同等条件饲养的奶牛,个体间的产奶量差异较大,产奶量受到较多因素的影响,包括奶牛遗传改良、生理阶段、饲养管理和饲粮组成等[1-3],但与泌乳相关重要代谢物差异是影响奶牛泌乳性能的重要因素[4-5]。因此,开展对奶牛产奶过程中变化显著的血浆代谢物和代谢通路的研究,对提高奶牛产奶量、获得最大的经济效益具有重要的实用价值和现实意义。
代谢组学是使用高通量技术对生物样品中的小内源性代谢物进行检测[6]。由于代谢组学技术具有较高的分辨率和灵敏度等特点,近年来,在筛选瘤胃代谢物生物标志物方面被广泛应用,从而揭示饲粮对动物健康和生产性能的影响[7],研究疾病或生理学的潜在代谢物生物标记物[8-9],并检查饮食诱导的瘤胃代谢谱的代谢改变及其对反刍动物产奶相关性状的影响[6, 10-11]。利用代谢组学技术对产奶牛能力不同的奶牛进行血浆代谢物检测,比较分析代谢的差异,便于了解不同产奶量奶牛体内物质代谢途径的变化[12]。本试验采用液相色谱串联质谱(LC-MS/MS)分析高产与低产奶牛的血浆差异代谢物,旨在研究高产与低产奶牛血浆代谢组特征,从代谢角度探索高产与低产奶牛血浆代谢物特点,为开展饲粮营养调控奶牛产奶性能提高理论依据。
1 材料与方法 1.1 试验动物与管理试验选择健康、胎次[(2.04±0.81)胎]相近、泌乳天数[(23.58±8.80) d]相近、体重相近的高产[产奶量(40.56±1.57) kg/d]和低产[产奶量(25.17±1.66) kg/d]荷斯坦奶牛各12头,分为高产组(Hca组)和低产组(Lca组)。在饲养期间,奶牛自由饮水,饲喂全混合日粮(TMR),饲粮组成及营养水平见表 1。
|
|
表 1 饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the diet (DM basis) |
在晨饲前,用含肝素钠的一次性真空采血管尾根静脉采血,1 500×g离心10 min,将收集到的上层血浆置于液氮速冻后,在-80 ℃冰箱中冻存,用于后续样品分析。
1.3 血浆代谢物提取将在4 ℃下解冻的每个样品取100 μL,加入100 μL预冷水和800 μL预冷的甲醇/乙腈1 ∶ 1,混合均匀,冰水浴中超声60 min后,于-20 ℃孵育1 h进行蛋白质沉淀,16 000×g、4 ℃离心20 min,取上清液在高速离心机中挥干。加入100 μL乙腈-水1 ∶ 1混合溶液用于质谱检测时的复溶,离心15 min(4 ℃、14 000×g),取上清液用于样品分析。
1.4 LC-MS/MS分析 1.4.1 色谱分离采用1290 Infinity LC超高效液相色谱系统(UHPLC,安捷伦科技有限公司)对放于4 ℃进样器的样品进行色谱分离,设定5 μL的进样量,25 ℃的温度,0.3 mL/min的流速;水、乙酸铵(25 mmol/L)、氨水(25 mmol/L)为流动相A,乙腈为流动相B。梯度洗脱程序:0~0.5 min,流动相B的线性为95%;0.5~7.0 min,流动相B的线性从95%降至65%;7.0~9.0 min,流动相B的线性从65%降至40%;9.0~10.0 min,流动相B的线性保持在40%;10.0~11.1 min,流动相B的线性从40%增至95%;11.1~16.0 min,流动相B的线性保持在95%。
1.4.2 质谱数据采集采用电喷雾电离(ESI)对每例样品进行正离子和负离子模式检测。采用Triple-TOF5600质谱仪(美国AB SCIEX公司)对UHPLC分离后的样品进行分析。ESI源条件:离子源气体1为60 psi,离子源气体2为60 psi,帘式气体(CUR)为30 psi,源温度为600 ℃,离子空间电压浮动(ISVF)±5 500 V(正、负2种模式);飞行时间质谱(TOF-MS)扫描范围:质荷比(m/z)60~1 200,生产扫描范围:m/z 25~1 200,TOF-MS扫描累积时间0.15 s/谱,产品离子扫描累积时间0.03 s/谱。
1.4.3 数据处理和分析峰面积的提取以及峰对齐、保留时间的校正均来自于格式转换后的原始数据经MSDIAL软件的XCMS程序。通过相关方式检索MassBank、HMDB等自建的代谢物标准品库和公共数据库来鉴定代谢物的结构。
采用SIMCA-P 14.1软件对删除数据组内缺失值>50%离子峰后的正负离子模式进行识别,通过多维统计分析经Pareto scaling预处理后的数据,包括无监督主成分分析(PCA)、有监督偏最小二乘法判别分析(PLS-DA)和正交偏最小二乘法判别分析(OPLS-DA)。
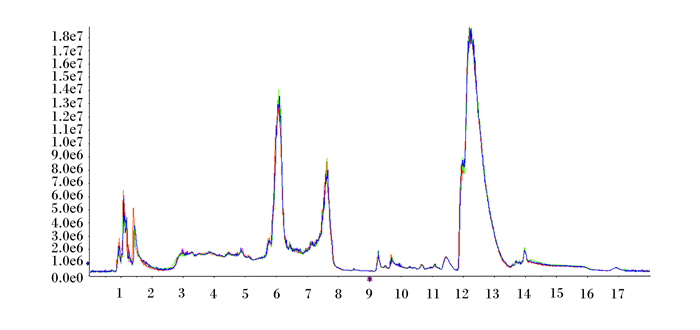
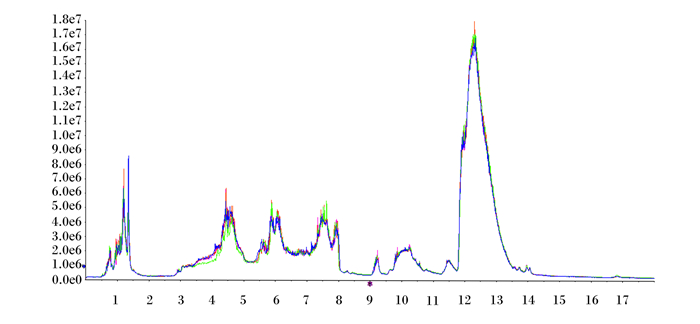
2 结果与分析 2.1 血浆样品代谢质谱及数据分析由图 1和图 2可知,正、负离子检测模式下质量控制(QC)样品各色谱峰的响应强度和保留时间基本重叠,总粒子流图(TIC)重叠图的重叠程度可以说明仪器稳定,且所得数据有效。
|
横坐标为正离子检测模式下质量控制样品各色谱峰的保留时间(min),纵坐标为正离子检测模式下质量控制样品各色谱峰的响应强度。 The abscissa is the retention time (min) of each chromatographic peak of the QC sample in the positive ion detection mode, and the ordinate is the response intensity of each chromatographic peak of the QC sample in the positive ion detection mode. 图 1 质量控制样品正离子总粒子流图 Fig. 1 QC sample positive ion TIC |
|
横坐标为负离子检测模式下质量控制样品各色谱峰的保留时间(min),纵坐标为负离子检测模式下质量控制样品各色谱峰的响应强度。 The abscissa is the retention time (min) of each chromatographic peak of the QC sample in the negative ion detection mode, and the ordinate coordinate is the response intensity of each chromatographic peak of the QC sample in the negative ion detection mode. 图 2 质量控制样品负离子总粒子流图 Fig. 2 QC sample negative ion TIC |
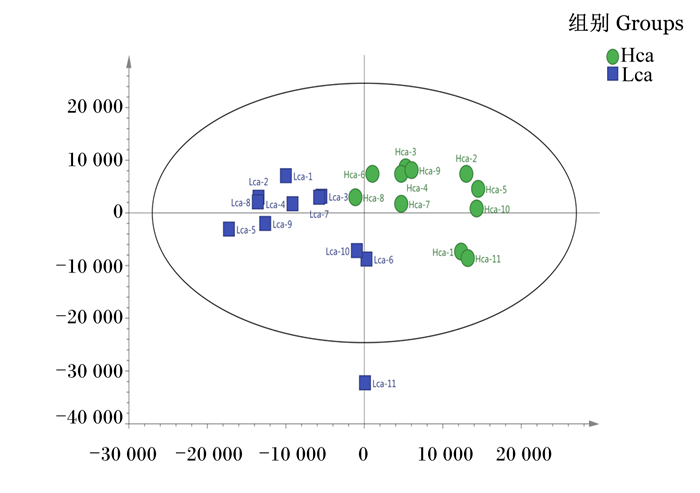
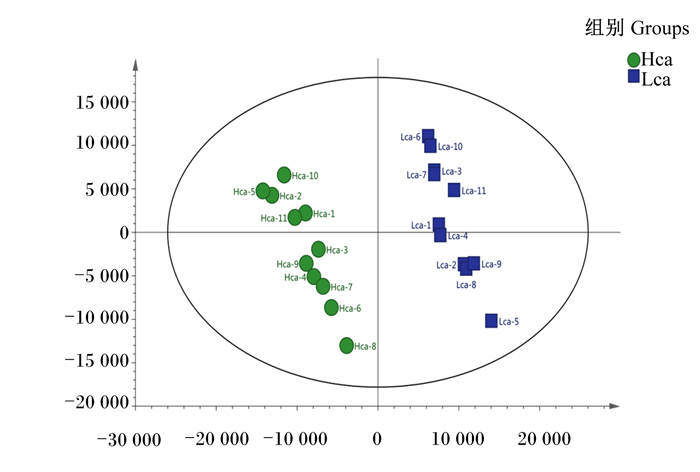
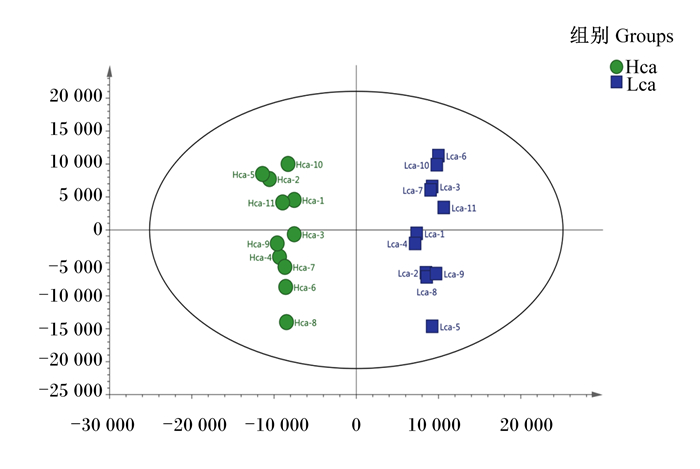
利用PCA对色谱峰的数据进行分析,由PCA得分图(图 3)可知,Hca组和Lca组血浆样品之间有堆叠,但表现出明显的分离趋势,Hca组和Lca组之间模型解释率(R2X)为0.325。进一步使用有监督PLS-DA和OPLS-DA来评估Hca组和Lca组之间的血浆样品差异,进而判断LC-Q/TOF-MS的X变量(峰强度)与Y变量(分组信息)之间的相关性。为了预测样品差异,本试验通过PLS-DA和OPLS-DA建立代谢物表达量与样品差异之间的关系模型,结果见图 4,2模型的R2Y、Q2均大于0.5且趋近于1,说明该模型预测能力较高且稳定性良好。
|
图中每个点代表了1个样品。横坐标为第1主成分,纵坐标为第2主成分,2点之间在横、纵坐标上的距离代表了样品受第1主成分或第2主成分影响下的相似性距离。 Each point in the figure represents a sample. The abscissa is the first principal component, the ordinate is the second principal component, and the distance between the two points on the abscissa and ordinate represents the similarity distance of the sample under the influence of the first principal component or second principal component. 图 3 Hca组和Lca组血浆样品PCA得分图 Fig. 3 PCA scoring diagram of plasma sample of Hca group and Lca group |
|
横坐标为第1主成分,纵坐标为第2主成分,R2Y为0.997,Q2为0.838。 The abscissa is the first principal component, and the ordinate is the second principal component. R2Y is 0.997, and Q2 is 0.838. 图 4 Hca组和Lca组血浆样品PLS-DA得分图 Fig. 4 PLS-DA scoring diagram of plasma sample of Hca group and Lca group |
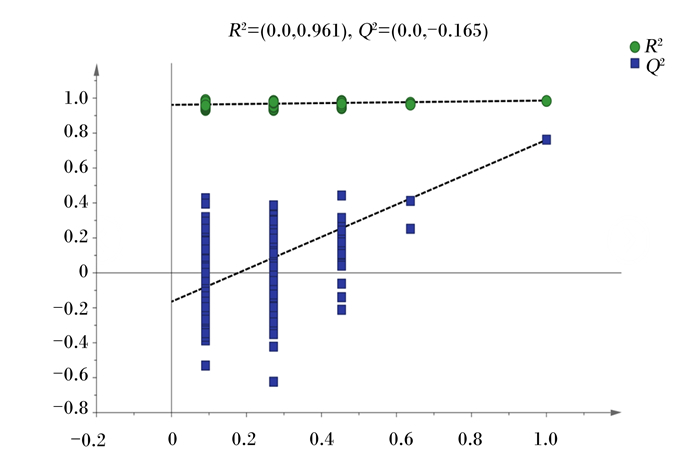
OPLS-DA是在PLS-DA的基础上,进行了正交变换的矫正,可以滤除与分类信息无关的噪音,提高了模型的解析能力和有效性。并将得到的OPLS-DA模型进行置换检验用来检测构建的模型是否“过拟合”(图 5)。在OPLS-DA模型下,Hca组和Lca组血浆样品区分状况良好(图 6)。其中,R2X为0.197,R2Y为0.986,表明利用19.7%的LC-Q/TOF-MS数据可以反映2组血浆样品之间的差异,但Q2为0.761,高于0.5,表明模型具有较高的可预测性。所以,由图 3和图 6可知,Hca组和Lca组奶牛血浆样品代谢物有明显差异。
|
横坐标代表随机分组的Y与原始分组Y的相关性,纵坐标代表R2和Q2的得分。7次置换检验。 The abscissa represents the correlation of randomly grouped Y to the original grouping Y, and the ordinate represents the scores of R2 and Q2. Seven permutation tests. 图 5 Hca组和Lca组血浆样品OPLS-DA置换检验图 Fig. 5 OPLS-DA permutation test diagram of plasma sample of Hca group and Lca group |
|
图中横坐标表示预测主成分,纵坐标表示正交主成分,百分比表示该成分对数据集的解释率。R2Y为0.986,Q2为0.761。 The abscissa represents the predicted principal component, the ordinate represents the orthogonal principal component, the percentage represents the interpretation rate of the component to the dataset. R2Y is 0.986, and Q2 is 0.761. 图 6 Hca组和Lca组血浆样品OPLS-DA得分图 Fig. 6 OPLS-DA scoring diagram of plasma sample of Hca group and Lca group |
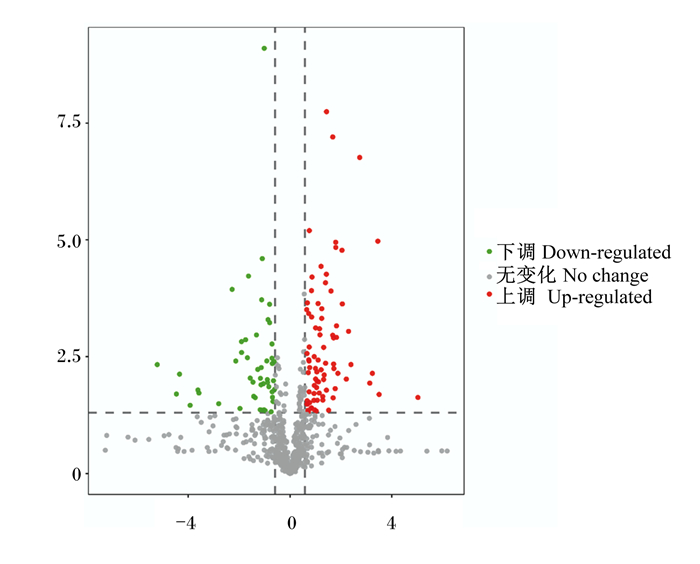
利用t检验的P值和差异倍数(fold change,FC)对预处理后的数据绘制火山图。由图 7可知,差异代谢物的分布说明筛选鉴定该数据可行。由表3可知,本试验中,2组奶牛血浆中共筛选出27个差异代谢物,与Lca组比较,Hca组奶牛血浆中牛磺酸、胆酸、猪胆酸、甘氨熊脱氧胆酸、脱氧胆酸、L-亮氨酸、吲哚-3-甲醇、吲哚-3-乙酰胺等18个代谢物上调,而苯乙酰甘氨酸、苯乙酸及、对羟基苯乙醇等9个代谢物下调。
|
横坐标为log2(差异倍数),纵坐标为t检验显著性检验P值的负对数-log10(P值)。 The abscissa is log2 (FC), and the ordinate is the negative logarithm-log10 (P-value) of the P-value of the t test significance test. 图 7 Hca组和Lca组血浆代谢组火山图 Fig. 7 Volcanic map of plasma metabolism group of Hca group and Lca group |
|
|
表 2 Hca组和Lca组血浆差异代谢物 Table 2 Plasma differential metabolites of Hca group and Lca group |
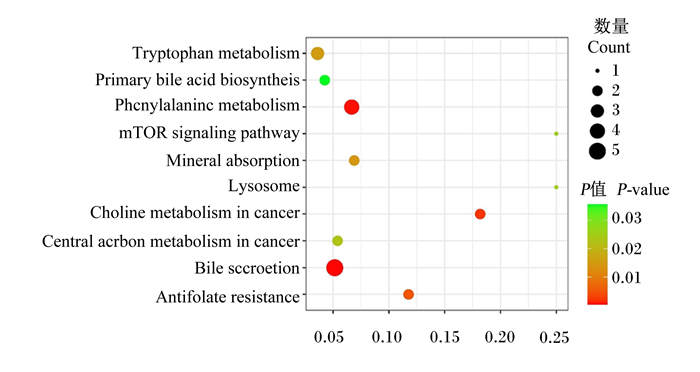
对差异代谢物富集的通路进行KEGG数据库分析(图 8),其是利用P值或Q值最小的前10个KEGG通路来作图,纵坐标为Pathway,横坐标为富集因子,大小表示数量多少,颜色越红P/Q值越小。经通路富集分析共得到10条代谢通路,筛选出的代谢通路为:色氨酸代谢、苯丙氨酸代谢、初级胆汁酸生物合成、胆汁分泌、矿物质吸收以及叶酸拮抗剂抗性等。由KEGG数据库分析可知,Hca组和Lca组奶牛血浆中苯丙氨酸代谢、胆汁分泌代谢以及叶酸拮抗剂抗性差异极显著(P < 0.01),色氨酸代谢、初级胆汁酸生物合成等代谢途径差异显著(P < 0.05)。且Hca组奶牛血浆中胆汁分泌、初级胆汁酸生物合成、哺乳动物雷帕霉素靶蛋白信号通路和矿物质吸收相对于Lca组增强,而苯丙氨酸代谢、叶酸拮抗剂抗性以及溶酶体代谢通路相对于Lca组减弱。
|
Tryptophan metabolism:色氨酸代谢;Primary bile acid biosynthesis:初级胆汁酸生物合成;Phenylalanine metabolism:苯丙氨酸代谢;mTOR signaling pathway:哺乳动物雷帕霉素靶蛋白信号通路;Mineral absorption:矿物质吸收;Lysosome:溶酶体;Choline metabolism in cancer:癌症中的胆碱代谢;Central carbon metabolism in cancer:癌症的中枢碳代谢;Bile secretion:胆汁分泌;Antifolate resistance:叶酸拮抗剂抗性。 图中每个图形为一个KEGG通路,纵坐标为KEGG通路,横坐标为富集因子,大小表示数量多少,颜色越红P/Q值越小。 Each graph in the figure is a KEGG pathway, the ordinate is the KEGG pathway, the abscissa is the enrichment factor, the size indicates the number, and the more red the color, the smaller the P/Q value. 图 8 Hca组和Lca组差异代谢通路富集分析图 Fig. 8 Enrichment analysis diagram of differential metabolic pathway of in Hca group and Lca group |
胆汁分泌产生胆酸,主要是胆汁酸,其是生命活动的重要调节因子,参与葡萄糖、脂肪和能量代谢的调节,与肠道激素、微生物群和能量平衡密切相关[13]。肝细胞色素胆固醇7α-羟化酶(CYP7A1)催化胆固醇转化为7α-羟基胆固醇,在线粒体中3β-羟基-Δ5-C27-羟基类固醇脱氢酶的催化下产生7α-羟基-4-胆甾烯-3-酮,经过一系列反应最终生成胆酸,并迅速结合牛磺酸或甘氨酸,形成结合的胆汁酸,然后从胆管流入十二指肠。初级胆汁酸转化为次级游离胆汁酸[14-15],主要是脱氧胆酸,并在肝脏和肠道代谢后发生硫酸化、糖基酯化和糖基化等反应。有研究发现,奶牛血浆中的胆固醇含量显著降低,胆固醇分解代谢增强,导致初级胆汁酸的合成增加[16]。本研究中,与低产奶牛相比,高产奶牛血浆中的胆酸、猪胆酸、甘氨熊脱氧胆酸和脱氧胆酸显著增加。初级胆汁酸和甘氨酸或牛磺酸结合,产生甘胆酸及牛磺胆酸,利用胆酰辅酶A,并协助脂肪在奶牛小肠中消化和吸收[17],脂肪的消化产物甘油通过糖异生作用进入糖酵解和三羧酸循环(TCA)[18-19],为奶牛泌乳供能,增加奶牛产奶量。有研究发现,肠道菌群对胆汁酸进行去共轭化、脱羟基化和差向异构化等生物学修饰,进而影响胆汁酸的合成与代谢[20-22]。同时,肠道菌群也可通过对胆汁酸合成途径中关键酶调节影响胆汁酸合成[23]。又有研究表明,胆汁酸在肠道中乳化脂肪的同时也能对肠道部分微生物起到抑制作用[24],进而提高奶牛泌乳量,改善能量负平衡,而汪悦等[25]研究发现,奶牛“肠道-乳腺通路”存在,关于血浆胆酸与肠道微生物及奶牛产奶量的关系需要深入研究。
3.2 氨基酸代谢对产奶量的影响本研究分析表明,苯丙氨酸途径在2组间的差异显著,与Lca组相比较,Hca组苯丙氨酸代谢中的主要底物中3种下调代谢产物所致(苯乙酰甘氨酸、苯乙酸及对羟基苯乙醇)。苯乙酸和苯乙醇用于合成苯乙酰辅酶A,而苯乙酰辅酶A是合成乙酰辅酶A的重要代谢物,且乙酰辅酶A合成量下降会促进糖酵解或者糖异生过程,使机体ATP含量增加[26]。ATP能在胞内传输化学能进行新陈代谢,为奶牛产奶过程提供能量,在本试验中,与Lca组奶牛相比,Hca组奶牛由于苯丙氨酸代谢途径中下游代谢产物苯乙酰甘氨酸、苯乙酸及对羟基苯乙醇含量下调导致生成的ATP增加,从而造成奶牛的产奶量增加。除了提供能源外,基本代谢途径之一的糖酵解的部分中间产物能用于其他物质的合成,以关联碳水化合物、蛋白质和脂肪代谢[27]。例如,丙酮酸可转变为丙氨酸和乙酰辅酶A,而乙酰辅酶A是合成脂肪酸的原料,这对奶牛的泌乳维持也非常重要[28]。
色氨酸代谢与碳水化合物代谢、蛋白质的合成、脂肪代谢等途径关系密切。在吲哚胺-2, 3-双加氧酶或色氨酸-2, 3-双加氧酶的催化下,色氨酸转变为犬尿氨酸(KYN),通过多级酶促反应后,生成喹啉酸、吡啶羧酸类及烟酰胺腺嘌呤二核苷酸(NAD)和烟酰胺腺嘌呤二核苷酸磷酸(NADP)等[29]。Viljoen等[30]研究表明,色氨酸经氧化生成烟酸,其不仅是合成NAD和NADP的前体,也是参与机体的氧化还原反应无氧脱氢酶的辅酶,进而参与机体能量代谢、糖脂代谢、氧化应激和炎症调控[31-32]。有研究表明,色氨酸合成烟酸的速度加快,体内糖原含量减少,使腺苷酸环化酶活性增加,导致为机体提供的ATP增加。与低产奶牛相比,高产奶牛色氨酸代谢途径中血浆代谢物浓度上调,致使为奶牛泌乳提供的能量增加,造成蛋白质合成速度加快,产奶量增加。L-色氨酸在多种微生物参与下,经过脱氨和脱羧作用生成吲哚、吲哚乙酸及吲哚丙酮酸等[33]。与低产组相比,高产奶牛血浆中吲哚-3-甲醇和吲哚-3-乙酰胺含量上调,经研究发现,在该代谢通路中,吲哚-3-甲醇和吲哚-3-乙酰胺可以用于合成乙酰辅酶A,而乙酰辅酶A可以参与TCA及脂肪酸的合成,增加了奶牛泌乳所需的能量,导致产奶量上升[34-35]。这说明奶牛色氨酸代谢途径中血浆代谢物浓度上调会使产奶量增加,苯丙氨酸代谢与色氨酸均为含有苯环的氨基酸,本研究发现,二者在奶牛体内均参与乙酰辅酶A的合成,乙酰辅酶A作为体内高能化合物,可以作为脂肪酸合成原料,也可以作为脂肪酸合成过程中ATP的来源,对于促进乳脂合成和泌乳能量提供发挥重要作用,苯丙氨酸代谢与色氨酸互作及协同代谢对于高产奶牛产奶的调控作用需要深入研究。
3.3 叶酸拮抗剂抗性对产奶量的影响在机体中,一碳单位参与蛋白质、核苷酸、泛酸的合成及分子的甲基化,而叶酸是介导一碳单位的辅助因子[36],在NADPH参与下,叶酸被其还原酶还原成四氢叶酸(THFA或FH4),参与机体合成嘌呤及嘧啶,进而参与DNA合成[37]。而叶酸拮抗剂是一类抗代谢类抗癌药物[38],在恶性、微生物、寄生虫和慢性炎症性疾病的药物治疗中发挥了关键作用,其与叶酸结构相似,并能抑制叶酸代谢中的关键酶,导致嘌呤和胸腺嘧啶的生物合成中断,抑制DNA复制和细胞死亡。叶酸是DNA合成的重要物质,而肿瘤细胞的快速增殖需要以更多DNA为原料,所以,肿瘤细胞增殖可以被叶酸拮抗剂有效抑制[39]。本研究中,与Lca组奶牛相比,Hca组奶牛叶酸拮抗剂抗性增强,有效抑制了炎症等疾病的DNA复制和肿瘤细胞的增殖,使更多能量用于奶牛泌乳,这可能是增加奶牛泌乳能力的一个因素。
4 结论① 通过对高产与低产奶牛血浆代谢组特征的研究表明,高产与低产奶牛血浆中共筛选出27个差异代谢物,与Lca组相比,Hca组奶牛血浆中牛磺酸、胆酸、猪胆酸、甘氨熊脱氧胆酸、脱氧胆酸、L-亮氨酸、吲哚-3-甲醇、吲哚-3-乙酰胺等18个代谢物含量显著增加,而苯乙酰甘氨酸、苯乙酸及、对羟基苯乙醇等9个代谢物含量显著下降。
② 代谢通路富集分析表明,与Lca组相比,Hca组奶牛胆汁分泌、色氨酸代谢、初级胆汁酸生物合成显著增强,而苯丙氨酸代谢、叶酸拮抗剂抗性相对减弱。
③ 研究结果为通过饲粮营养成分调控奶牛产奶潜力发挥提供了理论依据,苯丙氨酸、色氨酸以及叶酸等营养物质具有一定研究潜力,胆汁酸分泌调控对于奶牛产奶性能发挥也具有一定理论研究价值。
| [1] |
任伟忠. 奶牛干奶前期饲喂不同粗饲料日粮对其生产性能、血液指标及瘤胃发酵的影响[D]. 硕士学位论文. 保定: 河北农业大学, 2019. REN W Z. Effects of different roughage diets fed to the dry cows during the first phase of the dry period on its performance, blood indexes and rumen fermentation[D]. Master's Thesis. Baoding: Hebei Agricultural University, 2019. (in Chinese) |
| [2] |
欧四海, 冯建丽, 付云宝, 等. 德系弗莱维赫牛与高产荷斯坦奶牛杂交一代生产性能研究[J]. 中国乳业, 2019(10): 55-57. OU S H, FENG J L, FU Y B, et al. Study on the production performance of the first generation of German Fleivich cattle and high-yield Holstein cows[J]. China Dairy, 2019(10): 55-57 (in Chinese). |
| [3] |
姜晓鹃. 鲁西牛、中国西门塔尔牛和荷斯坦牛的经济效益分析[D]. 硕士学位论文. 北京: 中国农业科学院, 2011. JIANG X J. The economic benefit analysis of Luxi, Chinese Simmental and Holstein[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2011. (in Chinese) |
| [4] |
DE LA TORRE-SANTOS S, ROYO L J, MARTÍNEZ-FERNÁNDEZ A, et al. The mode of grass supply to dairy cows impacts on fatty acid and antioxidant profile of milk[J]. Foods, 2020, 9(9): 1256. DOI:10.3390/foods9091256 |
| [5] |
DAVIS H, STERGIADIS S, CHATZIDIMITRIOU E, et al. Meeting breeding potential in organic and low-input dairy farming[J]. Frontiers in Veterinary Science, 2020, 7: 544149. DOI:10.3389/fvets.2020.544149 |
| [6] |
SUN H Z, WANG D M, WANG B, et al. Metabolomics of four biofluids from dairy cows: potential biomarkers for milk production and quality[J]. Journal of Proteome Research, 2015, 14(2): 1287-1298. DOI:10.1021/pr501305g |
| [7] |
HUMER E, KRÖGER I, NEUBAUER V, et al. Supplementing phytogenic compounds or autolyzed yeast modulates ruminal biogenic amines and plasma metabolome in dry cows experiencing subacute ruminal acidosis[J]. Journal of Dairy Science, 2018, 101(10): 9559-9574. DOI:10.3168/jds.2018-14744 |
| [8] |
TANG C H, ZHANG K, ZHAN T F, et al. Metabolic characterization of dairy cows treated with gossypol by blood biochemistry and body fluid untargeted metabolome analyses[J]. Journal of Agricultural and Food Chemistry, 2017, 65(42): 9369-9378. DOI:10.1021/acs.jafc.7b03544 |
| [9] |
AMETAJ B N, ZEBELI Q, SALEEM F, et al. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows[J]. Metabolomics, 2010, 6(4): 583-594. DOI:10.1007/s11306-010-0227-6 |
| [10] |
MAO S Y, HUO W J, ZHU W Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model[J]. Environmental Microbiology, 2016, 18(2): 525-541. DOI:10.1111/1462-2920.12724 |
| [11] |
ZHAO S, ZHAO J, BU D, et al. Metabolomics analysis reveals large effect of roughage types on rumen microbial metabolic profile in dairy cows[J]. Letters in Applied Microbiology, 2014, 59(1): 79-85. DOI:10.1111/lam.12247 |
| [12] |
SUN L W, ZHANG H Y, WU L, et al. (1)H-nuclear magnetic resonance-based plasma metabolic profiling of dairy cows with clinical and subclinical ketosis[J]. Journal of Dairy Science, 2014, 97(3): 1552-1562. DOI:10.3168/jds.2013-6757 |
| [13] |
LI T G, APTE U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer[J]. Advances in Pharmacology, 2015, 74: 263-302. |
| [14] |
BATTA A K, SALEN G, ARORA R, et al. Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids[J]. Journal of Biological Chemistry, 1990, 265(19): 10925-10928. DOI:10.1016/S0021-9258(19)38535-7 |
| [15] |
RIDLON J M, KANG D J, HYLEMON P B. Bile salt biotransformations by human intestinal bacteria[J]. Journal of Lipid Research, 2006, 47(2): 241-259. DOI:10.1194/jlr.R500013-JLR200 |
| [16] |
PACÍFICO C, STAUDER A, REISINGER N, et al. Distinct serum metabolomic signatures of multiparous and primiparous dairy cows switched from a moderate to high-grain diet during early lactation[J]. Metabolomics, 2020, 16(9): 96. DOI:10.1007/s11306-020-01712-z |
| [17] |
SHERIHA G M, WALLER G R, CHAN T, et al. Composition of bile acids in ruminants[J]. Lipids, 1968, 3(1): 72-78. DOI:10.1007/BF02530972 |
| [18] |
刘艳利, 孙文强, 韩迪, 等. 基于GC-MS代谢组学技术对叶酸调控鸡原代肝细胞代谢的研究[J]. 畜牧兽医学报, 2018, 49(9): 1919-1927. LIU Y L, SUN W Q, HAN D, et al. The study on folic acid regulating metabolism of chicken primary hepatocytes by GC-MS metabolomics[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(9): 1919-1927 (in Chinese). |
| [19] |
ZHAO M, YUAN M M, YUAN L, et al. Chronic folate deficiency induces glucose and lipid metabolism disorders and subsequent cognitive dysfunction in mice[J]. PLoS One, 2018, 13(8): e0202910. DOI:10.1371/journal.pone.0202910 |
| [20] |
杨鑫, 汪水平, 谭支良, 等. 胆汁酸菌群修饰介导肠道黏膜免疫的研究进展[J]. 动物营养学报, 2021, 33(7): 3702-3712. YANG X, WANG S P, TAN Z L, et al. Gut microbiota-dominated bioconversion of bile acids on intestinal mucosal immunity: a systemic review[J]. Chinese Journal of Animal Nutrition, 2021, 33(7): 3702-3712 (in Chinese). |
| [21] |
DEVLIN A S, FISCHBACH M A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids[J]. Nature Chemical Biology, 2015, 11(9): 685-690. DOI:10.1038/nchembio.1864 |
| [22] |
JAROCKI P, TARGOÑSKI Z. Genetic diversity of bile salt hydrolases among human intestinal bifidobacteria[J]. Current Microbiology, 2013, 67(3): 286-292. DOI:10.1007/s00284-013-0362-1 |
| [23] |
SAYIN S I, WAHLSTRÖM A, FELIN J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metabolism, 2013, 17(2): 225-235. DOI:10.1016/j.cmet.2013.01.003 |
| [24] |
杨汉博. 胆汁酸——脂肪消化促进剂[J]. 饲料广角, 2008(11): 49-50. YANG H B. Bile acid-fat digestion promoter[J]. Feed China, 2008(11): 49-50 (in Chinese). |
| [25] |
汪悦, 王炳, 童津津, 等. 牛奶微生物群落与奶牛乳腺健康和犊牛胃肠道发育的关系[J]. 动物营养学报, 2018, 30(2): 458-467. WANG Y, WANG B, TONG J J, et al. Relationship between milk microbial community and mammary gland health of dairy cows as well as gastrointestinal development of calves[J]. Chinese Journal of Animal Nutrition, 2018, 30(2): 458-467 (in Chinese). |
| [26] |
MALLOY C R, SHERRY A D, JEFFREY F M. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy[J]. The Journal of Biological Chemistry, 1988, 263(15): 6964-6971. DOI:10.1016/S0021-9258(18)68590-4 |
| [27] |
HE W H, MIAO F J P, LIN D C H, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors[J]. Nature, 2004, 429(6988): 188-193. DOI:10.1038/nature02488 |
| [28] |
SUN H Z, SHI K, WU X H, et al. Lactation-related metabolic mechanism investigated based on mammary gland metabolomics and 4 biofluids' metabolomics relationships in dairy cows[J]. BMC Genomics, 2017, 18(1): 936. |
| [29] |
XU K, LIU H N, BAI M M, et al. Redox properties of tryptophan metabolism and the concept of tryptophan use in pregnancy[J]. International Journal of Molecular Sciences, 2017, 18(7): 1595. |
| [30] |
VILJOEN M, SWANEPOEL A, BIPATH P. Antidepressants may lead to a decrease in niacin and NAD in patients with poor dietary intake[J]. Medical Hypotheses, 2015, 84(3): 178-182. |
| [31] |
魏筱诗, 姚军虎, 王翀. 烟酸和烟酰胺在反刍动物机体代谢及生产研究中的异同性[J]. 动物营养学报, 2020, 32(12): 5509-5515. WEI X S, YAO J H, WANG C. Similarities and differences of niacin and niacinamide in study on metabolism and production of ruminant[J]. Chinese Journal of Animal Nutrition, 2020, 32(12): 5509-5515 (in Chinese). |
| [32] |
WEI X S, CAI C J, HE J J, et al. Effects of biotin and nicotinamide supplementation on glucose and lipid metabolism and milk production of transition dairy cows[J]. Animal Feed Science and Technology, 2018, 237: 106-117. |
| [33] |
DESLANDES B, GARIÉPY C, HOUDE A. Review of microbiological and biochemical effects of skatole on animal production[J]. Livestock Production Science, 2001, 71(2/3): 193-200. |
| [34] |
孟德超. 集约化牛场体况管理对泌乳奶牛代谢、生产性能和健康的影响[D]. 硕士学位论文. 大庆: 黑龙江八一农垦大学, 2021. MENG D C. Effects of intensive cattle farm body condition management on metabolism, production performance and health of lactating dairy cows[D]. Master's Thesis. Daqing: Heilongjiang Bayi Agricultural University, 2021. (in Chinese) |
| [35] |
朱玉哲. 泌乳量与日粮能量水平对泌乳奶牛生殖机能的影响[D]. 硕士学位论文. 大庆: 黑龙江八一农垦大学, 2009. ZHU Y Z. The effect of milk yield and dietary energy levels on reproductive function of lactating cows[D]. Master's Thesis. Daqing: Heilongjiang Bayi Agricultural University, 2009. (in Chinese) |
| [36] |
许丽惠, 谢丽曲, 林丽花. 叶酸的研究进展[J]. 福建畜牧兽医, 2013, 35(2): 34-36. XU L H, XIE L Q, LIN L H. Research progress of folic acid[J]. Fujian Journal of Animal Husbandry and Veterinary Medicine, 2013, 35(2): 34-36 (in Chinese). |
| [37] |
BÄSSLER K H. Enzymatic effects of folic acid and vitamin B12[J]. International Journal for Vitamin and Nutrition Research, 1997, 67(5): 385-388. |
| [38] |
薛良敏, 王锰, 刘俊义, 等. 新型叶酸拮抗剂吡啶并嘌呤衍生物的环合反应研究[J]. 化学通报, 2018, 81(7): 653-656. XUE L M, WANG M, LIU J Y, et al. Cyclization reaction of pyridanopurine derivatives as novel antifolate[J]. Chemistry, 2018, 81(7): 653-656 (in Chinese). |
| [39] |
方芳, 薛良敏, 丛婧, 等. 2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2111-2120. FANG F, XUE L M, CONG J, et al. Synthesis and anti-tumor activity evaluation of a series of 2-or 4-substituted pyrido[3, 2-d] pyrimidines as nonclassical antifolates[J]. Chemical Journal of Chinese Universities, 2019, 40(10): 2111-2120 (in Chinese). |




