2. 河北农业大学山区研究所, 保定 071000;
3. 杨凌泓雁换羽研究所, 杨凌 712100;
4. 陕西省佳县店镇区域农牧技术推广站, 佳县 719208
2. Mountain Research Institute, Hebei Agricultural University, Baoding 071000, China;
3. Yangling Hongyan Moulting Research Institute, Yangling 712100, China;
4. Regional Agricultural and Animal Husbandry Technology Extension Station in Dian Town, Jiaxian County, Shaanxi Province, Jiaxian 719208, China
蛋鸡的利用年限一般为1个产蛋周期,即养到72周龄则全部淘汰,通常认为2个产蛋周期是不经济的[1]。为此,采取换羽技术可以使蛋鸡在短期内集中换羽,换羽期缩短一半,使老龄蛋鸡生殖系统得到统一复苏,提高生产性能,改善蛋品质,延长蛋鸡利用年限[2-4]。Tiwary等[5]研究表明,换羽技术可以提高蛋鸡的产蛋率,延长蛋鸡的产蛋高峰期。Andreatti等[6]研究表明,换羽组的生产性能、蛋重、哈氏单位和蛋壳品质等显著高于对照组。马淑雪等[7]研究表明,换羽可以提高蛋鸡的生殖激素水平,从而改善蛋鸡的生产性能。目前国内多采用禁食法对蛋鸡进行换羽,但禁食会导致蛋鸡机体受到一定程度的损伤[8-9],并且与其他换羽方法相比,死亡率较高[10],不符合动物福利。故本试验采用非禁食法对蛋鸡进行换羽,并与不进行集中换羽(负对照组)和禁食法换羽(正对照组)进行对比,观察分析其对蛋鸡生产性能、蛋品质、卵泡数量和生殖激素的影响,以期缓解换羽期蛋鸡的应激反应,并为非禁食法换羽技术的应用提供理论依据。
1 材料与方法 1.1 试验动物选取180只精神状态良好、体重接近、产蛋率[(76±2)%]相近的90周龄大午金凤蛋鸡,随机分为3组,每组5个重复,每个重复12只蛋鸡。本试验以双层笼养的形式在河北农业大学环境控制舱内进行。试验组(非禁食组)采用非禁食法对蛋鸡进行换羽,负对照组不进行集中换羽处理,正对照组(禁食组)采用禁食法进行换羽。试验期共86 d,其中预试期7 d,正试期79 d。
1.2 试验设计试验开始前1天,由非禁食组和禁食组各随机选择30只蛋鸡进行标记并称重,换羽期间每隔1 d称重,记录2组蛋鸡的体重以及下降情况。
非禁食组实施方案见表 1。整个换羽期饲喂换羽料,正常供给饮水,观察并记录标记蛋鸡的减重率,当减重率达25%左右时,恢复饲喂基础饲粮。换羽期内鸡舍维持光照在8 h/d,恢复饲喂基础饲粮后每天增加0.5 h光照直至每天维持在16 h。
|
|
表 1 非禁食组实施方案 Table 1 Implementation plan of non-fasting group |
禁食组实施方案见表 2。试验前7 d对蛋鸡进行停料不停水处理,第8~9天鸡舍停料停水。换羽期观察标记蛋鸡的体重下降情况,待体重下降到25%左右时恢复饲喂基础饲粮。换羽期内鸡舍维持光照在8 h/d,恢复饲喂基础饲粮后每天增加0.5 h光照直至每天维持在16 h。整个试验期内负对照组蛋鸡均饲喂基础饲粮,鸡舍维持光照16 h/d。
|
|
表 2 禁食组实施方案 Table 2 Implementation plan of fasting group |
基础饲粮、换羽料组成及营养水平见表 3。
|
|
表 3 基础饲粮和换羽料组成及营养水平(风干基础) Table 3 Composition and nutrient levels of basal diet and moulting diet (air-dry basis) |
为观察蛋鸡在整个换羽过程中的生理变化,样品的采集分3次进行,即:第Ⅰ阶段,换羽期间产蛋率为0且减重率达25%左右;第Ⅱ阶段,恢复饲喂基础饲粮后产蛋率达50%左右;第Ⅲ阶段,恢复饲喂基础饲粮后产蛋率稳定在85%以上,即达到二次产蛋高峰期。
1.3.1 生产性能称量并记录整个换羽期非禁食组和禁食组标记蛋鸡的体重,观察减重情况。在相同管理条件下,以重复为单位,记录每天产蛋总数和总蛋重,产蛋率下降至0所需天数,产蛋率恢复到50%所需天数,产蛋率维持在85%以上所需天数以及整个试验期的死淘情况。
1.3.2 蛋品质分别于试验开始时(第0天)、第Ⅱ阶段、第Ⅲ阶段,每个重复随机收集3枚鸡蛋,测定蛋重、蛋壳强度、蛋壳厚度、蛋白高度、哈氏单位、蛋黄颜色,计算蛋形指数等指标。
1.3.3 卵泡数量统计分别于第Ⅰ阶段、第Ⅱ阶段、第Ⅲ阶段,每个重复随机选取1只蛋鸡,共计15只,进行屠宰试验。打开蛋鸡腹腔,无菌操作下取出卵巢,根据卵泡直径进行计数,蛋鸡卵泡分级标准见表 4。
|
|
表 4 蛋鸡卵泡分级标准 Table 4 Laying hen follicle grading standard[11] |
分别于第Ⅰ阶段、第Ⅱ阶段、第Ⅲ阶段,每个重复随机选取2只蛋鸡,翅静脉采血收集于离心管中。将采集的血液3 000 r/min离心15 min,分离并收集上层清液于-20 ℃保存,待测促卵泡生成素(FSH)、促黄体生成素(LH)、雌激素(E)和孕酮(Prog)的含量。上述指标所用试剂盒均购于上海江莱生物科技有限公司,严格按说明书要求测定。
1.4 数据整理及分析试验数据首先用Excel 2010进行初步整理,再用SPSS 19.0软件进行单因素方差分析(one-way ANOVA),并采用Duncan氏法进行组间多重比较,结果用平均值±标准差表示,对于不符合正态分布的数据采用非参数检验(Kruskal-Wallis H检验)进行分析,结果用中位数表示,以P<0.05表示差异显著。
2 结果与分析 2.1 非禁食法换羽对蛋鸡生产性能的影响 2.1.1 换羽期蛋鸡产蛋情况由图 1可知,非禁食组换羽期持续时间较长,前期产蛋率下降不稳定,后期产蛋率下降逐渐趋于平缓,于第16天停止产蛋;禁食组产蛋率下降非常迅速,其中第4天产蛋率下降最为明显,达30.1%,第5天即停止产蛋。
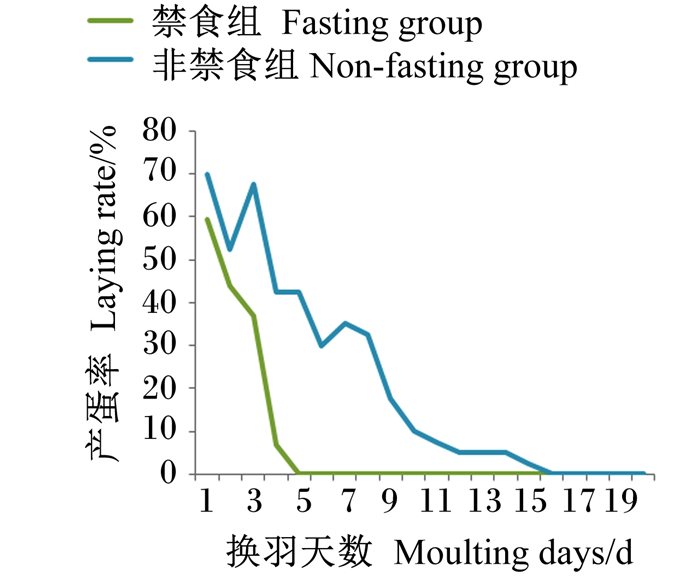
|
图 1 换羽期产蛋率 Fig. 1 Laying rate during moulting period |
由图 2可知,非禁食组在恢复饲喂基础饲粮后第14天开始产蛋,产蛋率达3.6%,之后产蛋率迅速恢复,第20天产蛋率达48.8%,第29天达二次产蛋高峰期,产蛋率为89.6%;禁食组在恢复饲喂基础饲粮第9天开始产蛋,产蛋率达1.9%,之后产蛋率迅速升高,第16天产蛋率达61.0%,第21天达二次产蛋高峰期,产蛋率为90.3%,此后维持在85%以上。
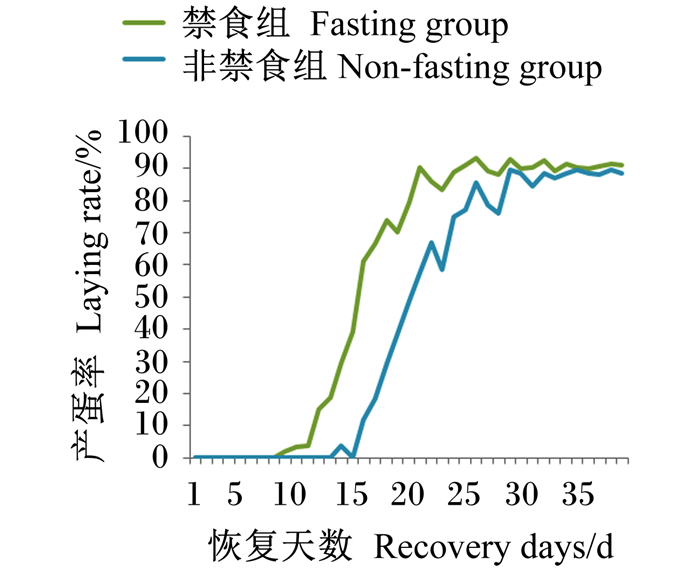
|
图 2 恢复期产蛋率 Fig. 2 Laying rate during recovery period |
由图 3可知,非禁食组和禁食组分别在试验第40天和第10天体重下降到标准范围内,前者减重率达24.40%,后者为25.40%;非禁食组在第3~4天体重下降最快,平均减重率为3.45%,第5~12天下降率稍有减慢,平均减重率为0.94%,第13~40天体重下降最为缓慢,平均减重率仅为0.38%;禁食组在前4天体重下降最快,平均减重率为3.65%,之后体重下降稍有减缓。
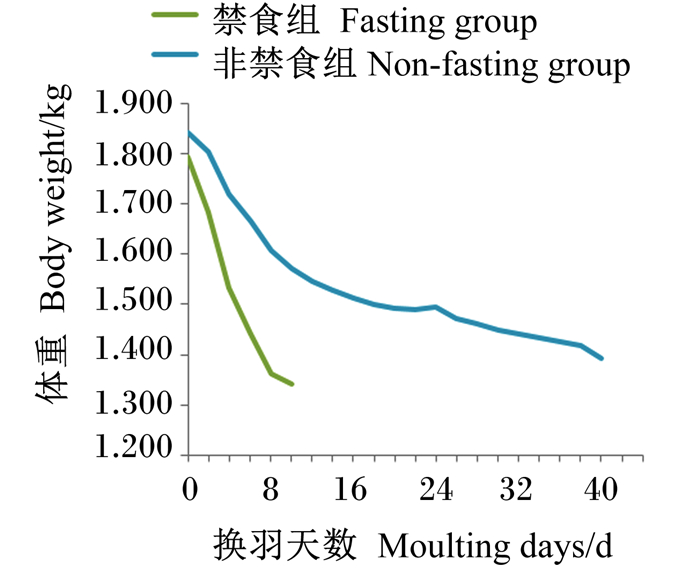
|
图 3 换羽期蛋鸡体重变化 Fig. 3 Body weight change of laying hens during moulting period |
由表 5可知,换羽期间禁食组蛋鸡死亡率为1.7%,其他时期无死亡现象;非禁食组和负对照组整个试验期均无死亡现象。
|
|
表 5 试验期死淘率 Table 5 Mortality rate during trial period% |
由表 6可知,在试验开始时(第0天)对3组的蛋品质进行测定,结果显示3组间无显著差异(P>0.05);在第Ⅱ阶段,在蛋重、蛋壳强度、蛋壳厚度、蛋黄颜色、蛋白高度和哈氏单位上3组间均无显著差异(P>0.05),但在蛋形指数上非禁食组和负对照组显著小于禁食组(P<0.05);在第Ⅲ阶段,在蛋重、蛋形指数、蛋壳厚度上3组间均无显著差异(P>0.05),但非禁食组、禁食组与负对照组相比蛋重和蛋壳厚度均有一定程度的增加,非禁食组的蛋壳强度与禁食组无显著差异(P>0.05),但显著大于负对照组(P<0.05),非禁食组蛋黄颜色显著低于负对照组和禁食组(P<0.05),在蛋白高度和哈氏单位上非禁食组与禁食组之间无显著差异(P>0.05),但均显著大于负对照组(P<0.05)。
|
|
表 6 非禁食法换羽对蛋鸡蛋品质的影响 Table 6 Effects of non-fasting method moulting on egg quality of laying hens |
由表 7可知,在第Ⅰ阶段,负对照组的排卵前卵泡和小黄卵泡数量均显著大于非禁食组和禁食组(P<0.05);在大白卵泡数量上,非禁食组显著小于负对照组(P<0.05)。在第Ⅱ阶段,非禁食组的排卵前卵泡、小黄卵泡、中白卵泡和小白卵泡的数量与禁食组、负对照组无显著差异(P>0.05);在大白卵泡数量上,非禁食组显著小于其他2组(P<0.05);在第Ⅲ阶段时,卵巢机能逐渐恢复,3组的各级卵泡数量均无显著差异(P>0.05)。
|
|
表 7 非禁食法换羽对蛋鸡卵泡数量的影响 Table 7 Effects of non-fasting method moulting on follicle number of laying hens |
由表 8可知,在第Ⅰ阶段,非禁食组的血清促卵泡生成素、促黄体生成素、雌激素和孕酮的含量与禁食组无显著差异(P>0.05),但2组的上述激素含量均显著低于负对照组(P<0.05);在第Ⅱ阶段,血清促黄体生成素和雌激素含量3组间无显著差异(P>0.05),非禁食组的血清促卵泡生成素和孕酮含量与禁食组无显著差异(P>0.05),但显著大于负对照组(P<0.05);在第Ⅲ阶段,非禁食组与禁食组的血清促卵泡生成素、促黄体生成素、雌激素和孕酮含量均显著大于负对照组(P<0.05)。
|
|
表 8 非禁食法换羽对蛋鸡血清生殖激素含量的影响 Table 8 Effects of non-fasting method moulting on serum reproductive hormone contents of laying hens |
研究表明,采用禁食法换羽时,产蛋率下降迅速,一般在第7天左右产蛋停止[12];采用非禁食法对换羽时,蛋鸡产蛋量下降速度稍缓于禁食组,在第15天左右停止产蛋[13]。本试验结果与上述研究一致,禁食组产蛋率下降非常迅速,仅用5 d产蛋即停止,非禁食组前期产蛋下降情况不稳定,第16天产蛋停止。蹇东东[14]对蛋鸡进行禁食法换羽的研究表明,在恢复喂料后1周左右即可见蛋,此后1周产蛋率即可达到50%。Bozkurt等[15]对白来航蛋鸡进行非禁食法换羽的研究表明,在恢复饲喂基础饲粮9 d后开始见蛋。本试验结果表明,非禁食组在恢复饲喂基础饲粮第14天开始产蛋,第20天产蛋率达48.8%,第29天达二次产蛋高峰期;禁食组在恢复饲喂基础饲粮第9天开始产蛋,第16天产蛋率达61.0%,第21天达二次产蛋高峰期。非禁食组恢复产蛋时间较长推测可能与换羽期持续时间较长,卵巢萎缩程度较大有关。本试验存在非禁食法换羽期持续时间较长的问题,推测可能与换羽料能量过高或者饲喂量偏大有关,这个问题有待进一步研究。
减重率是评判蛋鸡人工换羽技术成功与否的一项重要指标。一般成功的换羽技术,以蛋鸡的减重率在25%~35%为宜[16]。Gordon等[17]表明,换羽期逐渐增加海兰W36蛋鸡的减重率至25%~35%,会提高蛋鸡后期的产蛋量以及蛋壳质量。McDaniel等[18]表明,换羽期减重率在25%~35%时,换羽后的蛋鸡可以获得最优的生产性能。本试验结果表明,非禁食组在试验第40天减重率达24.4%,禁食组在试验第10天体重下降至25.5%,禁食组体重下降速度明显快于非禁食组,推测可能与非禁食组换羽料营养水平较高或饲喂量较大有关。研究表明,换羽期间蛋鸡体重下降速度还与饲养方式、初始体重、蛋鸡周龄、不同遗产基础的蛋鸡等因素有关[19-22]。同时,本试验中非禁食组蛋鸡换羽时间较长,导致换羽后蛋鸡的周龄大于禁食组,但已有研究发现这并不会降低换羽后蛋鸡的生产性能。Chanaksorn等[23]研究表明,换羽时间的长短不会影响换羽后蛋鸡的产蛋率、饲料转化率、蛋壳品质等。余慧芳等[10]研究表明,换羽时间较长的组二次产蛋高峰期产蛋率更高,持续时间更长。
通常换羽期死亡率以低于3%为标准[24]。匡刚[25]研究表明,换羽期前7天死亡率不超过1%,前10天不超过1.5%,35 d内不超过2.5%。余慧芳等[10]采用不同方式对蛋鸡进行换羽,结果表明,化学法的死亡率最低,其次为饥饿与化学综合法,最大的为禁食法。刘龙等[26]通过对比非禁食法与禁食法对海兰褐蛋鸡换羽发现,非禁食组死亡率显著低于禁食组。本试验结果表明,非禁食组换羽期应激小,无死亡现象;禁食组死淘率为1.7%。
3.2 非禁食法换羽对蛋鸡蛋品质的影响换羽技术可一定程度上改善蛋壳品质。研究表明,饲喂木薯粉诱导海兰褐蛋鸡进行换羽,鸡蛋的蛋壳厚度和蛋壳强度得到明显改善[27]。Bozkurt等[15]对白来航鸡的研究发现,与对照组相比,换羽组可显著提高蛋壳厚度、蛋壳强度和哈氏单位,改善蛋品质。由换羽对蛋鸡生产性能影响的Meta分析表明,非禁食法换羽后,第2周期的破蛋率显著减少,并且,与禁食法换羽相比,非禁食法能更有效地提高产蛋量和蛋重[28]。本试验结果表明,第Ⅲ阶段时,针对蛋壳强度、蛋白高度和哈氏单位等指标,非禁食组显著提高,蛋重有增加趋势,蛋壳厚度则有一定程度的改善。
3.3 非禁食法换羽对蛋鸡卵泡数量的影响研究表明,当机体能量充足时,可以促进卵泡发育,提高蛋鸡的生产性能[29-30];反之,蛋鸡的繁殖机能和产蛋性能则会受到抑制[31]。蛋鸡的人工换羽技术实际上就是一个机体能量和营养成分重新分配的过程。Renema等[32]发现,与限饲肉种鸡相比,能量充足的肉种鸡有更多的优势卵泡,并且小黄卵泡的闭锁率更低。研究表明,换羽后卵泡对性激素的敏感性增强,卵泡的摄取能力提高[33]。本试验结果表明,第Ⅰ阶段时,非禁食组的排卵前卵泡、小黄卵泡和大白卵泡数量显著低于负对照组,但与禁食组无显著差异;换羽后,卵巢机能逐渐恢复,卵泡数量增多,至第Ⅲ阶段3组的卵泡数量无显著差异。
3.4 非禁食法换羽对蛋鸡血清生殖激素含量的影响禽类生产性能的高低与生殖激素的含量有关。促卵泡生成素可以促进卵泡颗粒层增生分化,促黄体生成素可以加快血液循环,两者共同作用分泌雌激素,促进卵泡成熟和排卵[34]。雌二醇是一种卵巢类固醇激素,具有调节蛋壳形成的钙代谢、调节输卵管的钙代谢、诱导蛋白合成、刺激卵黄前体合成以及抑制红细胞合成等功能[13]。林小辉等[35]研究表明,卵巢退化的老龄蛋鸡血浆中的促黄体生成素含量降低。Kobayashi等[2]研究表明,禁食会使日本鹌鹑垂体中促黄体生成素β亚基和促卵泡生成素β亚基mRNA表达降低。研究表明,光照时间的缩短也会抑制促黄体生成素和促卵泡生成素的合成与释放[36-38]。换羽期间蛋鸡受到应激,卵巢退化,卵泡萎缩,促黄体生成素和促卵泡生成素生成减少,甚至停止分泌雌激素[39-40]。Hoshino等[41]对海兰蛋鸡进行换羽,结果表明,换羽期间血浆中雌二醇和孕酮含量降低,之后逐渐恢复到正常水平,Braw-Tal等[42]对罗曼蛋鸡进行换羽也得到相同的结论。本试验发现,第Ⅰ阶段,非禁食组所测血清生殖激素含量均下降并显著低于负对照组,之后各生殖激素含量逐渐回升,至第Ⅲ阶段时显著高于负对照组,但与禁食组无显著差异。
4 结论综上所述,非禁食法换羽可改善蛋鸡第二产蛋周期前期的产蛋性能,提高产蛋率,增加蛋壳强度,改善蛋壳品质,同时还可以通过调节血清生殖激素含量改善蛋鸡的生产性能。非禁食法换羽与禁食法换羽效果相当,但非禁食法换羽死亡率更低,更符合动物福利,因此,非禁食法换羽将会拥有更广阔的发展前景并将逐步取代禁食法换羽。
| [1] |
武翠枝. 强制换羽研究进展[J]. 中国畜牧兽医文摘, 2014, 30(8): 81-83. WU C Z. Research progress on forced moulting[J]. Chinese Animal Husbandry and Veterinary Abstracts, 2014, 30(8): 81-83 (in Chinese). |
| [2] |
KESHAVARZ K, QUIMBY F W. An investigation of different molting techniques with an emphasis on animal welfare[J]. Journal of Applied Poultry Research, 2002, 11(1): 54-67. DOI:10.1093/japr/11.1.54 |
| [3] |
AZIZ S R, SHAKER A, KIRKUKI S M S. Changes in external egg traits of chickens during pre- and post-molting periods[J]. Poultry Science, 2017, 5(2): 91-95. |
| [4] |
KOELKEBECK K W, ANDERSON K E. Molting layers—alternative methods and their effectiveness[J]. Poultry Science, 2007, 86(6): 1260-1264. DOI:10.1093/ps/86.6.1260 |
| [5] |
TIWARY A K, SHARMA S, KANDEL M, et al. Effectiveness of induced molting in laying hens-a feed removal approach[J]. International Journal of Applied Sciences and Biotechnology, 2019, 7(4): 429-433. DOI:10.3126/ijasbt.v7i4.25771 |
| [6] |
ANDREATTI FILHO R L, MILBRADT E L, OKAMOTO A S, et al. Salmonella enteritidis infection, corticosterone levels, performance and egg quality in laying hens submitted to different methods of molting[J]. Poultry Science, 2019, 98(10): 4416-4425. DOI:10.3382/ps/pez248 |
| [7] |
马淑雪, 刘幸, 赵鹏涛, 等. 饥饿法强制换羽对蛋鸡免疫功能、血液生化指标及生殖激素的影响[J]. 中国家禽, 2020, 42(6): 65-71. MA S X, LIU X, ZHAO P T, et al. Effect of hunger-induced forced moulting on immune function, blood biochemical indicators and reproductive hormone content in laying hens[J]. China Poultry, 2020, 42(6): 65-71 (in Chinese). DOI:10.16372/j.issn.1004-6364.2020.06.012 |
| [8] |
KOGUT M H, GENOVESE K J, STANKER L H. Effect of induced molting on heterophil function in White Leghorn hens[J]. Avian Diseases, 1999, 43(3): 538-548. DOI:10.2307/1592654 |
| [9] |
ANWAR H, RAHMAN Z U, JAVED I, et al. Effect of protein, probiotic, and symbiotic supplementation on serum biological health markers of molted layers[J]. Poultry Science, 2012, 91(10): 2606-2613. DOI:10.3382/ps.2012-02172 |
| [10] |
余慧芳, 潘懿, 玉耀贤. 不同强制换羽方法对蛋鸡产蛋率、再产蛋高峰效果观察[J]. 广西畜牧兽医, 2018, 34(1): 15-16. YU H F, PAN Y, YU Y X. Observation on the effect of different forced moulting methods on egg production rate and re-production peak of laying hens[J]. Guangxi Journal of Animal Husbandry & Veterinary Medicine, 2018, 34(1): 15-16 (in Chinese). DOI:10.3969/j.issn.1002-5235.2018.01.006 |
| [11] |
JOHNSON A L. Ovarian follicle selection and granulosa cell differentiation[J]. Poultry Science, 2015, 94(4): 781-785. DOI:10.3382/ps/peu008 |
| [12] |
孟宝春. 蛋鸡的饥饿法强制换羽技术[J]. 中国畜禽种业, 2015, 11(12): 133-134. MENG B C. Forced moulting technology of laying hens by starvation method[J]. The Chinese Livestock and Poultry Breeding, 2015, 11(12): 133-134 (in Chinese). DOI:10.3969/j.issn.1673-4556.2015.12.107 |
| [13] |
GONGRUTTANANUN N, KOCHAGATE P, POONPAN K, et al. Effects of an induced molt using cassava meal on body weight loss, blood physiology, ovarian regression, and postmolt egg production in late-phase laying hens[J]. Poultry Science, 2017, 96(6): 1925-1933. DOI:10.3382/ps/pew457 |
| [14] |
蹇东东. 浅谈蛋鸡饥饿法强制换羽技术[J]. 中国畜禽种业, 2018, 14(6): 151. JIAN D D. Talking about the forced moulting technology of laying hens by starvation method[J]. The Chinese Livestock and Poultry Breeding, 2018, 14(6): 151 (in Chinese). DOI:10.3969/j.issn.1673-4556.2018.06.133 |
| [15] |
BOZKURT M, BINTAŞ E, KIRKAN Ş, et al. Comparative evaluation of dietary supplementation with mannan oligosaccharide and oregano essential oil in forced molted and fully fed laying hens between 82 and 106 weeks of age[J]. Poultry Science, 2016, 95(11): 2576-2591. DOI:10.3382/ps/pew140 |
| [16] |
陈丽珠, 卞红春, 吴婷婷, 等. 蛋鸡饥饿法强制换羽技术的研究与应用实践[J]. 山东畜牧兽医, 2020, 41(11): 18-20. CHEN L Z, BIAN H C, WU T T, et al. Research and application practice of forced moulting technology by starvation method of laying hens[J]. Shandong Journal of Animal Science and Veterinary Medicine, 2020, 41(11): 18-20 (in Chinese). DOI:10.3969/j.issn.1007-1733.2020.11.007 |
| [17] |
GORDON R, BRYANT M M, ROLAND D A S. Performance and profitability of second-cycle laying hens as influenced by body weight and body weight reduction during molt[J]. Journal of Applied Poultry Research, 2009, 18(2): 223-231. DOI:10.3382/japr.2008-00014 |
| [18] |
MCDANIEL G R. Factors affecting broiler breeder performance 6.The relationship of premolt performance to postmolt performance[J]. Poultry Science, 1985, 64(12): 2267-2272. DOI:10.3382/ps.0642267 |
| [19] |
魏立东, 姜伟, 孙为, 等. 按体重分组的肉种鸡强制换羽法[J]. 中国家禽, 1998(8): 24-25. WEI L D, JIANG W, SUN W, et al. Forced moulting of broiler breeders by weight[J]. China Poultry, 1998(8): 24-25 (in Chinese). |
| [20] |
黄炎坤, 来明杰, 司玉亭. 不同品系蛋种鸡对强制换羽的反应[J]. 当代畜牧, 1999(4): 10-11. HUANG Y K, LAI M J, SI Y T. Responses of different strains of egg breeders to forced molting[J]. Contemporary Animal Husbandry, 1999(4): 10-11 (in Chinese). |
| [21] |
郭其华, 曹国春. 饥饿法对肉种鸡人工强制换羽的效果[J]. 畜牧兽医杂志, 2015, 34(2): 27-29. GUO Q H, CAO G C. Effects of starvation on artificial forced molting of broiler breeder[J]. Journal of Animal Science and Veterinary Medicine, 2015, 34(2): 27-29 (in Chinese). |
| [22] |
张浩, 周珍辉, 曹金元. 强制换羽对蛋鸡生产性能和蛋品质的影响[J]. 畜牧与饲料科学, 2020, 41(6): 46-49, 55. ZHANG H, ZHOU Z H, CAO J Y. Effect of forced moulting on productive performance and egg qualities of laying hens[J]. Animal Husbandry and Feed Science, 2020, 41(6): 46-49, 55 (in Chinese). |
| [23] |
CHANAKSORN M, BOONKAEWWAN C, KAYAN A, et al. Evaluation of molt induction using cassava meal varying the length of feeding period in older (90 week) laying hens[J]. Poultry Science, 2019, 98(9): 4131-4139. DOI:10.3382/ps/pez110 |
| [24] |
赵怀宝, 陈亮, 张宏福. 强制换羽在肉种鸡上的应用及营养调控策略[J]. 中国家禽, 2020, 42(9): 1-11. ZHAO H B, CHEN L, ZHANG H F. Application and nutritional control strategy on broiler breeder forced molting[J]. China Poultry, 2020, 42(9): 1-11 (in Chinese). DOI:10.16372/j.issn.1004-6364.2020.09.001 |
| [25] |
匡刚. 种母鸡人工强制换羽技术[J]. 现代畜牧科技, 2017(7): 31. KUANG G. Artificial forced moulting technology of breeding hens[J]. Modern Animal Husbandry Science & Technology, 2017(7): 31 (in Chinese). DOI:10.19369/j.cnki.2095-9737.2017.07.024 |
| [26] |
刘龙, 周宵, 宁中华. 非禁食与禁食强制换羽效果对比与分析[C]//第十五次全国家禽学术讨论会论文集. 广州: 中国畜牧兽医学会, 2011: 459-468. LIU L, ZHOU X, NING Z H. Comparison and analysis of the effects of forced moulting between non-fasting and fasting[C]//Proceedings of the 15th National Poultry Symposium. Guangzhou: Chinese Association of Animal Science and Veterinary Medicine, 2011: 459-468. (in Chinese) |
| [27] |
GONGRUTTANANUN N. Induced molt using cassava meal.2.Effects on eggshell quality, ultrastructure, and pore density in late-phase laying hens[J]. Poultry Science, 2018, 97(3): 1050-1058. DOI:10.3382/ps/pex365 |
| [28] |
AKBARI MOGHADDAM KAKHKI R, MOUSAVI Z, ANDERSON K E. An appraisal of moulting on post-moult egg production and egg weight distribution in white layer hens: Meta-analysis[J]. British Poultry Science, 2018, 59(3): 278-285. DOI:10.1080/00071668.2018.1432032 |
| [29] |
PÉREZ-BONILLA A, NOVOA S, GARCÍA J, et al. Effects of energy concentration of the diet on productive performance and egg quality of brown egg-laying hens differing in initial body weight[J]. Poultry Science, 2012, 91(12): 3156-3166. DOI:10.3382/ps.2012-02526 |
| [30] |
刘立文, 张立永, 穆秀明, 等. 日粮能量对育成柴鸡卵巢促卵泡素受体、促黄体素受体表达的影响[J]. 中国兽医学报, 2014, 34(6): 1023-1027. LIU L W, ZHANG L Y, MU X M, et al. Effects of diet energy on expression of FSHR, LHR in the ovary of replacement period Caiji-chicken[J]. Chinese Journal of Veterinary Science, 2014, 34(6): 1023-1027 (in Chinese). DOI:10.16303/j.cnki.1005-4545.2014.06.035 |
| [31] |
辛倩, 王晓鹃, 林海. 日粮能量水平对蛋鸡繁殖性能的影响[J]. 东北农业大学学报, 2021, 52(12): 72-81. XIN Q, WANG X J, LIN H. Effects of dietary energy level on reproductive performance of laying hens[J]. Journal of Northeast Agricultural University, 2021, 52(12): 72-81 (in Chinese). DOI:10.3969/j.issn.1005-9369.2021.12.010 |
| [32] |
RENEMA R A, ROBINSON F E. Defining normal: comparison of feed restriction and full feeding of female broiler breeders[J]. World's Poultry Science Journal, 2004, 60(4): 508-522. DOI:10.1079/WPS200434 |
| [33] |
孟晓彤. 强制换羽与日粮中添加大豆异黄酮对蛋鸡生产性能的影响[D]. 硕士学位论文. 泰安: 山东农业大学, 2013. MENG X T. Effects of forced molting and dietary supplementation of isolfavone on performance of laying hens[D]. Master's Thesis. Tai'an: Shandong Agricultural University, 2013. (in Chinese) |
| [34] |
ZHANG T Y, CHEN Y, WEN J H, et al. Transcriptomic analysis of laying hens revealed the role of aging-related genes during forced molting[J]. Genes, 2021, 12(11): 1767. DOI:10.3390/genes12111767 |
| [35] |
林小辉, 金艳梅, 曾卫东, 等. 黄体生成素促进鸡小黄卵泡膜细胞增殖的作用机理[J]. 中国兽医学报, 2008, 28(2): 212-215. LIN X H, JIN Y M, ZENG W D, et al. Signal transduction of luteinizing hormone in stimulating proliferation of cultured theca cells from chicken small yellow follicles[J]. Chinese Journal of Veterinary Science, 2008, 28(2): 212-215 (in Chinese). DOI:10.16303/j.cnki.1005-4545.2008.02.011 |
| [36] |
MADDINENI S R, OCÓN-GROVE O M, KRZYSIK-WALKER S M, et al. Gonadotropin-inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: potential role of GnIH in follicular maturation[J]. Reproduction, 2008, 135(2): 267-274. DOI:10.1530/REP-07-0369 |
| [37] |
BENTLEY G E, UBUKA T, MCGUIRE N L, et al. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system[J]. General and Comparative Endocrinology, 2008, 156(1): 34-43. DOI:10.1016/j.ygcen.2007.10.003 |
| [38] |
BENTLEY G E, PERFITO N, UKENA K, et al. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone[J]. Journal of Neuroendocrinology, 2003, 15(8): 794-802. DOI:10.1046/j.1365-2826.2003.01062.x |
| [39] |
CICCONE N A, SHARP P J, WILSON P W, et al. Changes in reproductive neuroendocrine mRNAs with decreasing ovarian function in ageing hens[J]. General and Comparative Endocrinology, 2005, 144(1): 20-27. DOI:10.1016/j.ygcen.2005.04.009 |
| [40] |
HOLMES D J, THOMSON S L, WU J L, et al. Reproductive aging in female birds[J]. Experimental Gerontology, 2003, 38(7): 751-756. DOI:10.1016/S0531-5565(03)00103-7 |
| [41] |
HOSHINO S, SUZUKI M, KAKEGAWA T, et al. Changes in plasma thyroid hormones, luteinizing hormone (LH), estradiol, progesterone and corticosterone of laying hens during a forced molt[J]. Comparative Biochemistry and Physiology-Part A: Comparative Physiology, 1988, 90(2): 355-359. |
| [42] |
BRAW-TAL R, YOSSEFI S, PEN S, et al. Hormonal changes associated with ageing and induced moulting of domestic hens[J]. British Poultry Science, 2004, 45(6): 815-822. DOI:10.1080/00071660400012782 |




