2. 西华师范大学生命科学学院, 南充 637009
2. College of Life Science, China West Normal University, Nanchong 637009, China
肠道屏障是抵抗肠道中病原微生物和有毒有害物质入侵的第1道防线,对于维持机体正常功能至关重要[1]。正常肠道屏障由微生物屏障、化学屏障、机械屏障和免疫屏障共同构成。其中机械屏障是维持肠道黏膜屏障正常的结构基础,是完整的彼此紧密相连的肠黏膜上皮结构,主要包括黏膜上皮细胞及上皮细胞间的各种连接复合体[2]。紧密连接(tight junction,TJ)是肠道黏膜上皮细胞间最重要的连接复合体,构成了相邻细胞间顶端复合连接,是肠道中维持黏膜上皮细胞间机械屏障的主要结构之一。TJ包括上皮细胞侧膜的闭合蛋白(claudins)[3]、咬合蛋白(occludins)[4]以及细胞质内膜表面的闭合小环蛋白(ZOs)[5]。TJ蛋白表达和分布的改变会影响TJ的结构和功能,进而影响肠黏膜机械屏障的正常生理作用。其中,claudins主要调控细胞旁电荷的选择性来调节肠道屏障渗透性,occludins通过自身磷酸化调节TJ复合体的定位及功能,ZOs参与调节细胞内物质转运和维持上皮细胞极性,三者共同维持肠黏膜上皮机械屏障稳态[6-7]。近年来,大量研究表明,肠道微生物群代谢物可通过调节TJ相关蛋白的表达和分布,调节肠黏膜机械屏障功能[8-9]。短链脂肪酸(short-chain fatty acids,SCFAs)是由肠道菌群通过糖酵解和磷酸戊糖途径降解不宜消化的膳食纤维所产生的代谢物。SCFAs是链长为1~6个碳原子的有机脂肪酸,主要包括乙酸盐、丙酸盐和丁酸盐,其中乙酸盐是肠道中含量最丰富的SCFAs[10-11]。研究发现,SCFAs可增强动物肠上皮TJ蛋白表达,减轻由病原微生物引起的肠上皮TJ损伤,修复肠黏膜机械屏障功能[12-14]。本文就SCFAs对动物肠上皮细胞TJ的影响及其机制研究进展作一简要综述。
1 SCFAs相关作用受体SCFAs可作为一些G蛋白偶联受体(G protein-coupled receptors,GPCRs)的配体,通过激活GPCRs发挥生物学功能[15]。SCFAs激活的受体主要包括GPR41、GPR43和GPR109A,广泛存在于脂肪[16-17]、骨髓[18]、肝脏[19]、肌肉[20]、肺脏[21]、脑[22-23]、心脏和外周交感神经元[21, 24]中,在肠道中尤为丰富。Wu等[25]在GPR43敲除与野生型的C57BL/6小鼠上发现,喂食野生型而不是GPR43敲除小鼠醋酸盐,促进了独立于T细胞的肠道免疫球蛋白A(IgA)反应,维持肠道屏障功能。Tazoe等[26]研究发现,GPR41在人结肠黏膜高表达,可以被乙酸盐、丙酸盐和丁酸盐不同程度激活,是SCFAs的有效受体之一。Borthakur等[27]在人肠道C2BBe1细胞和大鼠肠道IEC-6细胞中发现,丁酸盐通过激活GPR109A调节细胞能量代谢。由此可见,GPCRs在动物各组织器官广泛表达,SCFAs通过与肠道的不同GPCRs受体结合发挥其生物学功能。
2 SCFAs对肠上皮TJ的影响肠上皮TJ处于动态过程,它的开闭受到外界饮食、神经体液信号及细菌和病毒等的影响,是决定肠道通透性的关键,而肠道通透性是反映肠道屏障完整性的重要指标[28-29]。研究表明,SCFAs通过调节TJ相关基因和蛋白的表达增强肠上皮TJ,降低肠道通透性,保证肠道机械屏障完整性(表 1)。在人Caco-2细胞上研究发现,用0.5 mmol/L乙酸盐、0.01 mmol/L丙酸盐和0.01 mmol/L丁酸盐与脂多糖共同处理Caco-2细胞24 h,可显著增加ZO-1和occludin的蛋白表达,减轻脂多糖诱导的TJ损伤,逆转细胞间通透性增大,维持肠上皮细胞正常生理功能[30]。Elamin等[31]研究发现,2 mmol/L丁酸盐、4 mmol/L丙酸盐和8 mmol/L乙酸盐预处理人Coca-2细胞1 h,显著增加ZO-1和occludin的蛋白表达,缓解乙醇诱导的TJ损伤及肠道机械屏障功能障碍。在人未成熟的肠上皮细胞系H4细胞中也发现,20 mmol/L的丁酸盐预处理30 min上调了occludin、claudin-4、claudin-11和claudin-15等TJ相关基因的mRNA表达水平,降低由白细胞介素-1β(IL-1β)诱导的该细胞TJ损伤[32]。对C57BL/6J小鼠研究发现,1%(质量体积比)丙酸盐饮水预处理1周显著抑制ZO-1和occludin的蛋白表达,有效预防葡聚糖硫酸钠诱导的细胞旁通透性增大,防止结肠上皮TJ损伤[33]。Guo等[34]通过管饲法给予高尿酸血症模型C57BL/6J小鼠9.5 g/(kg·d)菊粉7周后发现,菊粉在肠道微生物群衍生下产生的SCFAs可上调ZO-1和occludin的mRNA和蛋白表达,提高肠上皮细胞间TJ,恢复肠道机械屏障功能。在早期断奶仔猪上研究发现,饲粮添加2 000 mg/kg丁酸钠显著上调ZO-1、occludin和claudin-3的mRNA表达和occludin、claudin-3的蛋白表达,降低肠道通透性,减轻腹泻,提高仔猪生长性能[12]。综上所述,适宜浓度SCFAs可以有效缓解外界抗原刺激引起的肠上皮TJ损伤,降低肠道通透性,保护肠道机械屏障完整性。
|
|
表 1 SCFAs对肠黏膜机械屏障的影响 Table 1 Effects of SCFAs on intestinal mucosal mechanical barrier |
AMPK是生物能量代谢的关键调节因子,其激活可促进TJ蛋白的表达,从而增强肠上皮TJ,改善肠道机械屏障功能[31, 35]。SCFAs作为肠上皮细胞主要的供能物质,可通过激活AMPK参与细胞能量代谢调节。通常细胞内能量传感器AMPK是无活性的,增加单磷酸腺苷(adenosine monophosphate,AMP)/ATP比例或增加钙/钙调素依赖性蛋白激酶激酶β(calcium/calmodulin-dependent protein kinase kinase β,CaMKKβ)的活性使AMPK苏氨酸172(Thr172)上的α亚基磷酸化,进而激活AMPK[36-37]。Yan等[38]研究发现,0.1 mmol/L丁酸盐增加IPEC-J2上皮细胞AMP/ATP比例,AMPK磷酸化,提高claudin-3和claudin-4的mRNA和蛋白表达,从而增强肠上皮TJ。对断奶仔猪饲粮补充膳食纤维(海藻酸寡糖)发现,盲肠和结肠发酵产生的SCFAs使AMPK磷酸化被激活,显著增加occludin和claudin-1的mRNA表达,增强肠上皮TJ作用,提高肠道机械屏障完整性[39]。由此可见,SCFAs作为肠上皮供能物质,保证宿主上皮细胞能量代谢,通过调节AMP/ATP比例激活AMPK,增加肠上皮细胞中TJ蛋白的表达量,保证肠道机械屏障完整性。
另外,细胞内钙离子(Ca2+)浓度升高可激活CaMKKβ,调节AMPK活性[36]。研究表明,SCFAs(主要是丁酸盐)通过增加细胞内Ca2+浓度激活CaMKKβ调节AMPK活性。在人Caco-2细胞上发现,丁酸盐通过调节钙池操纵性钙通道(store-operated calcium channel,SOCC)诱导胞外Ca2+流入胞内,激活Ca2+/CaMKKβ通路介导的AMPK,使肌球蛋白轻链激酶(myosin light chain kinase,MLCK)的丝氨酸815(Ser815)磷酸化,诱导MLCK失活,进而导致肌球蛋白Ⅱ调节轻链(myosin Ⅱ regulatory light chain,MLC2)在丝氨酸19(Ser19)磷酸化水平降低,促进TJ蛋白与蛋白相互作用,调节肠上皮通透性,对维持TJ的结构和功能至关重要;此外,激活的AMPK增加蛋白激酶Cβ2(protein kinase Cβ2,PKCβ2)在丝氨酸660(Ser660)的磷酸化水平,进一步发挥促进TJ重组的作用[40-42]。综上所述,适宜水平的SCFAs作为肠上皮细胞的供能物质,可通过改变细胞内AMP和ATP的水平或通过调节细胞内Ca2+浓度在不同程度上激活AMPK的表达,加强肠道上皮细胞TJ,保证肠机械屏障完整性(图 1)。
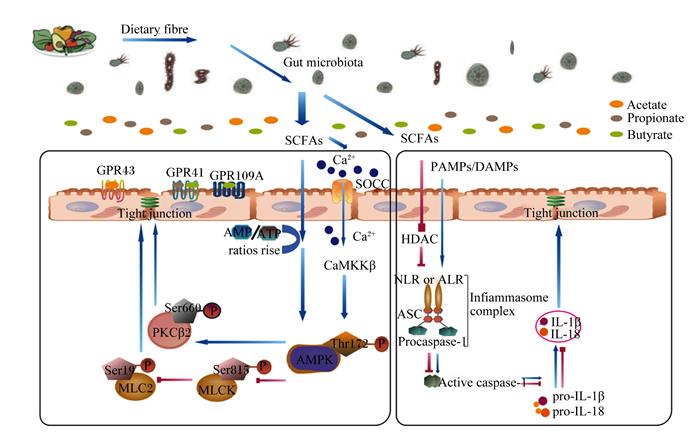
|
Dietary fibre:膳食纤维;Gut microbiota:肠道微生物;SCFAs:短链脂肪酸 short-chain fatty acids;Acetate:乙酸盐;Propionate:丙酸盐;Butyrate:丁酸盐;GPR43:G蛋白偶联受体43 G protein-coupled receptor 43;GPR41:G蛋白偶联受体41 G protein-coupled receptor 41;GPR109A:G蛋白偶联受体109A G protein-coupled receptor 109A;Ca2+:钙离子 calcium ion;SOCC:钙池操纵性钙通道 store-operated calcium channel;CaMKKβ:钙/钙调素依赖性蛋白激酶激酶 β calcium/calmodulin-dependent protein kinase kinase β;AMPK:单磷酸腺苷活化蛋白激酶 adenosine monophosphate activated protein kinase;PKCβ2:蛋白激酶Cβ2 protein kinase Cβ2;MLCK:肌球蛋白轻链激酶 myosin light chain kinase;MLC2:肌球蛋白Ⅱ调节轻链 myosin Ⅱ regulatory light chain; PAMPs:病原体相关分子模式 pathogen-associated molecular patterns;DAMPs:损伤相关的分子模式 damage-associated molecular patterns;HDAC:组蛋白去乙酰化酶 histone deacetylase;NLR:NOD样受体蛋白 NOD-like receptors;ALR:AIM2样受体蛋白 AIM2-like receptor;ASC:凋亡相关斑点样蛋白 apoptosis-associated speck-like protein containing CARD;procaspase-1:半胱天冬蛋白酶-1前体 cysteinyl aspartate specific proteinase-1 precursor;pro-IL-1β:白细胞介素-1β前体 interleukin-1β precursor;pro-IL-18:白细胞介素-18前体 interleukin-18 precursor;Tight junction:紧密连接。 图 1 SCFAs对肠上皮TJ的可能作用机制 Fig. 1 Potential mechanism of SCFAs on intestinal epithelial TJ |
炎症小体又称炎性小体或炎性体,是能够识别病原体和外界刺激的细胞质内多聚体蛋白复合物,可响应细胞扰动而组装。该组装会导致半胱天冬蛋白酶-1(cysteinyl aspartate specific proteinase-1,Caspase-1)激活,从而促进炎症细胞因子IL-1β和白细胞介素-18(IL-18)前体的成熟和释放,炎症细胞因子的过渡激活和释放最终导致炎症的发生并损伤肠上皮TJ破坏肠道屏障功能[43-44]。小鼠模型表明,外界刺激核苷酸结合寡聚化结构域样受体蛋白(nucleotide-binding oligomerization domain-like receptor protein,NLRP)3炎症小体活化产生的炎症细胞因子IL-1β的表达是诱导炎症发生造成肠上皮TJ损伤的重要原因[45-46]。因此,有效防止炎症小体的异常激活是保证肠上皮TJ完整性的关键。SCFAs作为肠道微生物代谢的主要产物,在抑制炎症小体活化维持肠上皮TJ中发挥着关键作用。研究表明,腹腔注射300 mg/kg丁酸钠溶液可有效抑制重度烫伤C57BL/6小鼠肠黏膜NLRP3炎症小体的激活,减少IL-1β和IL-18的产生,上调TJ蛋白ZO-1、occludin、claudin-1和claudin-2的表达,改善TJ蛋白ZO-1的分布异常,保护肠道机械屏障功能[47]。Tye等[48]发现,炎症小体NLRP1大量激活会增加IL-18的产生,可通过限制肠道中有益的产丁酸盐梭菌来加重葡聚糖硫酸钠诱导的C57BL/6小鼠结肠炎,而在补充2%(质量体积比)丁酸盐后可以缓解小鼠结肠炎的发生。因此,适宜浓度的SCFAs可通过抑制炎症小体的活性减轻肠道炎症,提高肠道屏障功能。SCFAs可作为组蛋白去乙酰化酶(histone deacetylase,HDAC)抑制剂,抑制炎症小体过度激活防止炎症产生,增强肠上皮TJ并维持肠道机械屏障完整性。研究表明,0.5 mmol/L醋酸盐、0.01 mmol/L丙酸盐和0.01 mmol/L丁酸盐单独或组合处理可抑制HDAC,显著抑制人Caco-2细胞中的NLRP3炎症小体的过度激活,并显著增加ZO-1和occludin的蛋白表达水平,提高肠上皮TJ,保护肠道机械屏障完整性[30]。Beisner等[49]研究也发现,适宜浓度丁酸盐作为HDAC的有效抑制剂可有效缓解C57BL/6小鼠结肠炎症损伤,并显著增加结肠中ZO-1、occludin、claudin-2和claudin-5的mRNA表达水平,增强肠上皮TJ,改善肠道机械屏障功能。综上所述,适宜浓度的SCFAs可作为HDAC抑制剂,从而抑制炎症小体活性,减少炎症细胞因子的发生,提高肠上皮细胞间TJ,保护肠道机械屏障完整性(图 1)。
4 小结综上所述,SCFAs可增强肠道上皮细胞间TJ并保护肠黏膜机械屏障完整性,其发挥作用的主要机制是分别作为供能物质和调节细胞内Ca2+浓度激活AMPK信号通路;作为HDAC抑制剂抑制炎症小体活化等。在常规畜禽养殖中,SCFAs不仅维持肠上皮TJ,而且对肠道屏障功能也有一定的调节作用,但其发挥具体作用是否与肠道微生物存在必然联系需要进一步探讨。此外,SCFAs对肠上皮TJ的影响在部分畜禽中研究较少,具体作用机制仍尚不明确。因此,还需要进一步广泛深入研究SCFAs在常规畜禽中的具体作用方式,以便为肠道疾病的预防和治疗提供参考。
| [1] |
BJARNASON I, MACPHERSON A, HOLLANDER D. Intestinal permeability: an overview[J]. Gastroenterology, 1995, 108(5): 1566-1581. DOI:10.1016/0016-5085(95)90708-4 |
| [2] |
REN Z H, GUO C Y, YU S M, et al. Progress in mycotoxins affecting intestinal mucosal barrier function[J]. International Journal of Molecular Sciences, 2019, 20(11): 2777. DOI:10.3390/ijms20112777 |
| [3] |
FURUSE M, FUJITA K, HⅡRAGI T, et al. Claudin-1 and -2:novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin[J]. Journal of Cell Biology, 1998, 141(7): 1539-1550. DOI:10.1083/jcb.141.7.1539 |
| [4] |
FURUSE M, HIRASE T, ITOH M, et al. Occludin: a novel integral membrane protein localizing at tight junctions[J]. Journal of Cell Biology, 1993, 123(Pt.2): 1777-1788. |
| [5] |
WITTCHEN E S, HASKINS J, STEVENSON B R. Protein interactions at the tight junction.Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3[J]. Journal of Biological Chemistry, 1999, 274(49): 35179-35185. DOI:10.1074/jbc.274.49.35179 |
| [6] |
王希, 廖吕钊, 江荣林. 肠上皮细胞紧密连接蛋白的结构功能及其调节[J]. 浙江医学, 2018, 40(8): 895-898. WANG X, LIAO L Z, JIANG R L. Structural function and regulation of tight junction proteins in intestinal epithelial cells[J]. Zhejiang Medical Journal, 2018, 40(8): 895-898 (in Chinese). |
| [7] |
STURGEON C, FASANO A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases[J]. Tissue Barriers, 2016, 4(4): e1251384. DOI:10.1080/21688370.2016.1251384 |
| [8] |
SINGH R, CHANDRASHEKHARAPPA S, BODDULURI S R, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway[J]. Nature Communications, 2019, 10(1): 89. DOI:10.1038/s41467-018-07859-7 |
| [9] |
LI J J, ZHANG L, WU T, et al. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier[J]. Journal of Agricultural and Food Chemistry, 2021, 69(5): 1487-1495. DOI:10.1021/acs.jafc.0c05205 |
| [10] |
MILLER T L, WOLIN M J. Fermentations by saccharolytic intestinal bacteria[J]. The American Journal of Clinical Nutrition, 1979, 32(1): 164-172. DOI:10.1093/ajcn/32.1.164 |
| [11] |
CANI P D, EVERARD A, DUPARC T. Gut microbiota, enteroendocrine functions and metabolism[J]. Current Opinion in Pharmacology, 2013, 13(6): 935-940. DOI:10.1016/j.coph.2013.09.008 |
| [12] |
FENG W Q, WU Y C, CHEN G X, et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner[J]. Cellular Physiology and Biochemistry, 2018, 47(4): 1617-1629. DOI:10.1159/000490981 |
| [13] |
YUE X, WEN S, LONG-KUN D, et al. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction[J]. BMC Immunology, 2022, 23(1): 19. DOI:10.1186/s12865-022-00495-3 |
| [14] |
JI C L, LU F H, WU Y C, et al. Rhubarb enema increasing short-chain fatty acids that improves the intestinal barrier disruption in CKD may be related to the regulation of gut dysbiosis[J]. BioMed Research International, 2022, 2022: 1896781. |
| [15] |
PINGITORE A, GONZALEZ-ABUIN N, RUZ-MALDONADO I, et al. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: role of free fatty acid receptor 2[J]. Diabetes, Obesity and Metabolism, 2019, 21(2): 330-339. DOI:10.1111/dom.13529 |
| [16] |
IVÁN J, MAJOR E, SIPOS A, et al. The short-chain fatty acid propionate inhibits adipogenic differentiation of human chorion-derived mesenchymal stem cells through the free fatty acid receptor 2[J]. Stem Cells and Development, 2017, 26(23): 1724-1733. DOI:10.1089/scd.2017.0035 |
| [17] |
JOCKEN J W E, GONZÁLEZ HERNÁNDEZ M A, HOEBERS N T H, et al. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model[J]. Frontiers in Endocrinology, 2018, 8: 372. DOI:10.3389/fendo.2017.00372 |
| [18] |
TOLHURST G, HEFFRON H, LAM Y S, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2[J]. Diabetes, 2012, 61(2): 364-371. DOI:10.2337/db11-1019 |
| [19] |
DEN BESTEN G, LANGE K, HAVINGA R, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2013, 305(12): G900-G910. DOI:10.1152/ajpgi.00265.2013 |
| [20] |
PLUZNICK J L, PROTZKO R J, GEVORGYAN H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(11): 4410-4415. DOI:10.1073/pnas.1215927110 |
| [21] |
KIMURA I, ICHIMURA A, OHUE-KITANO R, et al. Free fatty acid receptors in health and disease[J]. Physiological Reviews, 2020, 100(1): 171-210. DOI:10.1152/physrev.00041.2018 |
| [22] |
MISHRA S P, KARUNAKAR P, TARAPHDER S, et al. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view[J]. Biomedicines, 2020, 8(6): 154. DOI:10.3390/biomedicines8060154 |
| [23] |
BINDELS L B, PORPORATO P E, DUCASTEL S, et al. Ffar2 expression regulates leukaemic cell growth in vivo[J]. British Journal of Cancer, 2017, 117(9): 1336-1340. DOI:10.1038/bjc.2017.307 |
| [24] |
KIMURA I, INOUE D, MAEDA T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(19): 8030-8035. DOI:10.1073/pnas.1016088108 |
| [25] |
WU W, SUN M, CHEN F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43[J]. Mucosal Immunology, 2017, 10(4): 946-956. DOI:10.1038/mi.2016.114 |
| [26] |
TAZOE H, OTOMO Y, KARAKI S I, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon[J]. Biomedical Research, 2009, 30(3): 149-156. DOI:10.2220/biomedres.30.149 |
| [27] |
BORTHAKUR A, PRIYAMVADA S, KUMAR A, et al. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2012, 303(10): G1126-G1133. DOI:10.1152/ajpgi.00308.2012 |
| [28] |
PARADA VENEGAS D, DE LA FUENTE M K, LANDSKRON G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases[J]. Frontiers in Immunology, 2019, 10: 277. DOI:10.3389/fimmu.2019.00277 |
| [29] |
谢婷, 高峻, 李兆申. 肠道通透性研究进展[J]. 国际消化病杂志, 2008, 28(2): 145-147. XIE T, GAO J, LI Z S. Progress in intestinal permeability[J]. International Journal of Digestive Diseases, 2008, 28(2): 145-147 (in Chinese). |
| [30] |
FENG Y H, WANG Y, WANG P, et al. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy[J]. Cellular Physiology and Biochemistry, 2018, 49(1): 190-205. DOI:10.1159/000492853 |
| [31] |
ELAMIN E E, MASCLEE A A, DEKKER J, et al. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers[J]. The Journal of Nutrition, 2013, 143(12): 1872-1881. DOI:10.3945/jn.113.179549 |
| [32] |
GAO Y N, DAVIS B, ZHU W S, et al. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2021, 320(4): G521-G530. DOI:10.1152/ajpgi.00279.2020 |
| [33] |
TONG L C, WANG Y, WANG Z B, et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress[J]. Frontiers in Pharmacology, 2016, 7: 253. |
| [34] |
GUO Y J, YU Y N, LI H L, et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice[J]. European Journal of Nutrition, 2021, 60(4): 2217-2230. DOI:10.1007/s00394-020-02414-x |
| [35] |
HARDIE D G, HAWLEY S A. AMP-activated protein kinase: the energy charge hypothesis revisited[J]. BioEssays, 2001, 23(12): 1112-1119. DOI:10.1002/bies.10009 |
| [36] |
OAKHILL J S, STEEL R, CHEN Z P, et al. AMPK is a direct adenylate charge-regulated protein kinase[J]. Science, 2011, 332(6036): 1433-1435. DOI:10.1126/science.1200094 |
| [37] |
ANAND S K, SHARMA A, SINGH N, et al. Activation of autophagic flux via LKB1/AMPK/mTOR axis against xenoestrogen Bisphenol-A exposure in primary rat hepatocytes[J]. Food and Chemical Toxicology, 2020, 141: 111314. DOI:10.1016/j.fct.2020.111314 |
| [38] |
YAN H, AJUWON K M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway[J]. PLoS One, 2017, 12(6): e0179586. DOI:10.1371/journal.pone.0179586 |
| [39] |
WAN J, ZHANG J, CHEN D W, et al. Alterations in intestinal microbiota by alginate oligosaccharide improve intestinal barrier integrity in weaned pigs[J]. Journal of Functional Foods, 2020, 71: 104040. DOI:10.1016/j.jff.2020.104040 |
| [40] |
MIAO W, WU X J, WANG K, et al. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2[J]. International Journal of Molecular Sciences, 2016, 17(10): 1696. DOI:10.3390/ijms17101696 |
| [41] |
PENG L Y, LI Z R, GREEN R S, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers[J]. The Journal of Nutrition, 2009, 139(9): 1619-1625. DOI:10.3945/jn.109.104638 |
| [42] |
CUNNINGHAM K E, TURNER J R. Myosin light chain kinase: pulling the strings of epithelial tight junction function[J]. Annals of the New York Academy of Sciences, 2012, 1258(1): 34-42. DOI:10.1111/j.1749-6632.2012.06526.x |
| [43] |
SHARMA B R, KANNEGANTI T D. NLRP3 inflammasome in cancer and metabolic diseases[J]. Nature Immunology, 2021, 22(5): 550-559. DOI:10.1038/s41590-021-00886-5 |
| [44] |
SONG M Y, WANG J X, SUN Y L, et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages[J]. Acta Pharmacologica Sinica, 2022, 43(5): 1274-1284. DOI:10.1038/s41401-021-00693-6 |
| [45] |
ZHANG B W, XU Y C, ZHAO C Y, et al. Protective effects of bioactive peptides in foxtail millet protein hydrolysates against experimental colitis in mice[J]. Food & Function, 2022, 13(5): 2594-2605. |
| [46] |
LI X, YANG C B, GULIFEIRE T, et al. Ulinastatin protects intestinal mucosal barrier by inhibiting the activation of intestinal NLRP3 inflammasomes in septic rats[J]. Chinese Critical Care Medicine|Chin Crit Care Med, 2021, 33(2): 192-197. |
| [47] |
梁晶冰, 王裴, 冯燕海, 等. 丁酸钠对严重烫伤小鼠肠道屏障的作用与相关机制[J]. 中华烧伤杂志, 2020, 36(1): 48-53. LIANG J B, WANG P, FENG Y H, et al. Effects of sodium butyrate on intestinal barrier of severe scald mice and the related mechanism[J]. Chinese Journal of Burns, 2020, 36(1): 48-53 (in Chinese). |
| [48] |
TYE H, YU C H, SIMMS L A, et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease[J]. Nature Communications, 2018, 9(1): 3728. DOI:10.1038/s41467-018-06125-0 |
| [49] |
BEISNER J, FILIPE ROSA L, KADEN-VOLYNETS V, et al. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides[J]. Frontiers in Immunology, 2021, 12: 678360. DOI:10.3389/fimmu.2021.678360 |




