2. 湖南农业大学动物科技学院, 长沙 410128;
3. 中国科学院亚热带农业生态研究所, 长沙 410125
2. College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China;
3. Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China
呕吐毒素,又名脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON),是由禾谷镰刀菌(F. graminearum)产生的次级代谢产物[1-5]。这些镰刀菌在全世界范围内广泛的分布,主要污染谷物与饲料,如玉米、小麦和大麦[1, 6-7]。人和动物摄入被DON污染的食物后,会产生一系列不良的反应,如呕吐、体重减轻、免疫毒性和血液生化指标的变化[8-9]。在细胞水平的研究表明,DON能诱导巨噬细胞和单核细胞中丝裂原活化蛋白激酶(MAPK)的激活,促进炎性细胞因子释放,最终导致细胞凋亡[10-13]。仔猪动物试验研究表明,DON首先攻击肠道,诱导核转录因子-κB(NF-κB)激活,触发胞内活性氧与许多免疫相关炎性因子过量释放,导致肠黏膜充血、水肿,绒毛萎缩与隐窝细胞坏死或凋亡,抑制紧密连接蛋白的合成,引起肠道通透性增加,最终导致仔猪生长性能降低与血液生理生化指标变化,甚至死亡[14-16]。
目前,通过外源添加酵母细胞壁、寡糖、益生菌、功能性氨基酸、中药提取物等均可以在一定程度上缓解DON导致的仔猪生长抑制与生理生化指标紊乱[12, 16-20]。杜仲是湖南湘西地区重要的植物资源,含有大量抗氧化活性物质,其中黄酮类化合物是其主要的抗氧化成分。研究表明,杜仲黄酮(Eucommia ulmoides flavones, EUF)能通过调节白细胞介素-2(IL-2)和肿瘤坏死因子-α(TNF-α)等细胞因子的分泌,增强荷瘤小鼠免疫功能,提高机体抗氧化能力[21]。在敌草快诱导的仔猪氧化应激模型中,EUF能显著提高仔猪生长性能,降低肠道氧化应激损伤与炎症反应[22]。根据文献报道,我们推测EUF能缓解DON导致的仔猪生长性能抑制和生理生化指标紊乱。为验证此推测,本试验就EUF对DON诱导的断奶仔猪生长性能、脏器指数、血液抗氧化功能与肠道免疫炎性因子释放的干预效应进行研究,以期为指导仔猪健康生产提供参考。
1 材料与方法 1.1 试验材料F. graminearum R6576菌株由华中农业大学廖玉才教授研究组提供。杜仲黄酮由吉首大学医学院提取制备,以芦丁为校准标准,采用紫外分光光度法测定了EUF粉中总黄酮的含量,其含量为83.61%[22]。AgraQuant DON酶联免疫吸附检测(ELISA)试剂盒购自ROMER公司。
1.2 霉变玉米准备与处理将保存于PDA斜面中的F. graminearum R6576菌株接种于PDA平板上,25~28 ℃黑暗条件下活化培养5 d,再将菌丝接种于CMC液体培养基中,用接种针将菌丝打碎,持续光照诱导条件下置于摇床上振荡培养(28 ℃,200 r/min)5 d,产生分生孢子后,用无菌双层纱布过滤,用血球计数板计数,将分生孢子的浓度调为5×105个/mL左右接种于玉米粉中,进行14 d的发酵培养[12]。将霉变玉米与基础饲粮按照1 ∶ 1比例均匀混合。整个试验周期60 d,共处理饲粮4次,每次每组取样3份进行DON浓度的测定。DON浓度测定方法参考AgraQuant DON酶联免疫检测试剂盒检测说明书进行检测。
1.3 试验动物与饲养管理选择初始体重(6.50±0.28) kg的健康三元杂交(杜×长×大)断奶仔猪24头,随机分为3组,每组8头,每头为1个重复。3个组分别为对照组(Control组,基础饲粮)、DON组(饲粮中含1.5 mg/kg DON)、DON+EUF组(饲粮中含1.5 mg/kg DON和100 mg/kg EUF)。DON和EUF的剂量参考文献[22-23]。试验仔猪全部单栏饲养,自由采食与饮水,预试期7 d,正试期21 d。试验期间每天准确记录每头猪的实际采食量,观察猪群整体健康状况。基础饲粮参考NRC(2012)标准进行配制,其组成及营养水平见表 1。
|
|
表 1 基础饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal diet (air-dry basis) |
在试验第21天晨饲前每组随机选取6头仔猪进行前腔静脉采血。使用医用真空促凝一次性采血管采集血液10 mL,室温静置2 h后以3 000 r/min离心10 min制备血清,分装于1.5 mL灭菌离心管中,用于后续血清生化指标、细胞因子含量和抗氧化指标的检测。采血后电击致死,剖开腹腔,分离心脏、肝脏、脾脏和肾脏等主要脏器,剥离器官表面黏附的脂肪,用0.9%生理盐水洗净器官表面血渍并用滤纸吸干表面水分,最后用电子分析天平进行准确称量。
1.5 指标测定及方法 1.5.1 生长性能与脏器指数在试验第1天和第21天对所有试验断奶仔猪进行空腹称重,记录为初始体重和终末体重,并计算得出平均日增重(ADG)。记录21 d全程试验期间的采食量,计算平均日采食量(ADFI)。根据ADG和ADFI计算料重比(F/G)。各脏器相对指数按照如下公式计算:
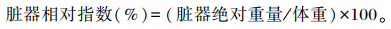
|
用Beckman公司的CX4型全自动血液生化分析仪进行血清白蛋白(ALB)含量与碱性磷酸酶(ALP)、谷丙转氨酶(ALT)、谷草转氨酶(AST)活性测定。
1.5.3 血清抗氧化指标测定血清超氧化物歧化酶(SOD)活性采用黄嘌呤氧化酶法测定,丙二醛(MDA)含量采用比色法测定,谷胱甘肽过氧化物酶(GSH-Px)活性采用化学比色法测定,总抗氧化能力(T-AOC)采用铁还原/抗氧化反应法测定,活性氧(ROS)含量采用化学荧光法测定。所有试剂盒均购自于南京建成生物工程研究所,具体测定方法均按试剂盒说明书进行。
1.5.4 血清细胞因子含量测定采用ELISA法测定血清白细胞介素-6(IL-6)、白细胞介素-1β(IL-1β)和肿瘤坏死因子-α(TNF-α)含量。所有试剂盒均购自于南京建成生物工程研究所,具体测定方法均按试剂盒说明书进行。
1.6 数据统计与分析先用Excel 2013对试验数据进行初步处理,再采用SPSS 19.0统计分析软件进行单因素方差分析(one-way ANOVA)和Duncan氏法多重比较。结果以平均值和均值标准误(SEM)表示,P < 0.05为差异显著。
2 结果与分析 2.1 添加EUF对饲喂DON污染饲粮断奶仔猪生长性能和脏器相对指数的影响由表 2可知,与Control组相比,DON组的终末体重、ADG和ADFI显著降低(P < 0.05),F/G显著增加(P < 0.05),DON+EUF组的终末体重、ADG、ADFI和F/G与对照组均无显著差异(P>0.05)。
|
|
表 2 添加EUF对饲喂DON污染饲粮断奶仔猪生长性能的影响 Table 2 Effects of EUF supplementation on growth performance of weaned piglets fed DON-contaminated diet (n=6) |
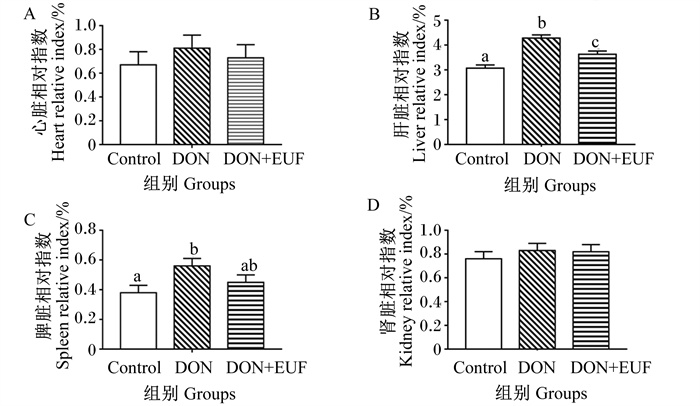
由图 1可知,与Control组相比,DON组的肝脏和脾脏相对指数显著升高(P < 0.05),其余脏器相对指数无显著变化(P>0.05)。DON+EUF组的肝脏相对指数虽较DON组显著降低(P < 0.05),但仍显著高于Control组(P < 0.05)。
|
数据柱形标注不同小写字母表示差异显著(P < 0.05),无字母标注表示差异不显著。 Value columns with different small letters mean significant difference (P < 0.05), while with no letter mean no significant difference (P>0.05). 图 1 添加EUF对饲喂DON污染饲粮断奶仔猪脏器相对指数的影响 Fig. 1 Effects of EUF supplementation on organ relative indexes of weaned piglets fed DON-contaminated diet (n=6) |
由表 3可知,与Control组相比,DON组的血清ALP、ALT和AST活性显著提高(P < 0.05);DON+EUF组的血清AST活性显著提高(P < 0.05),血清ALP和ALT活性无显著变化(P < 0.05)。此外,DON+EUF组的血清ALP活性显著低于DON组(P < 0.05)。
|
|
表 3 添加EUF对饲喂DON污染饲粮断奶仔猪血清生化指标的影响 Table 3 Effects of EUF supplementation on serum biochemical indexes of weaned piglets fed DON-contaminated diet (n=6) |
由表 4可知,与Control组相比,DON组血清SOD、GSH-Px活性和T-AOC显著降低(P < 0.05),而血清MDA和ROS含量显著提高(P < 0.05);与DON组相比,DON+EUF组血清SOD、GSH-Px活性和T-AOC显著提高(P < 0.05),血清MDA和ROS含量显著降低(P < 0.05)。
|
|
表 4 添加EUF对饲喂DON污染饲粮断奶仔猪血清抗氧化指标的影响 Table 4 Effects of EUF supplementation on serum antioxidant indexes of weaned piglets fed DON-contaminated diet (n=6) |
由表 5可知,与Control组相比,DON组血清中IL-6、IL-1β和TNF-α含量显著提高(P < 0.05);与DON组相比,DON+EUF组血清中IL-6、IL-1β和TNF-α含量显著降低(P < 0.05)。
|
|
表 5 添加EUF对饲喂DON污染饲粮断奶仔猪血清细胞因子含量的影响 Table 5 Effects of EUF supplementation on serum cytokine contents of weaned piglets fed DON-contaminated diet (n=6) |
传统中药在替抗与缓解DON导致的生长抑制方面扮演着重要的角色[15-16, 22]。EUF是从杜仲中提取的黄酮类化合物,在替抗和促进动物生长中表现出一定的优势。研究表明,0.01%EUF作为抗生素生长促进剂的潜在替代品,可以改善断奶仔猪的生长性能和肠道形态,减少大肠菌群的定植和腹泻指数[24];同时,100 mg/kg EUF能显著提高敌草快诱导的仔猪生长性能降低[22]。镰刀菌毒素污染饲粮会导致断奶仔猪肝脏和脾脏的损伤[25]。同时,跟EUF类似的黄酮类中药黄芩苷、黄芩苷锌络合物和黄芩苷铜络合物在缓解DON导致的生长抑制和脏器损伤方面也表现出一定的积极作用[26-29]。本研究中DON造成断奶仔猪终末体重、ADG和ADFI显著降低,且肝脏和脾脏相对指数显著提高,而添加EUF能够在一定程度上缓解DON诱导的断奶仔猪生长性能的降低与脏器相对指数的升高。尽管本研究表明EUF在缓解DON诱导的仔猪生长性能降低方面具有一定的效果,但是由于本试验的样本量还是太小,在未来的研究中应该进行大规模饲养试验,以验证EUF作为断奶仔猪生长促进剂的潜力。
3.2 EUF对饲喂DON污染饲粮断奶仔猪血清生化指标的影响血液生理生化指标与代谢、营养及健康状况有着密切的联系,是反映动物机体生理机能的重要指标,也是临床医疗和科学研究的重要依据。机体血清ALP、AST和ALT活性在一定程度上可以反映肝脏功能情况。有研究表明,DON能够增加仔猪血清ALP的活性,从而加重DON诱导的全身毒性,导致肝脏代谢产物的异常排泄[30]。据报道,血清AST和ALT活性是肝脏损伤的敏感指标,其活性增加临床上反映了受损肝细胞的渗漏[11]。研究表明,饲粮中添加0.01%EUF能够显著改善抗生素导致的断奶仔猪血清ALP、ALT和AST活性变化,促进仔猪健康生长[24]。与前人的研究结果一致,本研究中DON能导致肝脏功能变化,使肝细胞向血液中释放更多的ALP、ALT和AST,而DON+EUF组血清ALP和AST活性显著低于DON组,表明饲粮添加EUF能够改善DON导致的肝脏功能变化,减少肝细胞分泌ALP和AST,有利于缓解断奶仔猪肝脏功能。与Control组相比,DON组的血清AST和ALT活性显著升高,表明这种损伤机制是在摄入DON后触发的,其结果与肝脏相对指数的变化是一致的。
3.3 EUF对饲喂DON污染饲粮断奶仔猪血清抗氧化指标的影响黄酮类化合物的抗氧化机制是通过抑制氧化酶活性、上调或下调抗氧化保护屏障以及清除细胞内的ROS来实现的[22, 26]。DON可以刺激细胞或动物体内产生ROS,并抑制抗氧化酶(如SOD和GSH-Px)的活性[11-12, 17]。在正常情况下,抗氧化酶在体内处于动态平衡状态[26]。有许多类型的抗氧化酶,包括SOD、GSH-Px和CAT,通过这些酶,SOD将超氧自由基转化为过氧化氢(H2O2),H2O2通过GSH-Px和CAT转化为水[27]。氧化应激源于抗氧化酶不能清除自由基,导致机体内自由基失去平衡。黄酮类化合物抗氧化能力的机制包括通过抑制参与其产生的酶、清除ROS、上调或保护抗氧化防御来抑制ROS的形成[22]。DON可刺激细胞产生ROS,抑制SOD和GSH-Px等抗氧化酶的活性[6, 15-17, 26]。研究表明,外源性添加EUF、白藜芦醇、表没食子儿茶素没食子酸酯等多种抗氧化中药成分,重建机体组织的氧化还原平衡,是缓解DON所致肠道氧化应激损伤的有效途径之一[22, 26, 28-29]。与前人的研究结果一致,本研究中DON能显著降低仔猪血清SOD和GSH-Px活性,而DON+EUF组显著降低了仔猪血清MDA和ROS含量,表明外源添加EUF能通过增加血液中抗氧化酶活性来中和DON导致的ROS失衡,从而显著降低DON诱导的机体氧化应激,促进仔猪体内氧化还原平衡。
3.4 EUF对饲喂DON污染饲粮断奶仔猪血清细胞因子含量的影响炎性因子分泌水平的高低是反映机体炎症损伤程度的生理指标之一[25-26]。氧化应激和炎症之间的关系已经被以前许多的研究所证明[8, 14-15, 17, 22, 29-30]。研究表明,DON能诱导NF-κB的激活,触发炎症反应,选择性的诱导一系列细胞因子、趋化因子以及其他免疫相关炎性因子mRNA的上调[11-12, 16-17, 26]。NF-κB信号通路在机体抗氧化应激和抗炎中起着重要作用,相关研究也证实了两者之间的相关性[30-35]。同时也有研究表明,外源添加EUF、精氨酸、黄芩苷、盐酸小檗碱等能缓解DON导致的炎性因子分泌增加,包含显著降低小肠和血清中白细胞介素-8(IL-8)、IL-1β、IL-6和IFN-γ的蛋白质和基因表达水平,最终提高动物肠道或机体的健康水平[12, 16, 22, 26, 29]。有研究表明,肠道和血清中过量的抗氧化酶可能与相应部位的促炎和抗炎细胞因子的产生有关[18, 22]。有证据表明,自由基可能作为二级信使刺激促炎细胞因子分泌[17]。当然,不同的动物模型、不同的中药提取物和不同的喂养时间导致细胞因子的基因和蛋白质表达水平不一致[15]。与前人的研究结果一致,本研究中DON组仔猪血清中IL-6、IL-1β和TNF-α含量较Control组显著提高,而DON+EUF组血清中IL-6、IL-1β和TNF-α含量则较DON组显著降低,表明外源添加EUF能显著降低了DON诱导的炎性因子分泌,减轻了仔猪机体炎性损伤。
4 结论饲粮中添加1.5 mg/kg DON能导致断奶仔猪生长抑制,肝脏和脾脏病变,出现氧化应激和炎性损伤,而添加EUF能缓解DON诱导的断奶仔猪的生长抑制与肝脏、脾脏增生以及抗氧化能和抗炎能力的降低,对缓解DON诱导的毒害作用具有一定的效果。
| [1] |
MISHRA S, SRIVASTAVA S, DEWANGAN J, et al. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey[J]. Critical Reviews in Food Science and Nutrition, 2020, 60(8): 1346-1374. DOI:10.1080/10408398.2019.1571479 |
| [2] |
GRUBER-DORNINGER C, JENKINS T, SCHATZMAYR G. Global mycotoxin occurrence in feed: a ten-year survey[J]. Toxins, 2019, 11(7): 375. DOI:10.3390/toxins11070375 |
| [3] |
WU L, LI J J, LI Y H, et al. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from different province in China[J]. Journal of Animal Science and Biotechnology, 2016, 7: 63. DOI:10.1186/s40104-016-0122-8 |
| [4] |
ZHANG J, QIN X J, GUO Y P, et al. Enzymatic degradation of deoxynivalenol by a novel bacterium, Pelagibacterium halotolerans ANSP101[J]. Food and Chemical Toxicology, 2020, 140: 111276. DOI:10.1016/j.fct.2020.111276 |
| [5] |
LIU J, SUN L H, ZHANG J C, et al. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from central China[J]. Food Additives & Contaminants.Part B, Surveillance, 2016, 9(2): 91-97. |
| [6] |
LEE S Y, WOO S Y, TIAN F, et al. Occurrence of deoxynivalenol, nivalenol, and their glucosides in Korean market foods and estimation of their population exposure through food consumption[J]. Toxins, 2020, 12(2): 89. DOI:10.3390/toxins12020089 |
| [7] |
LIU Y P, LU Y, WANG L Y, et al. Occurrence of deoxynivalenol in wheat, Hebei Province, China[J]. Food Chemistry, 2016, 197(Pt.B): 1271-1274. |
| [8] |
PAYROS D, MÉNARD S, LAFFITTE J, et al. The food contaminant, deoxynivalenol, modulates the Thelper/Treg balance and increases inflammatory bowel diseases[J]. Archives of Toxicology, 2020, 94(9): 3173-3184. DOI:10.1007/s00204-020-02817-z |
| [9] |
黄凯, 黄明明, 朱祖贤, 等. 呕吐毒素毒性研究进展[J]. 饲料博览, 2013(12): 8-11. HUANG K, HUANG M M, ZHU Z X, et al. Research progress on toxicity of DON[J]. Feed Review, 2013(12): 8-11 (in Chinese). |
| [10] |
HOOFT J M, BUREAU D P. Deoxynivalenol: mechanisms of action and its effects on various terrestrial and aquatic species[J]. Food and Chemical Toxicology, 2021, 157: 112616. DOI:10.1016/j.fct.2021.112616 |
| [11] |
WU L, LIAO P, HE L Q, et al. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)-challenged growing pigs[J]. BMC Veterinary Research, 2015, 11: 144. DOI:10.1186/s12917-015-0449-y |
| [12] |
WU L, LIAO P, HE L Q, et al. Dietary L-arginine supplementation protects weanling pigs from deoxynivalenol-induced toxicity[J]. Toxins, 2015, 7(4): 1341-1354. DOI:10.3390/toxins7041341 |
| [13] |
LIAO P, LIAO M F, LI L, et al. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells[J]. Toxicology Research, 2017, 6(6): 866-877. DOI:10.1039/C7TX00202E |
| [14] |
KANG R F, LI R N, DAI P Y, et al. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production[J]. Environmental Pollution, 2019, 251: 689-698. DOI:10.1016/j.envpol.2019.05.026 |
| [15] |
HOLANDA D M, KIM S W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol[J]. Toxins, 2021, 13(2): 171. DOI:10.3390/toxins13020171 |
| [16] |
TANG M, YUAN D X, LIAO P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets[J]. Environmental Pollution, 2021, 289: 117865. DOI:10.1016/j.envpol.2021.117865 |
| [17] |
WANG S, WU K T, XUE D F, et al. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: insights from mitochondrial dysfunction[J]. Food and Chemical Toxicology, 2021, 153: 112214. DOI:10.1016/j.fct.2021.112214 |
| [18] |
JIA R, SADIQ F A, LIU W B, et al. Protective effects of Bacillus subtilis ASAG 216 on growth performance, antioxidant capacity, gut microbiota and tissues residues of weaned piglets fed deoxynivalenol contaminated diets[J]. Food and Chemical Toxicology, 2021, 148: 111962. DOI:10.1016/j.fct.2020.111962 |
| [19] |
YANG Y X, YU S, JIA B X, et al. Metabolomic profiling reveals similar cytotoxic effects and protective functions of quercetin during deoxynivalenol- and 15-acetyl deoxynivalenol-induced cell apoptosis[J]. Toxicology in Vitro, 2020, 66: 104838. DOI:10.1016/j.tiv.2020.104838 |
| [20] |
HOLANDA D M, YIANNIKOURIS A, KIM S W. Investigation of the efficacy of a postbiotic yeast cell wall-based blend on newly-weaned pigs under a dietary challenge of multiple mycotoxins with emphasis on deoxynivalenol[J]. Toxins, 2020, 12(8): 504. DOI:10.3390/toxins12080504 |
| [21] |
袁带秀, 舒丽霞, 黄蓉. 杜仲黄酮对H22小鼠的抑瘤作用及其机制[J]. 中国老年学杂志, 2016, 36(2): 291-293. YUAN D X, SHU L X, HUANG R. Antitumor effect of Flavonoids from Eucommia ulmoides on H22 mice and its mechanism[J]. Chinese Journal of Gerontology, 2016, 36(2): 291-293 (in Chinese). DOI:10.3969/j.issn.1005-9202.2016.02.016 |
| [22] |
YUAN D X, HUSSAIN T, TAN B, et al. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models[J]. Oxidative Medicine and Cellular Longevity, 2017, 2017: 8140962. |
| [23] |
廖思梦, 齐鸣, 颜佳梦, 等. 氯喹抑制自噬对呕吐毒素诱导的仔猪生长性能和血液指标变化的影响[J]. 动物营养学报, 2019, 31(10): 4817-4824. LIAO S M, QI M, YAN J M, et al. Effects of chloroquine inhibiting autophagy on growth performance and blood indexes change of piglets induced by deoxynivalenol[J]. Chinese Journal of Animal Nutrition, 2019, 31(10): 4817-4824 (in Chinese). |
| [24] |
YUAN D X, WANG J, XIAO D F, et al. Eucommia ulmoides flavones as potential alternatives to antibiotic growth promoters in a low-protein diet improve growth performance and intestinal health in weaning piglets[J]. Animals, 2020, 10(11): 1998. DOI:10.3390/ani10111998 |
| [25] |
了荣波. 镰刀菌毒素对断奶仔猪肝脏和脾脏损伤的影响[D]. 硕士学位论文. 泰安: 山东农业大学, 2015. LIAO R B. Effects of Fusarium toxins on injury of liver and spleen in weaned piglets[D]. Master's Thesis. Tai'an: Shandong Agricultural University, 2015. (in Chinese) |
| [26] |
LIAO P, LI Y H, LI M J, et al. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-κB and increasing mTOR signaling pathways in piglets[J]. Food and Chemical Toxicology, 2020, 140: 111326. DOI:10.1016/j.fct.2020.111326 |
| [27] |
ZHA A D, YUAN D X, CUI Z J, et al. The evaluation of the antioxidant and intestinal protective effects of baicalin-copper in deoxynivalenol-challenged piglets[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 5363546. |
| [28] |
ZHA A D, CUI Z J, QI M, et al. Dietary baicalin zinc supplementation alleviates oxidative stress and enhances nutrition absorption in deoxynivalenol challenged pigs[J]. Current Drug Metabolism, 2020, 21(8): 614-625. DOI:10.2174/1389200221666200302124102 |
| [29] |
ZHA A D, CUI Z J, QI M, et al. Baicalin-copper complex modulates gut microbiota, inflammatory responses, and hormone secretion in DON-challenged piglets[J]. Animals, 2020, 10(9): 1535. DOI:10.3390/ani10091535 |
| [30] |
CHAYTOR A C, SEE M T, HANSEN J A, et al. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs[J]. Journal of Animal Science, 2011, 89(1): 124-135. DOI:10.2527/jas.2010-3005 |
| [31] |
BECKMAN K B, AMES B N. The free radical theory of aging matures[J]. Physiological Reviews, 1998, 78(2): 547-581. DOI:10.1152/physrev.1998.78.2.547 |
| [32] |
DA SILVA E O, GEREZ J R, HOHMANN M S N, et al. Phytic acid decreases oxidative stress and intestinal lesions induced by fumonisin B1 and deoxynivalenol in intestinal explants of Pigs[J]. Toxins, 2019, 11(1): 18. DOI:10.3390/toxins11010018 |
| [33] |
LING K H, WAN M L Y, EL-NEZAMI H, et al. Protective capacity of resveratrol, a natural polyphenolic compound, against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation[J]. Chemical Research in Toxicology, 2016, 29(5): 823-833. DOI:10.1021/acs.chemrestox.6b00001 |
| [34] |
PESTKA J J. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae[J]. Toxins, 2010, 2(6): 1300-1317. DOI:10.3390/toxins2061300 |
| [35] |
VALLABHAPURAPU S, KARIN M. Regulation and function of NF-kappaB transcription factors in the immune system[J]. Annual Review of Immunology, 2009, 27: 693-733. |




