73(4): 511-524. DOI:10.1189/jlb.0402179 |
| [33] |
ZHU X D, LEAROYD J, BUTT S, et al. Regulation of eosinophil adhesion by lysophosphatidylcholine via a non-store-operated Ca2+ channel[J]. American Journal of Respiratory Cell and Molecular Biology, 2007, 36(5): 585-593. DOI:10.1165/rcmb.2006-0391OC |
| [34] |
HAILEMARIAM D, MANDAL R, SALEEM F, et al. Identification of predictive biomarkers of disease state in transition dairy cows[J]. Journal of Dairy Science, 2014, 97(5): 2680-2693. DOI:10.3168/jds.2013-6803 |
| [35] |
JANSEN T, DAIBER A. Direct antioxidant properties of bilirubin and biliverdin.Is there a role for biliverdin reductase?[J]. Frontiers in Pharmacology, 2012, 3: 30. |
| [36] |
AHMED F H, MOHAMED A E, CARR P D, et al. Rv2074 is a novel F420H2-dependent biliverdin reductase in Mycobacterium tuberculosis[J]. Protein Science, 2016, 25(9): 1692-1709. DOI:10.1002/pro.2975 |
| [37] |
PHILLIPS S M. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia[J]. Advances in Nutrition, 2015, 6(4): 452-460. DOI:10.3945/an.115.008367 |
| [38] |
王月兵, 刘庆春, 李军, 等. 抗阻力运动和肌酸在肌肉衰减综合征中的研究进展[J]. 军事医学, 2017, 41(8): 703-706. WANG Y B, LIU Q C, LI J, et al. Research progress in creatine supplementation and resistance training in sarcopenia[J]. Military Medical Sciences, 2017, 41(8): 703-706 (in Chinese). |
| [39] |
向瑾, 余勤, 梁茂植, 等. LC-MS/MS法测定小鼠血浆中的肌酐[J]. 华西药学杂志, 2018, 33(1): 70-71. XIANG J, YU Q, LIANG M Z, et al. Determination of creatinine in rat plasma by LC-MS/MS[J]. West China Journal of Pharmaceutical Sciences, 2018, 33(1): 70-71 (in Chinese). |




