呕吐毒素(deoxynivalenol,DON)是多种镰刀菌产生的霉菌毒素之一,具有2种乙酰化形式,分别为3-乙酰呕吐毒素(3-acetyldeoxynivalenol,3-Ac-DON)和15-乙酰呕吐毒素(15-acetyldeoxynivalenol,15-Ac-DON。DON和乙酰化DON主要产生于禾谷镰刀菌和镰刀菌污染的玉米和小麦中,也广泛存在于受污染的食品和动物饲料中,影响人体和动物健康[1]。2017年,研究人员对来源于我国不同地域的579个小麦样品和606个玉米样品的检测发现,DON在小麦中的检出率高达100%,在玉米中的检出率达到99.83%,且3-Ac-DON在小麦和玉米中的检出率分别为4.15%和13.53%[2]。摄入DON污染的食物会使人和动物出现呕吐、腹泻等胃肠道不良反应。DON能够损害肠上皮细胞,影响营养物质的吸收和机体健康[3-4]。研究表明,DON能够造成内质网应激和肠上皮细胞凋亡,破坏肠屏障功能[5]。比较而言,3-Ac-DON的极性比DON小,因而更容易被十二指肠和空肠吸收并造成肠道损伤[6]。猪对3-Ac-DON敏感性高于其他动物,因此饲粮中的3-Ac-DON污染对养猪生产的影响较大[7-9]。寻找适宜的缓解物质,减轻3-Ac-DON对猪肠道健康的影响,是养猪业急需解决的难题。
姜酮是生姜中的天然提取物,在生姜中的含量占比约为9.25%,属于甲氧基苯酚家族及其相关衍生物的成员,有1个碱性酚环,苯环上带有1个甲氧基。姜酮也可以通过姜酚发生逆醛醇反应生成[10]。姜酮的药理性质广泛,具有抗氧化剂、抗炎等多种生物学活性[11-12]。研究报道,姜酮能够改善葡聚糖硫酸钠诱导的小鼠试验性结肠炎[13]。尽管如此,姜酮能否缓解3-Ac-DON造成的肠上皮细胞损伤和屏障功能障碍尚未见报道。因此,本试验以猪肠上皮细胞IPEC-1为研究对象,探究姜酮对3-Ac-DON引起的肠上皮细胞凋亡、内质网应激和肠屏障功能的影响。
1 材料与方法 1.1 试验材料DMEM/F12培养基、胰蛋白酶、青霉素-链霉素和胎牛血清(FBS)购于美国Gibco公司;3-Ac-DON购于青岛普瑞邦生物工程有限公司;姜酮购于美国MedChemExpress公司;细胞培养板购于无锡耐思生物科技有限公司;核糖核酸酶A购于上海索莱宝生物技术有限公司;Annexin V-FITC细胞凋亡检测试剂盒购于北京嘉美臻元生物技术有限公司;Cell Counting Kit-8(CCK-8)细胞活力检测试剂盒购于北京庄盟国际生物基因科技有限公司;Transwell细胞培养小室购于美国Corning Costar公司;异硫氰酸荧光素葡聚糖(FITC-dextran)购于美国Sigma公司;细胞色素C(CytC)、需肌醇酶1α(IRE1α)抗体购于美国Cell Signaling Technology公司;闭锁小带蛋白-3(ZO-3)抗体购于美国Invitrogen公司;磷酸化的需肌醇酶1α(p-IRE1α)抗体购于美国Abcam公司;活化型半胱氨酸蛋白酶-3(cleaved-Caspase-3)、B细胞淋巴瘤2相关X蛋白(BAX)、闭锁小带蛋白-2(ZO-2)、闭合蛋白(occludin)、转录活化因子6(ATF6)、CCAAT/增强子结合蛋白同源蛋白(CHOP)抗体购于美国Proteintech Group公司;封闭蛋白-1(claudin-1)、封闭蛋白-4(claudin-4)和闭锁小带蛋白-1(ZO-1)抗体购于生工生物工程(上海)股份有限公司;甘油醛-3-磷酸脱氢酶(GAPDH)抗体购于上海翊圣生物科技有限公司;辣根过氧化物酶(HRP)标记的二抗、增强化学发光(ECL)超敏发光试剂盒购于北京华兴博创基因技术有限公司。
1.2 细胞培养IPEC-1细胞培养于37 ℃、5% CO2的培养箱,培养液为含10% FBS的DMEM/F12完全培养基。细胞达到80%融合度时,进行传代。选择10~30代的IPEC-1细胞用于后续试验。
1.3 细胞活力检测将细胞接种于96孔板,当细胞融合度达到60%左右时,将IPEC-1细胞经16 μmol/L的3-Ac-DON和0、80、160、320 μg/mL的姜酮共处理24 h,培养液更换为含5% FBS的DMEM/F12培养基,每组设6个复孔,试验重复3次。处理结束后,参考CCK-8试剂盒说明书进行操作,最后采用酶标仪在450 nm下测量吸光度值。
1.4 细胞凋亡检测将细胞接种于6孔板中,当细胞融合度达到60%左右,用16 μmol/L的3-Ac-DON和320 μg/mL的姜酮处理细胞24 h,细胞凋亡参考试剂盒说明书进行操作,经流式细胞仪上机检测并记录数据。
1.5 单层细胞电阻和通透性测定将细胞接种到Transwell细胞培养小室,置于24孔板中。当细胞电阻值达到500 Ω·cm2时,更换为5% FBS的DMEM/F12培养基,用16 μmol/L的3-Ac-DON和320 μg/mL的姜酮处理细胞。将FITC-dextran溶解于含有320 μg/mL姜酮或16 μmol/L 3-Ac-DON的培养液中,FITC-dextran的终浓度为1 mg/mL,加入到小室内。24孔板中,加入对应的含320 μg/mL姜酮或16 μmol/L 3-Ac-DON的培养液。测量相应时间单层细胞的电阻值,并通过酶标仪检测荧光强度,测定单层细胞通透性(激发波长490 nm,发射波长520 nm)。
1.6 蛋白免疫印迹检测收取处理后的细胞,加入适量的放射免疫沉淀(RIPA)裂解液,振荡破碎、4 ℃、13 800×g离心15 min,收集细胞上清液。利用二喹啉甲酸(BCA)法检测蛋白的浓度。制备样品,随后进行电泳、转膜、封闭和孵育抗体,最后进行显影。条带用Image J软件扫描灰度值,以GAPDH为内参。
1.7 数据统计试验数据采用GraphPad Prism 8软件中的one-way ANOVA模型进行统计分析,采用Tukey法进行多重比较,结果用“平均值±标准误”表示,P < 0.05表示差异显著。
2 结果与分析 2.1 姜酮缓解3-Ac-DON诱导的猪肠上皮细胞活力下降和细胞凋亡由图 1-A可知,160和320 μg/mL的姜酮显著缓解3-Ac-DON引起的IPEC-1细胞活力下降(P < 0.05),而姜酮单独处理不影响细胞活力(P>0.05)。根据细胞活力结果,选用浓度320 μg/mL的姜酮用于后续的试验。由图 1-B和图 1-C可知,与3-Ac-DON处理相比,姜酮显著降低了3-Ac-DON引起的猪肠上皮细胞凋亡上升(P < 0.05)。由图 1-D和图 1-E可知,与3-Ac-DON处理相比,姜酮显著下调了cleaved-Caspase-3、BAX和CytC的蛋白表达水平(P < 0.05)。以上结果表明,姜酮缓解了3-Ac-DON诱导的猪肠上皮细胞活力下降和凋亡。
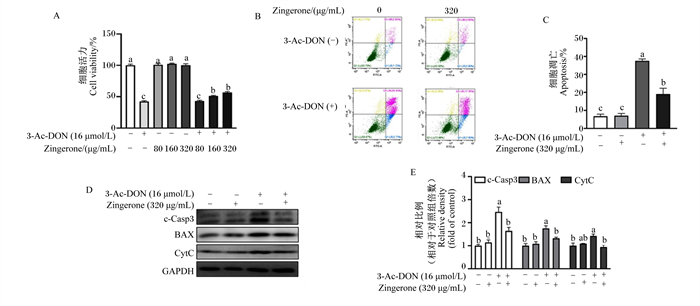
|
3-Ac-DON:3-乙酰呕吐毒素3-acetyldeoxynivalenol;Zingerone:姜酮;c-Casp3:活化型半胱氨酸蛋白酶-3 cleaved-Caspase-3;BAX:B细胞淋巴瘤2相关X蛋白B cell lymphoma 2 associated X protein;CytC:细胞色素C cytochrome C;GAPDH:甘油醛-3-磷酸脱氢酶glyceraldehyde-3-phosphate dehydrogenase。 数据柱标记不同字母表示差异显著(P < 0.05),下图同。Data columns marked with different letters indicated significant difference (P < 0.05), the same as below. 图 1 姜酮对3-Ac-DON诱导的IPEC-1细胞活力下降和细胞凋亡的影响 Fig. 1 Effects of zingerone on 3-Ac-DON-induced decrease in viability and apoptosis of IPEC-1 cells (n=3) |
由图 2可知,与3-Ac-DON处理相比,姜酮显著下调了3-Ac-DON引起的ATF6、p-IRE1α和CHOP的蛋白表达上升(P < 0.05)。以上结果表明,姜酮缓解了3-Ac-DON引起的IPEC-1细胞内质网应激反应。
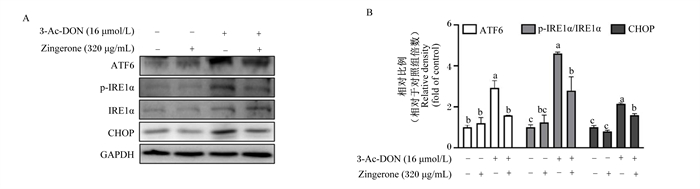
|
3-Ac-DON:3-乙酰呕吐毒素3-acetyldeoxynivalenol;Zingerone:姜酮;ATF6:转录活化因子6 activating transcription factor 6;p-IRE1α:磷酸化的需肌醇酶1α phosphorylated inositol-requiring transmembrane kinase/endoribonuclease 1α;IRE1α:需肌醇酶1α inositol-requiring transmembrane kinase/endoribonuclease 1α;CHOP:CCAAT/增强子结合蛋白同源蛋白CCAAT/enhancer binding protein homologous protein; GAPDH:甘油醛-3-磷酸脱氢酶glyceraldehyde-3-phosphate dehydrogenase。 图 2 姜酮对3-Ac-DON诱导的IPEC-1细胞内质网应激的影响 Fig. 2 Effects of zingerone on 3-Ac-DON-induced endoplasmic reticulum stress in IPEC-1 cells (n=3) |
由图 3可知,与未处理相比,姜酮单独处理细胞12和24 h,猪单层肠上皮细胞跨膜电阻显著下降(P < 0.05),这可能由于姜酮及其代谢产物对肠上皮屏障功能产生了影响,具体原因需要进一步探究。与3-Ac-DON处理相比,姜酮和3-Ac-DON共处理在12和24 h显著提高了单层肠上皮细胞跨膜电阻(P < 0.05),显著降低了单层肠上皮细胞的通透性(P < 0.05)。另外,姜酮显著上调了部分紧密连接蛋白的表达,如occludin和claudin-4(P < 0.05),但没有缓解ZO-1、ZO-2、ZO-3和claudin-1蛋白表达的下降(P>0.05)。以上结果进一步表明,姜酮缓解了猪肠上皮细胞屏障功能,并增强了部分紧密连接蛋白的表达。
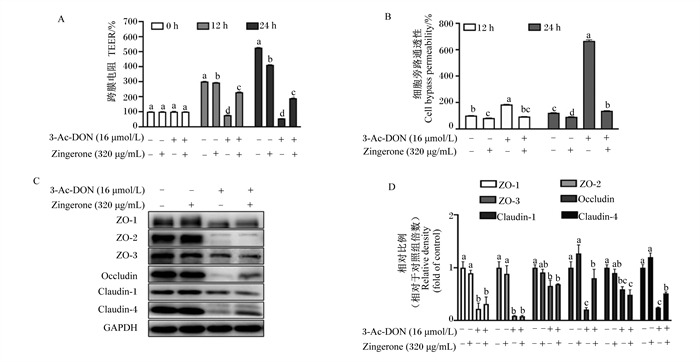
|
3-Ac-DON:3-乙酰呕吐毒素3-acetyldeoxynivalenol;Zingerone:姜酮;ZO:闭锁小带蛋白zonula occludens;occludin:闭合蛋白;claudin:封闭蛋白; GAPDH:甘油醛-3-磷酸脱氢酶glyceraldehyde-3-phosphate dehydrogenase。 图 3 姜酮对3-Ac-DON诱导的IPEC-1细胞屏障功能损伤的影响 Fig. 3 Effects of zingerone on 3-Ac-DON-induced barrier function injury in IPEC-1 cells (n=3) |
3-Ac-DON是食品和饲料中常见的霉菌毒素之一,能够引起肠上皮细胞凋亡,破坏肠道屏障的完整性,并降低营养物质吸收和转运[6]。针对3-Ac-DON对肠上皮细胞损伤作用,迫切的需要有效的缓解策略。姜酮作为生姜中的活性物质,能够减轻大鼠结肠炎并恢复肠道屏障功能[13]。本试验以猪肠上皮细胞为模型,探究姜酮对3-Ac-DON引起肠屏障功能损伤的缓解作用。
细胞增殖是反映细胞活力和更新的重要指标[14]。细胞凋亡是细胞程序性死亡,根据其发生机制可分为死亡受体途径和线粒体途径。细胞发生线粒途径细胞凋亡时,线粒体中的CytC释放到细胞质中,从而活化细胞凋亡蛋白半胱氨酸蛋白酶-3(Caspase-3),促进细胞凋亡。BAX属于B细胞淋巴瘤2家族的促凋亡成员,其蛋白表达升高通常代表细胞发生了线粒体途径的凋亡[15]。本试验中,姜酮恢复了3-Ac-DON引起的细胞活力下降和细胞凋亡升高,降低了凋亡相关蛋白BAX、cleaved-Caspase-3的表达,抑制了线粒体中CytC的释放。之前的研究表明,DON能够引起仔猪海马神经细胞发生线粒途径的凋亡[16]。DON处理引起猪肠上皮细胞IPEC-J2线粒体膜电位的下降,提示其发生线粒体途径的凋亡,白藜芦醇能抑制DON处理引起的凋亡蛋白的升高,从而缓解细胞凋亡[17]。本试验发现,姜酮缓解了3-Ac-DON诱导的线粒体途径凋亡,提示线粒体功能异常与3-Ac-DON引起的猪肠上皮细胞损伤密切相关。
内质网是蛋白质加工修饰的场所,也是外界各种应激的感受器。机体受到有害刺激时,细胞会发生未折叠蛋白反应,从而诱发内质网应激[18]。当细胞发生内质网应激时,IRE1α和ATF6会被激活,从而上调CHOP的蛋白表达,最终引起细胞发生线粒体途径凋亡[19]。最近的研究表明,DON能够增加牛乳腺上皮细胞中CHOP的表达,促进细胞发生线粒体途径的凋亡。蒲公英甾醇处理能缓解了DON引起的凋亡[20]。本试验与之前的研究结果一致,3-Ac-DON能够诱导猪肠上皮细胞发生内质网应激。姜酮通过下调p-IRE1α、ATF6和CHOP蛋白的表达,缓解3-Ac-DON引起的内质网应激。因此,内质网应激是3-Ac-DON造成猪肠上皮细胞损伤的重要途径,而姜酮能够通过恢复内质网稳态,保护猪肠上皮细胞。
紧密连接蛋白能够维持肠道上皮屏障功能和通透性,并且是调节肠道屏障功能的重要靶点[21]。本试验中,3-Ac-DON降低了单层肠上皮细胞的电阻,增加了肠上皮细胞的通透性,而姜酮处理细胞后,显著逆转了这一现象。与未处理相比,姜酮单独处理细胞12和24 h,单层细胞电阻值显著下降,这一点与预期相反,这可能与肠上皮细胞代谢姜酮产生的中间产物或终产物可能会影响肠上皮屏障功能,确切的机制需要进一步探究。本试验中,姜酮处理提高了紧密连接蛋白occludin、claudin-4的表达,缓解了3-Ac-DON引起的细胞屏障功能损伤,这一研究结果与之前的结果[22]相一致。
4 结论姜酮通过增强细胞活力、降低细胞线粒体途径的凋亡、缓解内质网应激和恢复肠上皮细胞屏障功能保护IPEC-1细胞免受3-Ac-DON诱导的损伤。
| [1] |
HE Y U, YIN X Y, DONG J J, et al. Transcriptome analysis of Caco-2 cells upon the exposure of mycotoxin deoxynivalenol and its acetylated derivatives[J]. Toxins, 2021, 13(2): 167. DOI:10.3390/toxins13020167 |
| [2] |
YAN P P, LIU Z Z, LIU S Q, et al. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017[J]. Toxins, 2020, 12(3): 200. DOI:10.3390/toxins12030200 |
| [3] |
SCHELSTRAETE W, DEVREESE M, CROUBELS S. Comparative toxicokinetics of Fusarium mycotoxins in pigs and humans[J]. Food and Chemical Toxicology, 2020, 137: 111140. DOI:10.1016/j.fct.2020.111140 |
| [4] |
HOOFT J M, BUREAU D P. Deoxynivalenol: mechanisms of action and its effects on various terrestrial and aquatic species[J]. Food and Chemical Toxicology, 2021, 112616. |
| [5] |
LIN J, HUANG F F, LIANG T Z, et al. EPA and DHA confer protection against deoxynivalenol-induced endoplasmic reticulum stress and iron imbalance in IPEC-1 cells[J]. The British Journal of Nutrition, 2021, 1-11. |
| [6] |
PAYROS D, ALASSANE-KPEMBI I, PIERRON A, et al. Toxicology of deoxynivalenol and its acetylated and modified forms[J]. Archives of Toxicology, 2016, 90(12): 2931-2957. DOI:10.1007/s00204-016-1826-4 |
| [7] |
BROEKAERT N, DEVREESE M, DE MIL T, et al. Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol in broiler chickens and pigs[J]. Journal of Agricultural and Food Chemistry, 2015, 63(39): 8734-8742. DOI:10.1021/acs.jafc.5b03270 |
| [8] |
MARESCA M. From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol[J]. Toxins, 2013, 5(4): 784-820. DOI:10.3390/toxins5040784 |
| [9] |
KADOTA T, FURUSAWA H, HIRANO S, et al. Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2[J]. Toxicology in Vitro, 2013, 27(6): 1888-1895. DOI:10.1016/j.tiv.2013.06.003 |
| [10] |
AHMAD B, REHMAN M U, AMIN I, et al. A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone)[J]. The Scientific World Journal, 2015, 2015: 816364. |
| [11] |
FANG J, ZHU H F, XU P C, et al. Zingerone suppresses proliferation, invasion, and migration of hepatocellular carcinoma cells by the inhibition of MTDH-mediated PI3K/Akt pathway[J]. Journal of Receptor and Signal Transduction Research, 2021, 1-9. DOI:10.1080/10799893.2021.1988970 |
| [12] |
BASHIR N, AHMAD S B, REHMAN M U, et al. Zingerone (4-(four-hydroxy-3-methylphenyl) butane-two-1) modulates adjuvant-induced rheumatoid arthritis by regulating inflammatory cytokines and antioxidants[J]. Redox Report, 2021, 26(1): 62-70. DOI:10.1080/13510002.2021.1907518 |
| [13] |
ZHANG Z C, CUI Y Q, LIU S Y, et al. Short-term treatment with zingerone ameliorates dextran sulfate sodium-induced mouse experimental colitis[J/OL]. Journal of the Science of Food and Agriculture, 2022. [2022-01-20]. https://onlinelibrary.wiley.com/doi/10.1002/jsfa.11850.DOI:10.1002/jsfa.11850.
|
| [14] |
ADAN A, KIRAZ Y, BARAN Y. Cell proliferation and cytotoxicity assays[J]. Current Pharmaceutical Biotechnology, 2016, 17(14): 1213-1221. DOI:10.2174/1389201017666160808160513 |
| [15] |
BOCK F J, TAIT S W G. Mitochondria as multifaceted regulators of cell death[J]. Nature Reviews Molecular Cell Biology, 2020, 21(2): 85-100. DOI:10.1038/s41580-019-0173-8 |
| [16] |
CAO L, JIANG Y J, ZHU L, et al. Deoxynivalenol induces caspase-8-mediated apoptosis through the mitochondrial pathway in hippocampal nerve cells of piglet[J]. Toxins, 2021, 13(2): 73. DOI:10.3390/toxins13020073 |
| [17] |
YANG J, ZHU C, YE J L, et al. Protection of porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway[J]. Journal of Agricultural and Food Chemistry, 2019, 67(6): 1726-1735. DOI:10.1021/acs.jafc.8b03662 |
| [18] |
OAKES S A, PAPA F R. The role of endoplasmic reticulum stress in human pathology[J]. Annual Review of Pathology, 2015, 10: 173-194. DOI:10.1146/annurev-pathol-012513-104649 |
| [19] |
HU H, TIAN M X, DING C, et al. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection[J]. Frontiers in Immunology, 2018, 9: 3083. |
| [20] |
WANG J X, ZHENG K X, JIN Y C, et al. Protective effects of taraxasterol against deoxynivalenol-induced damage to bovine mammary epithelial cells[J]. Toxins, 2022, 14(3): 211. DOI:10.3390/toxins14030211 |
| [21] |
SLIFER Z M, BLIKSLAGER A T. The integral role of tight junction proteins in the repair of injured intestinal epithelium[J]. International Journal of Molecular Sciences, 2020, 21(3): 972. DOI:10.3390/ijms21030972 |
| [22] |
RAJPUT S A, LIANG S J, WANG X Q, et al. Lycopene protects intestinal epithelium from deoxynivalenol-induced oxidative damage via regulating Keap1/Nrf2 signaling[J]. Antioxidants (Basel), 2021, 10(9): 1493. DOI:10.3390/antiox10091493 |




