2. 重庆市渝北区王家街道社区事务服务中心,重庆 401120
2. Community Affairs Service Center, Wangjia Street, Yubei District, Chongqing 401120, China
血管内皮损伤能够由高血脂和氧化应激引发,并且能与炎性反应相互作用共同导致动脉粥样硬化(atherosclerosis,AS)的形成[1-2]。黄酮类化合物是一类植物次生代谢产物,广泛存在于多种植物中,不仅数量、种类繁多,而且结构类型复杂多样。黄酮类化合物具有抗氧化活性[3],可通过直接清除自由基,抑制产生活性氧相关的酶活性,增加细胞分泌抗氧化介质,减少氧化应激[4-5]。此外,黄酮类化合物能够降低促炎性细胞因子的表达以及上调抗炎细胞因子的表达[6-7],发挥抗炎作用。
苜蓿素(tricin)又名麦黄酮,是一种甲基黄酮,最早在小麦中发现,随后在植物苜蓿中分离,得名麦黄酮、苜蓿素[8],别名为5, 7-二羟基-2-苯并吡喃-4-酮。苜蓿素作为黄酮类化合物,目前已发现其在牛乳腺上皮细胞[9]、小鼠肺泡巨噬细胞[10]、小鼠单核巨噬细胞[11-12]、外周血单核细胞[13-14]中都能够发挥抗炎作用。除了对细胞有抗炎效果外,苜蓿素在治疗小鼠结肠炎方面也具有显著的作用[15-16],但鲜有关苜蓿素对高脂饲粮诱发的血管内皮损伤治疗作用的报道。SD大鼠通常被作为研究血管疾病的动物模型[17-18]。因此,本研究以SD大鼠为动物模型,以苜蓿素为试验药物,探讨苜蓿素对高脂饲粮诱发的SD大鼠血管内皮损伤的治疗机制。
1 材料与方法 1.1 试验材料 1.1.1 主要药物与试剂苜蓿素(纯度>98.0%)为供试药物,购自上海某科技股份有限公司;辛伐他汀为阳性对照药物,购自北京某科技有限公司。
肿瘤坏死因子-α(TNF-α)、诱导型一氧化氮合酶(iNOS)、环氧化酶-2(COX-2)、白细胞介素-1(IL-1)、白细胞介素-6(IL-6)、总胆固醇(TC)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、甘油三酯(TG)、细胞间黏附分子-1(ICAM-1)、血管细胞黏附分子-1(VCAM-1)酶联免疫吸附测定(ELISA)试剂盒均购自重庆博诺恒生物科技有限公司。超氧化物歧化酶(SOD)、丙二醛(MDA)、乳酸脱氢酶(LDH)测定试剂盒均购自上海优选生物科技有限公司。聚偏二氟乙烯(PVDF)膜购自红荣微再生物工程技术有限公司;β-肌动蛋白(β-actin)、核因子-κB(NF-κB)p65蛋白(p65)、NF-κB抑制蛋白α(IKB)、磷酸化NF-κB p65蛋白(P-p65)、磷酸化NF-κB抑制蛋白α(P-IKB)、p38丝裂原活化蛋白激酶(p38MAPK)、c-Jun氨基末端激酶(JNK)、细胞外调节蛋白激酶1/2(ERK1/2)、磷酸化p38丝裂原活化蛋白激酶(P-p38)、磷酸化c-Jun氨基末端激酶(P-JNK)测定试剂盒及磷酸化细胞外调节蛋白激酶1/2(P-ERK1/2)兔抗鼠一抗、辣根过氧化物酶(HRP)标记山羊抗兔免疫球蛋白(IgG)二抗均购自北京博奥森生物技术有限公司;二喹啉甲酸(BCA)蛋白定量试剂盒购自碧云天生物技术有限公司。
1.1.2 试验动物SPF级SD大鼠(雌雄比例1 ∶ 1,体质量220~250 g,6周龄),许可证号:SCXK(湘)2019-0004。饲养环境:室温22~25 ℃,相对湿度65%~70%,光照周期12 h:12 h。
1.1.3 主要仪器设备美国Bio-Rad公司十二烷基硫酸钠垂直电泳系统,德国Sartorius公司高速冷冻离心机,北京六一生物科技有限公司WD-2102A型全自动酶标仪,上海巴拓仪器有限公司生物显微镜。
1.2 试验设计将50只试验SD大鼠随机分为5组,即空白对照组、高脂模型组、辛伐他汀治疗组[1.8 mg/(kg·d)]和低剂量苜蓿素治疗组[5 mg/(kg·d)]及高剂量苜蓿素治疗组[15 mg/(kg·d)],每组10个重复,每个重复1只。空白对照组给予普通饲粮喂养,其余各组大鼠均采用高脂饲粮(基础饲粮79.3%、蛋黄15%、猪油5%、胆固醇0.5%、胆酸钠0.2%)[19]喂养,构建血管内皮损伤模型。
1.3 样品采集及观察将大鼠用3.5%水合氯醛麻醉后,剪开大鼠胸腹部皮肤,分离出大鼠腹主动脉,取全血分离血清(4 ℃静置24 h,取上清,经3 000 r/min离心10 min后,再取上清),于4 ℃保存备用;剥取腹主动脉,一部分经生理盐水清洗后放于离心管并置于液氮中保存备用,另一部分经4%多聚甲醛液固定、常规脱水、石蜡包埋、切片、常规苏木精-伊红(HE)染色、复染和封片处理。切片制备完成后,用生物显微镜在200×下观察。
1.4 高脂模型建立、治疗及样品处理建模5周时,随机在空白对照组和高脂饲粮组中各选3只大鼠,禁食24 h,根据1.3分离血清并制备主动脉石蜡切片;建模完成后,每天09:00,各治疗组分别灌胃给药1次(剂量同1.2,每天仅1次),空白对照组和高脂模型组以等量生理盐水灌胃,共治疗5周,治疗阶段继续饲喂高脂饲粮。治疗完成后,每组选取6只,禁食24 h,根据1.3取大鼠血清,并制备主动脉石蜡切片。
1.5 血清中LDH和SOD活性及MDA含量检测按照试剂盒的操作步骤,采用微量法检测血清中LDH、SOD活性及MDA含量。
1.6 血清中各炎症相关因子及血脂相关指标的检测按照试剂盒的操作步骤,采用ELISA法检测血清中TNF-α、IL-1、IL-6、TC、HDL-C、LDL-C、TG、ICAM-1、VCAM-1含量及iNOS、COX-2活性。
1.7 腹主动脉血管炎症相关信号通路蛋白的表达检测采用Western blot法检测主动脉血管炎症相关信号通路蛋白的表达。各组分别称量冷冻动脉血管0.5 g,研磨,加入裂解液提取细胞总蛋白,BCA法测定蛋白浓度并定量,10% 十二烷基硫酸钠聚丙烯酰氨凝胶(SDS-PAGE)电泳后将蛋白电转到PVDF膜上,用5%的脱脂奶粉室温封闭1 h(磷酸化蛋白封闭4 h),一抗4 ℃孵育过夜,三羟甲基氨基甲烷盐酸盐聚山梨酯(TBST)室温避光洗涤5 min,洗涤6次,二抗室温避光孵育1 h,TBST洗涤同前,使用增强化学发光(ECL)显色液显色,使用化学发光成像系统拍照,采用Image Lab软件进行分析,目标条带吸收度与内参(β-actin)吸收度的比值为蛋白表达结果。
1.8 统计处理本试验所有数据均采用柱状图表示,采用GraphPad Prism 8.0.1统计分析软件中Column程序对数据进行单因素方差分析,并对组间数据进行多重比较,以P < 0.05作为差异显著水平,以P < 0.01作为差异极显著水平。
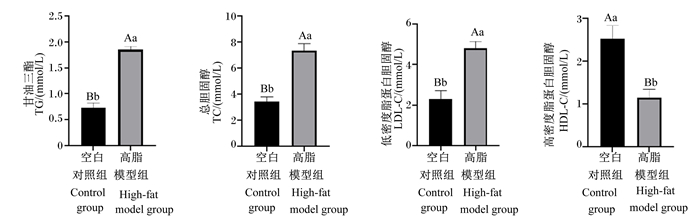
2 结果与分析 2.1 大鼠高脂模型建立图 1为空白对照组和高脂模型组大鼠在建模结束时(第36天)的血脂相关指标。与空白对照组相比,高脂模型组大鼠血清中TC、LDL-C、TG的含量均极显著升高(P<0.01),而血清中HDL-C的含量极显著降低(P<0.01)。
|
柱状图标注不同小写字母表示差异显著(P<0.05),不同大写字母表示差异极显著(P<0.01)。下图同。 Bars with different small letters mean significant difference (P < 0.05), and with different capital letters mean extremely significant difference (P < 0.01). The same as below. 图 1 空白对照组和高脂模型组大鼠血脂相关指标(第36天) Fig. 1 Serum lipid related indexes of rats in blank control group and high-fat model group (day 36) |
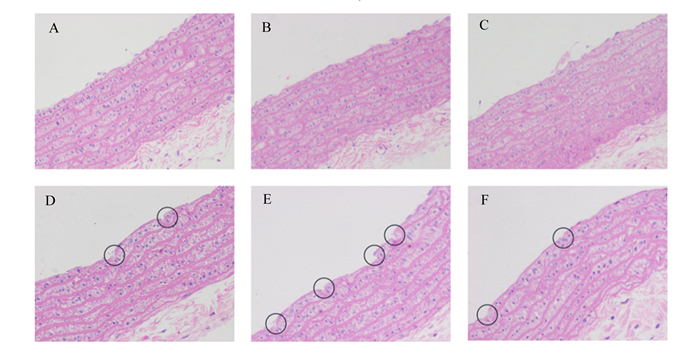
图 2位空白对照组和高脂模型组大鼠在建模结束时(第36天)血管内皮的损伤情况。空白对照组大鼠血管内皮完整,皮下组织厚度均匀排列整齐(图 2-A、图 2-B、图 2-C),而高脂模型组大鼠血管内皮可见连续多处损伤,血管内皮和皮下组织均明显增厚(图 2-D、图 2-E、图 2-F),且内皮局部向管腔明显隆起(图 2-E)。由此得出,大鼠血管内皮损伤模型制备成功。
|
A、B、C为空白对照组;D、E、F为高脂模型组。 A, B and C were blank control group; D, E and F were high-fat model group. 图 2 空白对照组和高脂模型组大鼠腹主动脉血管损伤情况(第36天) Fig. 2 Abdominal aortic vascular injury of rats in blank control group and high-fat model group (day 36, 200×) |
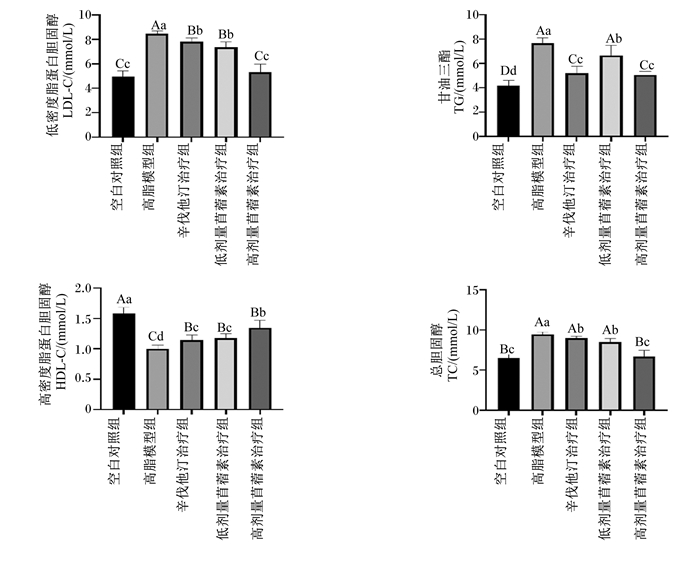
由图 3可知,治疗结束时,与空白对照组相比,高脂模型组大鼠血清中LDL-C、TC、TG的含量均极显著升高(P<0.01),而HDL-C的含量极显著降低(P<0.01);与高脂模型组相比,辛伐他汀治疗组、低剂量苜蓿素治疗组和高剂量苜蓿素治疗组大鼠血清中LDL-C、TC、TG的含量均显著降低(P<0.05),而HDL-C的含量极显著升高(P<0.01);与辛伐他汀治疗组相比,低剂量苜蓿素治疗组大鼠血清中LDL-C、TC、HDL-C的含量无显著差异(P>0.05),TG的含量显著提高(P<0.05),而高剂量苜蓿素治疗组血清中LDL-C、TC的含量极显著降低(P<0.01),HDL-C的含量显著升高(P<0.05),TG的含量无显著差异(P>0.05)。这说明苜蓿素具有降脂作用,而且本试验中,高剂量苜蓿素治疗组的降脂效果优于辛伐他汀治疗组。
|
空白对照组: blank control group; 高脂模型组:high-fat model group;辛伐他汀治疗组:simvastatin treatment group;低剂量苜蓿素治疗组:low-dose tricin treatment group;高剂量苜蓿素治疗组high-dose tricin treatment group。下图同the same as below。 图 3 各组大鼠血脂相关指标(第70天) Fig. 3 Serum lipid related indexes of rats in each group (day 70) |
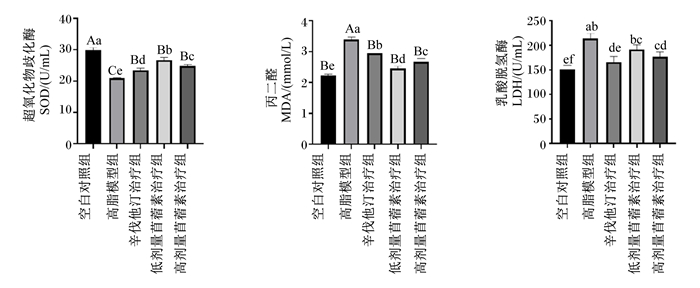
由图 4可知,与空白对照组相比,高脂模型组大鼠血清中SOD活性极显著降低(P<0.01);与高脂模型组相比,3个治疗组大鼠血清中SOD活性均极显著升高(P<0.01);与辛伐他汀治疗组相比,苜蓿素治疗组大鼠血清中SOD活性均显著升高(P<0.05)。与空白对照组相比,高脂模型组大鼠血清中LDH活性显著升高(P<0.05);与高脂模型组相比,辛伐他汀治疗组和高剂量苜蓿素治疗组大鼠血清中LDH活性均显著降低(P<0.05),而且这2组间无显著差异(P>0.05)。与空白对照组相比,高脂模型组大鼠血清中MDA的含量极显著升高(P<0.01);与模型组相比,3个治疗组大鼠血清中MDA的含量均极显著降低(P<0.01);而与辛伐他汀治疗组相比,2个苜蓿素治疗组大鼠血清中MDA的含量也均显著降低(P<0.05)。综上所述,苜蓿素具有显著的抗氧化作用。
|
图 4 各组大鼠血清中SOD和LDH的活性及MDA含量(第70天) Fig. 4 Activity of SOD and LDH and content of MDA in serum of rats in each group (day 70) |
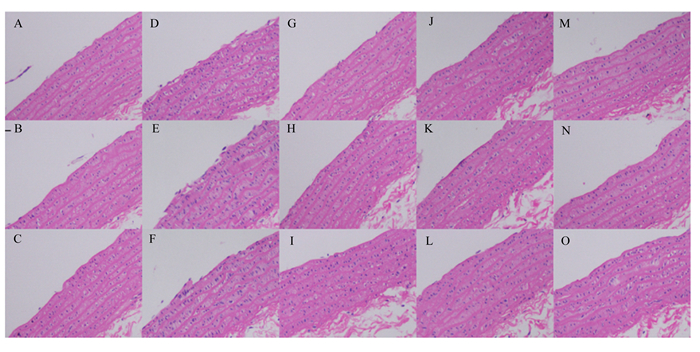
图 5为试验结束时大鼠主动脉的形态学变化。图中左上侧为血管内皮。观察可得,空白对照组大鼠血管内皮结构完整表面光滑,平滑肌细胞分布均匀,无胞核碎片,未见空泡变性和胶原纤维增生。与空白对照组相比,高脂模型组大鼠主动脉呈明显病变,血管内皮受损严重,可见连续多处的内皮损伤,皮下组织增厚,层次结构分布不清,平滑肌细胞大量增生,排列紊乱,内皮出现明显的隆起现象(图 5-D、图 5-E、图 5-F)。与高脂模型组相比,辛伐他汀治疗组、低剂量苜蓿素治疗组和高剂量苜蓿素治疗组大鼠均表现出血管内皮完整,厚度均匀,在形态结构上与空白对照组基本一致,并且3个治疗组之间没有明显差异,这说明苜蓿素能够治疗血管内皮损伤,并且高、低剂量的苜蓿素均能达到辛伐他汀的治疗水平。
|
A、B、C为空白对照组;D、E、F为高脂模型组;G、H、I为辛伐他汀治疗组;J、K、L为低剂量苜蓿素治疗组;M、N、O为高剂量苜蓿素治疗组。 A, B and C were blank control group; D, E and F were high-fat model group; G, H and I were simvastatin treatment group; J, K and L were low-dose tricin treatment group; M, N and O were high-dose tricin treatment group. 图 5 各组大鼠腹主动脉血管损伤情况(第70天) Fig. 5 Abdominal aortic vascular injury of rats in each group (day 70, 200×) |
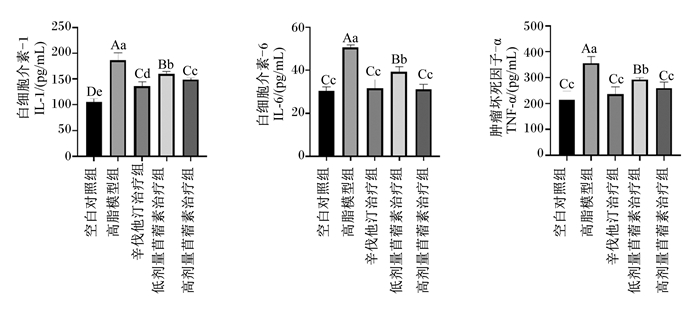
由图 6可知,与空白对照组相比,高脂模型组大鼠血清中IL-1、IL-6及TNF-α含量均极显著升高(P<0.01),而3个治疗组均比高脂模型组极显著降低(P<0.01)。比较3个治疗组大鼠血清中炎症因子含量,高剂量苜蓿素治疗组与辛伐他汀治疗组间无显著差异(P>0.05),但比低剂量苜蓿素治疗组极显著降低(P<0.01)。这说明高脂饲粮促进血清炎症因子的产生,苜蓿素能够抑制血清炎症因子(IL-1、IL-6及TNF-α)的产生,而且高剂量的苜蓿素抑制效果更好。
|
图 6 各组大鼠血清中IL-1、IL-6和TNF-α的含量(第70天) Fig. 6 Contents of IL-1, IL-6 and TNF-α in serum of rats in each group (day 70) |
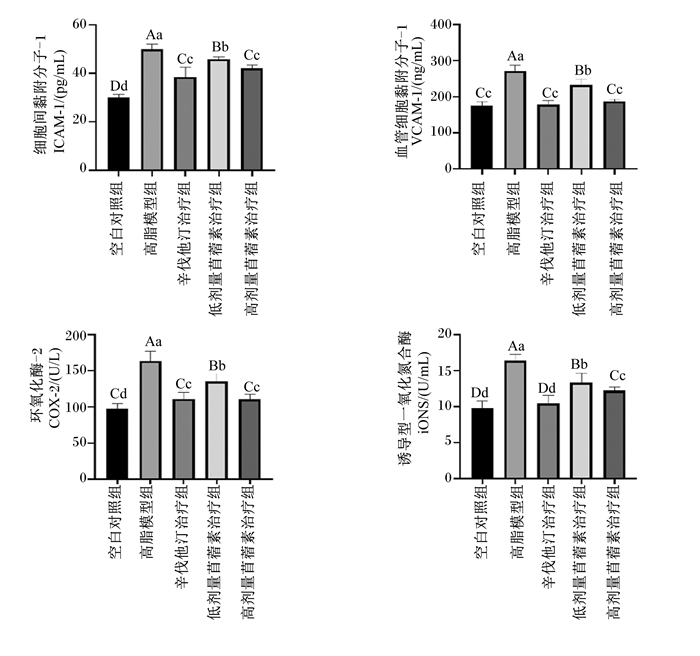
由图 7可知,与空白对照组相比,高脂模型组大鼠血清中ICAM-1、VCAM-1含量及iNOS、COX-2活性均极显著升高(P<0.01);与高脂模型组相比,辛伐他汀治疗组、低剂量苜蓿素治疗组和高剂量苜蓿素治疗组大鼠血清中ICAM-1、VCAM-1含量及iNOS、COX-2活性均极显著降低(P<0.01);与辛伐他汀治疗组相比,低剂量苜蓿素治疗组大鼠血清中ICAM-1、VCAM-1含量及iNOS、COX-2活性均极显著升高(P<0.01),高剂量苜蓿素治疗组大鼠血清中ICAM-1、VCAM-1含量及COX-2活性均与辛伐他汀治疗组无显著差异(P>0.05),而且高剂量苜蓿素治疗组血清ICAM-1、VCAM-1含量及iNOS、COX-2活性均极显著低于低剂量苜蓿素治疗组(P<0.01)。这说明苜蓿素能够抑制血清炎症因子(ICAM-1、VCAM-1、iNOS、COX-2)的产生,而且高剂量苜蓿素比低剂量苜蓿素效果更好。
|
图 7 各组大鼠血清中ICAM-1、VCAM-1含量及iNOS和COX-2活性(第70天) Fig. 7 Contents of ICAM-1, VCAM-1 and activity of iNOS and COX-2 in serum of rats in each group (day 70) |
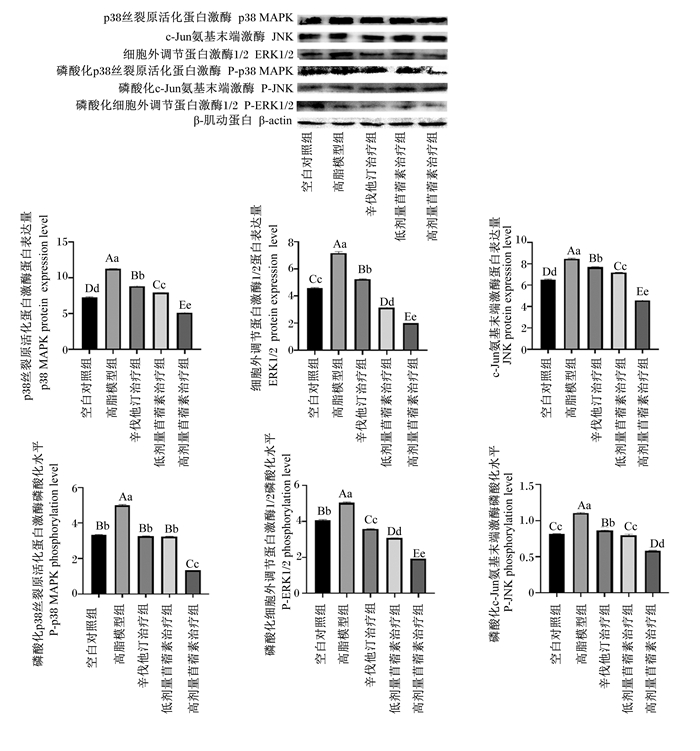
由图 8可知,与空白对照组相比,高脂模型组大鼠腹主动脉MAPK信号通路蛋白(p38 MAPK、JNK、ERK1/2)的表达量均极显著升高(P<0.01);与高脂模型组相比,3个治疗组大鼠腹主动脉MAPK信号通路蛋白的表达量均极显著降低(P<0.01);与辛伐他汀治疗组相比,除低剂量苜蓿素治疗组大鼠腹主动脉P-p38磷酸化水平无显著(P>0.05)变化外,2组苜蓿素治疗组其他MAPK信号通路蛋白的磷酸化水平均极显著降低(P<0.01),且高剂量苜蓿素治疗组大鼠腹主动脉MAPK信号通路磷酸化水平均极显著低于低剂量苜蓿素治疗组(P<0.01)。这说明苜蓿素能够抑制大鼠腹主动脉MAPK信号通路,其抑制效果优于辛伐他汀,而且高剂量的苜蓿素比低剂量的抑制效果好。
|
图 8 各组MAPK信号通路蛋白表达量及其磷酸化水平 Fig. 8 Expression level of MAPK signaling pathway protein and its phosphorylation level in each group |
由图 9可知,与空白对照组相比,高脂模型组大鼠腹主动脉NF-κB信号通路蛋白(p65、IKB)的表达量均极显著升高(P<0.01);与高脂模型组相比,3个治疗组大鼠腹主动脉NF-κB信号通路蛋白的表达量均极显著降低(P<0.01);与辛伐他汀组相比,2组苜蓿素治疗组大鼠腹主动脉NF-κB信号通路蛋白的磷酸化水平均极显著降低(P<0.01);与低剂量苜蓿素治疗组相比,高剂量苜蓿素治疗组大鼠腹主动脉NF-κB信号通路蛋白的磷酸化水平均极显著降低(P<0.01)。这说明苜蓿素能够抑制大鼠腹主动脉NF-κB信号通路,且效果优于辛伐他汀,而且高剂量的苜蓿素比低剂量的抑制效果好。
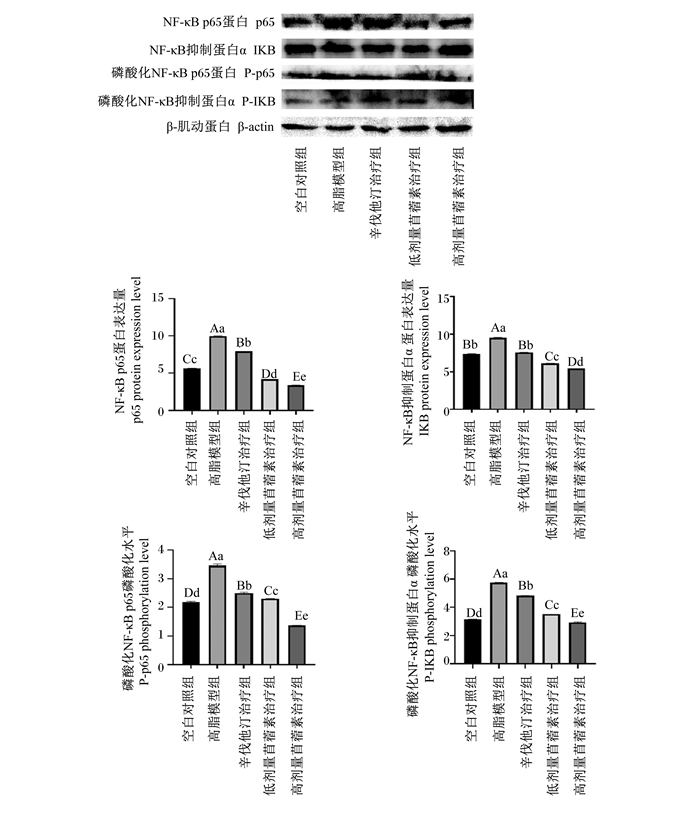
|
图 9 各组NF-κB信号通路蛋白表达量及其磷酸化水平 Fig. 9 Expression level of NF-κB signaling pathway protein and its phosphorylation level in each group |
血管内皮损伤与多种因素(包括病毒、内毒素、细胞因子、体液因子等)有关,研究发现,高脂血症使血管内皮受损后,慢性炎症能与受损的血管内皮相互作用,并伴随AS形成的全过程[20]。AS的形成过程主要包括血管内皮损伤、单核细胞和平滑肌细胞迁入血管内膜、泡沫细胞形成、新生血管生成以及斑块形成[21],在这一过程中,氧化应激引起生物膜脂过氧化、胞内蛋白及酶变性,导致血管张力调节障碍和黏附分子表达异常,并诱发动脉内膜发生慢性炎症,最终慢性炎症又促进AS的发展[22]。本试验中,建模完成时,大鼠血脂水平严重紊乱,同时血管内皮出现多处损伤且局部出现隆起,血管内皮和皮下组织均明显增厚,由此确定建模成功。治疗完成时,高脂模型组大鼠血清中TG、TC、LDL-C的含量远高于正常水平,HDL-C的含量远低于正常水平,而苜蓿素治疗组大鼠血清中这4项得到极大改善或恢复到正常水平,这说明苜蓿素能够改善脂质代谢异常。脂质代谢异常是指血液中的TG和TC含量超过正常值的上限,TC本身不能损伤血管内皮,但是LDL-C能进入血管内膜损伤血管内皮[23],因此,抗氧化对保护血管内皮具有重要意义。
SOD作为抗氧化酶能减轻血管氧化损伤,而MDA和LDH是能够加重血管氧化损伤的物质[24-26]。本试验中,高脂模型组大鼠血清中SOD活性极显著低于空白对照组,MDA的含量极显著高于空白对照组,LDH的活性显著高于空白对照组,而苜蓿素治疗组大鼠血清中SOD的活性极显著高于高脂模型组,MDA的含量极显著低于高脂模型组,LDH的活性显著低于高脂模型组,这与Park等[24]、Han等[25]及王志旺等[26]发现苜蓿素能够提高SOD活性及抑制MDA含量和LDH活性的结果一致,说明苜蓿素能够凭借其抗氧化特性消除氧化应激。氧化应激是导致血管内皮损伤的关键[22-23],因此,消除氧化应激在一定程度上也能够减轻血管内皮损伤。治疗结束时,苜蓿素治疗组大鼠血管内皮均呈现出无病变现象,而高脂模型组大鼠出现严重病变,并且比建模初期病变更严重,这说明苜蓿素能够治疗血管内皮损伤,这一结果与芹菜素和槲皮素(二者均为黄酮类化合物)均能够恢复血管内皮功能的结果一致[27-28],血管内皮损伤导致血管内皮功能异常,进而导致AS的形成[29],因此苜蓿素能通过修复血管内皮治疗AS。
氧化应激除了能损伤血管内皮外,还能够促进黏附因子和炎症因子的表达[22]。VCAM-1、ICAM-1是血管内皮细胞分泌的黏附因子,它们能促进炎性细胞(尤其是单核细胞)发生迁移浸润,并吸引单核细胞进入血管内皮加剧内皮损伤[30-31]。在试验过程中,采食高脂饲粮使大鼠血清中这2种炎性相关因子的含量均远高于空白对照组,而饲喂苜蓿素后其含量均极显著降低,这与Shalini等[14]发现苜蓿素能够降低人脐静脉内皮细胞中ICAM-1、VCAM-1含量的结果一致,说明苜蓿素还能够通过减少黏附因子产生来减轻内皮损伤。在降低炎症因子含量方面,苜蓿素也具有显著效果,试验结束时,高脂模型组大鼠血清中炎症相关因子TNF-α、IL-1、IL-6含量及iNOS、COX-2活性均极显著高于空白对照组,而苜蓿素组大鼠血清中TNF-α、IL-1、IL-6含量及iNOS、COX-2活性均极显著降低,这与Lee等[11]和Shalini等[13]发现苜蓿素能够抑制促炎因子表达的结果一致,说明抗炎也是苜蓿素治疗内皮损伤的重要手段。
有研究指出,抑制NF-κB和MAPK信号通路能够减轻炎症反应[32-33],而机体内的炎症通路主要是依靠炎症通路蛋白来传递信号。因此,抑制炎症通路蛋白的表达就能够阻断炎症通路,消除炎症反应。本试验结果表明,采食高脂饲粮时,大鼠腹主动脉血管中炎症通路蛋白(JNK、ERK、p38 MAPK、p65、IKB)的表达量均极显著升高,饲喂苜蓿素后,血管中炎症通路蛋白的表达量均极显著降低,这说明苜蓿素对大鼠腹主动脉NF-κB和MAPK信号通路具有明显的抑制作用,与Lee等[34]、Jiang等[35]以及Yue等[36]发现苜蓿素能够抑制这2条炎症通路的结果一致。炎症相关因子激活NF-κB和MAPK信号通路使机体组织发生炎症的同时[37],被激活的NF-κB和MAPK信号通路也能够调控炎症因子的表达[38],这些炎症因子进一步刺激局部组织或通过血液运输使机体其他组织发生炎症。因此,同时抑制炎症相关因子与炎症通路蛋白的表达才能够消除炎症反应。从本试验结果来看,苜蓿素能够同时抑制炎症相关因子和炎症通路蛋白表达,而且其抑制效果强于辛伐他汀。辛伐他汀主要通过竞争性抑制内源性胆固醇合成限速还原酶,使细胞内胆固醇合成减少,是市面上主要的降脂药物,而且在抗炎、抗氧化方面也具有显著作用[39]。辛伐他汀虽然拥有良好的降脂效果,但副作用也比较明显,常见的不良反应有肌痛、肝酶活性升高等,而苜蓿素虽然在降脂、抗氧化方面与辛伐他汀差异不明显,但是在抗炎方面效果更好。因此,苜蓿素有望成为替代辛伐他汀的药物。
4 结论苜蓿素能够通过降血脂、抗氧化、消炎等多种途径来修复血管内皮损伤,并且苜蓿素能够通过抑制NF-κB和MAPK信号通路消除炎症对动脉血管的损伤。
| [1] |
GUO Q, CAO W Z, ZHAO H, et al. Effect of Sancaijiangtang on plasma nitric oxide and endothelin-1 levels in patients with type 2 diabetes mellitus and vascular dementia: a single-blind randomized controlled trial[J]. Journal of Traditional Chinese Medicine, 2015, 35(4): 375-380. DOI:10.1016/S0254-6272(15)30112-6 |
| [2] |
MCALPINE C S, SWIRSKI F K. Circadian influence on metabolism and inflammation in atherosclerosis[J]. Circulation Research, 2016, 119(1): 131-141. DOI:10.1161/CIRCRESAHA.116.308034 |
| [3] |
PANCHE A N, DIWAN A D, CHANDRA S R. Flavonoids: an overview[J]. Journal of Nutritional Science, 2016, 5: e47. DOI:10.1017/jns.2016.41 |
| [4] |
DA POZZO E, COSTA B, CAVALLINI C, et al. The citrus flavanone naringenin protects myocardial cells against age-associated damage[J]. Oxidative Medicine and Cellular Longevity, 2017, 2017: 9536148. |
| [5] |
PENG K Z, YANG X D, ZHOU H L, et al. Safety evaluation, in vitro and in vivo antioxidant activity of the flavonoid-rich extract from Maydis stigma[J]. Molecules, 2015, 20(12): 22102-22112. DOI:10.3390/molecules201219835 |
| [6] |
YANG J H, CHOI M H, YANG S H, et al. Potent anti-inflammatory and antiadipogenic properties of bamboo (Sasa coreana Nakai) leaves extract and its major constituent flavonoids[J]. Journal of Agricultural and Food Chemistry, 2017, 65(31): 6665-6673. DOI:10.1021/acs.jafc.7b02203 |
| [7] |
TAGUCHI L, PINHEIRO N M, OLIVO C R, et al. A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases[J]. Respiratory Research, 2015, 16(1): 79. DOI:10.1186/s12931-015-0233-3 |
| [8] |
BICKOFF E M, LIVINGSTON A L, BOOTH A N. Tricin from alfalfa-isolation and physiological activity[J]. Journal of Pharmaceutical Sciences, 1964, 53(11): 1411-1412. DOI:10.1002/jps.2600531131 |
| [9] |
占今舜, 詹康, 陈小连, 等. 苜蓿素对脂多糖诱导下奶牛乳腺上皮细胞炎症和乳蛋白合成相关基因表达的影响[J]. 草业科学, 2018, 35(2): 441-448. ZHAN J S, ZHAN K, CHEN X L, et al. Lipopolysaccharide-induced effects of tricin on inflammatory and lacto-protein gene expression in bovine mammary epithelial cells[J]. Pratacultural Science, 2018, 35(2): 441-448 (in Chinese). |
| [10] |
殷玉婷, 刘丽丽, 刘利艳. 苜蓿素对哮喘小鼠肺泡巨噬细胞TLR4/MyD88/NF-κB通路的抑制作用[J]. 中成药, 2017, 39(3): 450-454. YIN Y T, LIU L L, LIU L Y. Tricin's inhibitory effects on TLR4/MyD88/NF-κB pathway of alveolar macrophages in asthma mice[J]. Chinese Traditional Patent Medicine, 2017, 39(3): 450-454 (in Chinese). DOI:10.3969/j.issn.1001-1528.2017.03.002 |
| [11] |
LEE D, IMM J Y. AMP kinase activation and inhibition of nuclear factor-kappa B (NF-κB) translocation contribute to the anti-inflammatory effect of tricin[J]. Journal of Food Biochemistry, 2017, 41(2): e12293. DOI:10.1111/jfbc.12293 |
| [12] |
KANG B M, AN B K, JUNG W S, et al. Anti-inflammatory effect of tricin isolated from Alopecurus aequalis Sobol. on the LPS-induced inflammatory response in RAW 264.7 cells[J]. International Journal of Molecular Medicine, 2016, 38(5): 1614-1620. DOI:10.3892/ijmm.2016.2765 |
| [13] |
SHALINI V, BHASKAR S, KUMAR K S, et al. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: possible role in the inflammatory signaling[J]. International Immunopharmacology, 2012, 14(1): 32-38. DOI:10.1016/j.intimp.2012.06.005 |
| [14] |
SHALINI V, JAYALEKSHMI A, HELEN A. Mechanism of anti-inflammatory effect of tricin, a flavonoid isolated from Njavara rice bran in LPS induced hPBMCs and carrageenan induced rats[J]. Molecular Immunology, 2015, 66(2): 229-239. DOI:10.1016/j.molimm.2015.03.004 |
| [15] |
TANAKA T, OYAMA T, SUGIE S. Dietary tricin suppresses inflammation-related colon carcinogenesis in mice[J]. Journal of Nutritional Science and Vitaminology, 2019, 65(Suppl.): S100-S103. |
| [16] |
OYAMA T, YASUI Y, SUGIE S. Dietary tricin suppresses inflammation-related colon carcinogenesis in male Crj: CD-1 mice[J]. Cancer Prevention Research, 2009, 2(12): 1031-1038. DOI:10.1158/1940-6207.CAPR-09-0061 |
| [17] |
RUSSELL J C. Evaluating micro- and macro-vascular disease, the end stage of atherosclerosis, in rat models[J]. Methods in Molecular Biology, 2009, 573: 17-44. |
| [18] |
RUSSELL J C, PROCTOR S D. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis[J]. Cardiovascular Pathology, 2006, 15(6): 318-330. DOI:10.1016/j.carpath.2006.09.001 |
| [19] |
张国刚. 水莲皂苷抗动脉粥样研究[D]. 博士学位论文. 长沙: 中南大学, 2006. ZHANG G G. Studies of reinioside's anti-atherosclerosis mechanism[D]. Ph. D. Thesis. Changsha: Central South University, 2006. (in Chinese) |
| [20] |
徐尤年, 马璞, 张曌, 等. 内皮细胞的活化/损伤与炎症反应[J]. 中国急救医学, 2010, 30(6): 552-554. XU Y N, MA P, ZHANG Z, et al. Activation/injury of the endothelial cells and the initiation and resolution of inflammation[J]. Chinese Journal of Critical Care Medicine, 2010, 30(6): 552-554 (in Chinese). DOI:10.3969/j.issn.1002-1949.2010.06.021 |
| [21] |
SHANTSILA E, KAMPHUISEN P W, LIP G Y H. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis[J]. Journal of Thrombosis and Haemostasis, 2010, 8(11): 2358-2368. DOI:10.1111/j.1538-7836.2010.04007.x |
| [22] |
吴彧, 孙琳, 黄彦生, 等. 氧化应激与炎症在动脉粥样硬化发生发展中的作用及相关治疗药物研究[J]. 中国实用神经疾病杂志, 2014, 17(21): 127-129. WU Y, SUN L, HUANG Y S, et al. The role of oxidative stress and inflammation in the occurrence and development of atherosclerosis and research on related therapeutic drugs[J]. Chinese Journal of Practical Nervous Diseases, 2014, 17(21): 127-129 (in Chinese). DOI:10.3969/j.issn.1673-5110.2014.21.086 |
| [23] |
CHERIYAN S, NANDAKUMARAN D G, ROY D D, et al. Oxidised LDL cholesterol (Ox-LDL-C) and Ox-LDL-C/HDL cholesterol (HDL-C) ratio in acute coronary syndrome patients versus chronic coronary artery disease patients on statin treatment[J]. Journal of Clinical and Diagnostic Research, 2019, 13(12): BC14-BC17. |
| [24] |
PARK S H, LEE S S, BANG M H, et al. Protection against UVB-induced damages in human dermal fibroblasts: efficacy of tricin isolated from enzyme-treated Zizania latifolia extract[J]. Bioscience, Biotechnology, and Biochemistry, 2019, 83(3): 551-560. DOI:10.1080/09168451.2018.1554424 |
| [25] |
HAN H J, PARK S K, KANG J Y, et al. Anti-melanogenic effect of ethanolic extract of Sorghum bicolor on IBMX-induced melanogenesis in B16/F10 melanoma cells[J]. Nutrients, 2020, 12(3): 832. DOI:10.3390/nu12030832 |
| [26] |
王志旺, 张扬, 程芳, 等. 五脉绿绒蒿总黄酮及其中苜蓿素的体外抗氧化活性[J]. 中国老年学杂志, 2011, 31(22): 4381-4383. WANG Z W, ZHANG Y, CHENG F, et al. Study on ant-oxidative activity of total flavones of radix angelicae sinensis and tricin in vitro[J]. Chinese Journal of Gerontology, 2011, 31(22): 4381-4383 (in Chinese). DOI:10.3969/j.issn.1005-9202.2011.22.041 |
| [27] |
CLAYTON Z S, HUTTON D A, BRUNT V E, et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging[J]. American Journal of Physiology: Heart and Circulatory Physiology, 2021, 321(1): H185-H196. DOI:10.1152/ajpheart.00118.2021 |
| [28] |
ZHANG F W, FENG J, ZHANG J Y, et al. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery[J]. Experimental and Therapeutic Medicine, 2020, 20(6): 280. |
| [29] |
DUAN H X U, ZHANG Q, LIU J, et al. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis[J]. Pharmacological Research, 2021, 168: 105599. DOI:10.1016/j.phrs.2021.105599 |
| [30] |
FENYO I M, GAFENCU A V. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis[J]. Immunobiology, 2013, 218(11): 1376-1384. DOI:10.1016/j.imbio.2013.06.005 |
| [31] |
ZUBOR P, DOKUS K, ZIGO I, et al. TNF α G308A gene polymorphism has an impact on renal function, microvascular permeability, organ involvement and severity of preeclampsia[J]. Gynecologic and Obstetric Investigation, 2014, 78(3): 150-161. DOI:10.1159/000364865 |
| [32] |
CHUN J M, NHO K J, KIM H S, et al. An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-кB and MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages[J]. BMC Complementary and Alternative Medicine, 2014, 14: 234. DOI:10.1186/1472-6882-14-234 |
| [33] |
GAO X J, GUO M Y, ZHANG Z C, et al. Bergenin plays an anti-inflammatory role via the modulation of MAPK and NF-κB signaling pathways in a mouse model of LPS-induced mastitis[J]. Inflammation, 2015, 38(3): 1142-1150. DOI:10.1007/s10753-014-0079-8 |
| [34] |
LEE J Y, PARK S H, JHEE K H, et al. Tricin isolated from enzyme-treated Zizania latifolia extract inhibits IgE-mediated allergic reactions in RBL-2H3 cells by targeting the Lyn/Syk pathway[J]. Molecules, 2020, 25(9): 2084. DOI:10.3390/molecules25092084 |
| [35] |
JIANG W L, XU Y, ZHANG S P, et al. Tricin 7-glucoside protects against experimental cerebral ischemia by reduction of NF-κB and HMGB1 expression[J]. European Journal of Pharmaceutical Sciences, 2012, 45(1/2): 50-57. |
| [36] |
YUE G G L, GAO S, LEE J K M, et al. A natural flavone tricin from grains can alleviate tumor growth and lung metastasis in colorectal tumor mice[J]. Molecules, 2020, 25(16): 3730. DOI:10.3390/molecules25163730 |
| [37] |
ZHENG X J, LIU H M, MA M Q, et al. Anti-thrombotic activity of phenolic acids obtained from Salvia miltiorrhiza f. alba in TNF-α-stimulated endothelial cells via the NF-κB/JNK/p38 MAPK signaling pathway[J]. Archives of Pharmacal Research, 2021, 44(4): 427-438. DOI:10.1007/s12272-021-01325-7 |
| [38] |
FANG F, XIE Z P, QUAN J Y, et al. Baicalin suppresses Propionibacterium acnes-induced skin inflammation by downregulating the NF-κB/MAPK signaling pathway and inhibiting activation of NLRP3 inflammasome[J]. Brazilian Journal of Medical and Biological Research, 2020, 53(12): e9949. DOI:10.1590/1414-431x20209949 |
| [39] |
孙涛, 张鹏, 程葆华, 等. 辛伐他汀对大鼠坐骨神经慢性压迫性损伤模型氧化应激和炎症的影响[J]. 解剖学研究, 2019, 41(3): 173-176. SUN T, ZHANG P, CHENG B H, et al. The effect of simvastatin on the oxidative stress and inflammation after chronic constriction injury of sciatic nerve in rats[J]. Anatomy Research, 2019, 41(3): 173-176 (in Chinese). |




