2. 山东省农业科学院 畜牧兽医研究所, 济南 250100;
3. 新疆畜牧科学院, 乌鲁木齐 830000
2. Institute of Animal Husbandry and Veterinary Science, Shandong Academy of Agricultural Sciences, Ji'nan 250100, China;
3. Xinjiang Academy of Animal Science, Urumqi 830000, China
初乳作为新生犊牛的第一营养来源,对犊牛的生长发育及机体免疫功能的建立起着关键作用[1],且富含多种免疫物质和非特异性抗菌物质,对于新生犊牛被动免疫十分重要[2],饲喂初乳可促进犊牛胃肠道发育和生长[3]。我国牧区肉牛养殖多采用放牧或放牧加补饲模式,犊牛随母哺乳,自然断奶。在四川藏区,牦牛多为春季产犊,母牦牛因冬季饲草短缺造成营养摄入不足,春季所产犊牛大多瘦弱。牧区牦牛饲养管理仍较为落后,加之“人畜争奶”现象频发,新生犊牛多未能采食足量的初乳,极易导致犊牛营养摄入不足,机体免疫能力差。犊牛未能采食足量的母乳,生长发育的关键时期营养供应不足,易导致犊牛出现发育迟缓[4]。
犊牛代乳粉作为母乳的替代产品可为早期断奶犊牛提供主要营养来源。王美美等[5]研究表明,荷斯坦犊牛饲喂含13%粗脂肪和22%粗蛋白质的代乳粉对其干物质、粗脂肪和粗蛋白质的消化有促进作用,有利于犊牛免疫力和抵抗力的提高。鲍宇红等[6]研究发现,与母带犊牛相比,饲喂代乳粉可提高早期断奶犊牦牛生长性能,饲喂含26%粗蛋白质的代乳粉更有利于提高犊牦牛机体免疫力和抗氧化能力。但是母带犊牛与饲喂代乳粉的离母犊牛在心理、营养摄入等方面均有较大差异,其机体代谢差异如何目前未见报道。因此,本试验采用超高效液相色谱-四极杆飞行时间质谱(UPLC-QTOF/MS)的代谢组学方法对母带犊牛和饲喂代乳粉的离母犊牛血清进行检测分析,比较母带犊牛和离母犊牛的血清代谢物和代谢途径差异,为犊牛早期断奶提供基础数据,为犊牛代乳粉的开发提供方向。
1 材料与方法 1.1 试验动物与试验设计试验于四川省广元市青川县某牛场内开展。挑选8头健康且体重接近的新生犊牛(牦牛×犏牛♀)为母带犊牛组,犊牛随母哺乳;另外挑选8头体重接近的新生犊牛(牦牛×犏牛♀)为离母犊牛组,犊牛饲喂代乳粉。2组犊牛10 d后自由采食相同饲粮。试验期90 d。
1.2 饲养管理母带犊牛组的犊牛出生后立即擦干被毛并对脐带消毒,随后进行称重,1.0~1.5 h内根据其体重灌服初乳3~4 L,然后跟随母牛哺乳,10 d后自由采食饲粮。
离母犊牛组的犊牛出生后饲喂代乳粉,代乳粉按照1∶8的比例用50 ℃左右温开水溶解并搅拌均匀,待温度冷却到37 ℃左右,分别于07:00和18:00采用奶瓶饲喂,奶瓶在每次饲喂结束后清洗并消毒。代乳粉的日饲喂量为体重的3%,并随着体重的增长每半个月进行1次调整。10 d后自由采食与母带犊牛相同的饲粮。
1.3 代乳粉代乳粉主要成分为脱脂奶粉、乳清粉、玉米蛋白粉等,其组成及营养水平见表 1[7]。
|
|
表 1 代乳粉组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the milk replacer (DM basis) |
试验第90天晨饲前,使用真空采血管在每头牛颈静脉采血10 mL,4 000×g离心10 min制备血清,随后立即保存于-80 ℃冰箱,直到进行代谢组学测定。
取100 μL解冻后的血清样品于EP管中,加入400 μL混有内标的提取溶剂(乙腈∶水=1∶1,内标浓度2 μg/mL),旋涡混匀30 s;转移至冰水中超声5 min;-20 ℃静置1 h;4 ℃条件下,将样品以12 000 r/min离心15 min;将上清(425 μL)转移至EP管中,置于真空浓缩器中进行干燥(无需进行加热),加入100 μL提取溶剂将干燥后的提取物再次溶解;旋涡30 s将其混合均匀,随后经水浴超声处理10 min;4 ℃条件下12 000 r/min离心15 min;将60 μL上清转移至2 mL进样瓶中,各样品均取10 μL充分混匀作为质控样本,另取60 μL进行检测。使用UPLC-QTOF/MS技术进行分析。
色谱条件:样品使用UPLC BEH Amide色谱柱(1.7 μm×2.1 μm×100 mm,Waters,美国)进行分离,进样量为1.5 μL。液相色谱流动相条件见表 2。
|
|
表 2 液相色谱流动相条件 Table 2 Liquid chromatography mobile phase conditions |
质谱条件:使用AB 6600 Triple TOF(AB Sciex,美国)质谱仪并根据其IDA功能作一级、二级质谱数据采集。在每个数据采集循环中,筛选出符合要求的分子离子(离子强度最强且大于100)进行采集对应的二级质谱数据。轰击能量:30 eV,15张二级谱图每50 ms。电喷雾离子源(ESI)参数:雾化气压为60 psi,辅助气压为60 psi,气帘气压为35 psi,温度为650 ℃,喷雾电压为5 000 V(正离子模式)或-4 000 V(负离子模式)。
1.5 数据处理质谱分析后得到的原始数据文件通过Proteo Wizard软件转换为mzXML格式,随后使用基于R语言设计的程序包XCMS 3.2进行数据处理。数据预处理结果到的了一个数据矩阵,其中包括质量电荷比(m/z)值、保留时间(RT)以及峰值强度。数据处理结束后,根据R包CAMERA对峰值进行标注,随后采用自建MS2数据库进行血清代谢物鉴定。
对得到的原始数据进行过滤用以剔除噪声数据,作归一化处理。然后进行主成分分析(PCA),使用R包ROPLS进行正交二乘法-判别分析(OPLS-DA)。根据OPLS-DA获得变量重要性投影(VIP),再结合t检验P值与代谢物的差异倍数(FC)筛选出离母犊牛组和母带犊牛组之间差异代谢物,筛选标准为FC<0.5或FC>1.5、VIP>1和P < 0.01。随后根据KEGG对筛选出的差异代谢物进行功能与代谢途径分析,使用MetaboAnalyst 5.0在线数据库作聚类分析,并作通路拓扑分析用于分析差异代谢物所富集的代谢通路。
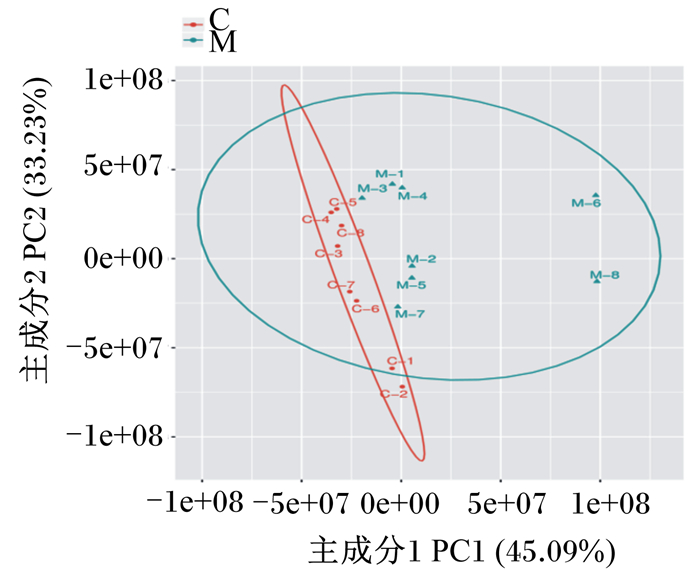
2 结果 2.1 主成分分析2组主成分分析结果见图 1。血清样本基本处于95%置信区间内,样本点分离清晰,2组之间能清楚的区分开来,样本间未出现相互重叠。
|
M:母带犊牛组calves with dam group;C:离母犊牛组calves without dam group。图 3同the same as Fig. 3. 图 1 母带犊牛组与离母犊牛组的主成分分析得分散点图 Fig. 1 Score scatter plot of PCA for calves with dam group and calves without dam group |
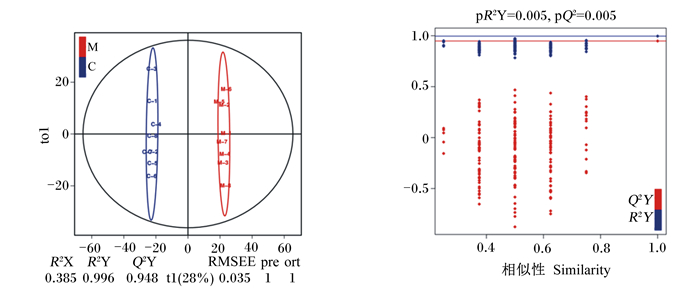
由图 2可见,OPLS-DAS得分结果中,当Q2和R2Y越接近于1时表明模型越稳定可靠,可根据该模型进行差异代谢物筛选。一般而言当Q2>0.5时即可为判定该模型有效,当Q2>0.9时可判定该模型预测能力优异。本试验中,OPLS-DA模型得分对应结果R2Y=0.996、Q2Y=0.998,表明模型稳定性极好。
|
图 2 OPLS-DA得分图及模型排列验证图 Fig. 2 OPLS-DA score chart and model permutation test chart |
采用排列验证对OPLS-DA模型的有效性和预测能力进行评估。红蓝横线分别对应为OPLS-DAS模型Q2和R2,红点表示经Y置换后模型的Q2′,蓝点表示经Y置换后模型的R2′。当Q2′和R2′均小于原始模型的Q2和R2,即相应的线都在其对应点之上时,则表明模型预测能力可靠,可按照各血清化合物的VIP进行差异代谢物的分析筛选。由以上结果可知,该模型可靠,可进行差异代谢物的筛选。
2.3 差异代谢物的筛选根据OPLS-DA获得VIP,再结合t检验P值与代谢物的FC筛选出离母犊牛组和母带犊牛组之间差异代谢物。按照FC<0.5或FC>1.5、P < 0.01和VIP>1的标准进行差异代谢物筛选,2组犊牛共筛选出30种差异代谢物。由表 3可知,与离母犊牛组相比,母带犊牛组血清中代谢物含量上调的主要有DL-3-苯乳酸、橙皮素、D-山梨糖醇等21种代谢物,下调的主要有戊二酸、二十一烷酸、二十酸等9种代谢物。
|
|
表 3 母带犊牛组和离母犊牛组之间差异代谢物 Table 3 Significant metabolics between calves with dam group and calves without dam group |
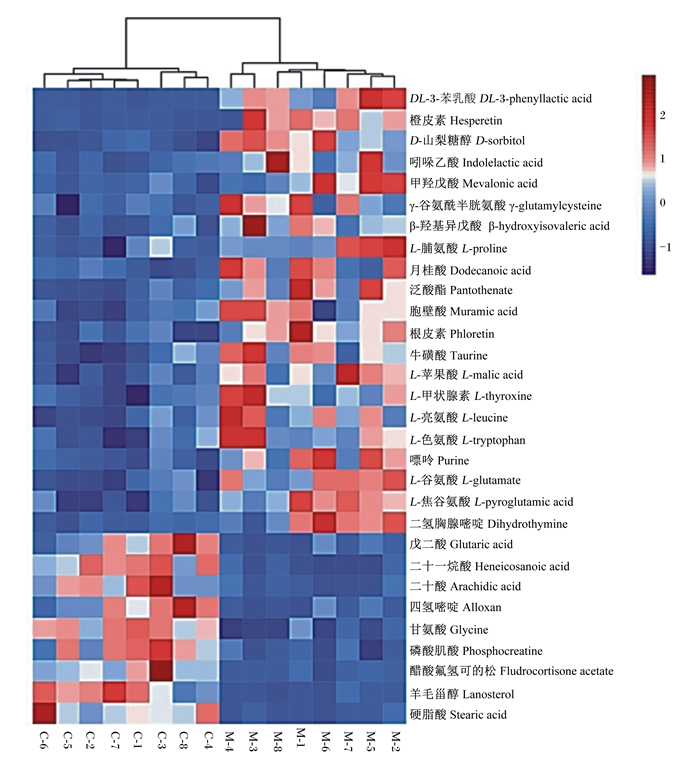
通过对筛选出来的差异代谢物进行层次聚类分析,以热图的方式呈现,直观的展现各差异代谢物在2组之间的差异。由图 3可见,分析结果表明,离母犊牛组与母带犊牛组可明显聚为2类,组间差异明显,组内8个重复间无明显差异,所筛选的差异代谢物合理。
|
图 3 差异代谢物聚类热图 Fig. 3 Clustering heat map of significant metabolites |
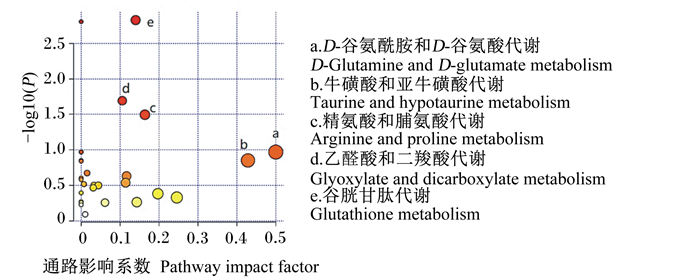
使用Metabo Analyst 5.0在线分析数据库以影响系数(Impact)和P值分析差异代谢物所富集相关代谢通路,结果见图 4。以Impact>0.1为筛选标准进行相关代谢通路筛选,结果见表 4。综合富集分析的显著性P值和通路影响系数(Impact>0.1)发现,与饲喂代乳粉的离母犊牛的血清代谢物的改变最为相关是D-谷氨酰胺和D-谷氨酸代谢、牛磺酸和亚牛磺酸代谢、精氨酸和脯氨酸代谢、乙醛酸和二羧酸代谢以及谷胱甘肽代谢5条代谢通路。
|
图 4 差异代谢物富集图 Fig. 4 Enrichment map of significant metabolites |
|
|
表 4 通路富集 Table 4 Pathway enrichment |
本试验在母带犊牛和离母犊牛血清中共筛选出了30种差异显著的代谢物,其中离母犊牛血清中牛磺酸、L-谷氨酸和橙皮素等代谢物含量显著低于母带犊牛,血清中硬脂酸、二十酸和二十一烷酸等代谢物含量显著高于母带犊牛。血清差异代谢物主要对犊牛的抗菌、抗氧化能力和脂类代谢产生影响。
3.1 离母犊牛抗菌能力弱于母带犊牛本试验结果表明,离母犊牛血清中具有抑菌作用的物质如DL-3-苯乳酸、月桂酸和L-苹果酸的含量显著低于母带犊牛。DL-3-苯乳酸是由部分乳酸菌分泌产生的一种化合物,有着很强的抑菌作用,能够有效抑制犊牛腹泻和肠胃炎的致病菌如大肠杆菌、金黄色葡萄球菌和嗜水气单胞菌[8],以及产生霉菌毒素的真菌[9]。月桂酸及其衍生物对部分病原菌有良好的抑制效果,如革兰氏阳性菌和某些病毒及真菌。Skrivanová等[10]研究表明,月桂酸对产气荚膜梭菌有很强的抑制作用,该菌可导致犊牛发生气性坏疽和病毒性腹泻。月桂酸衍生物单月桂酸甘油单酯(GML)是母乳的常见成分,具有良好的抗菌活性,对易诱发机体炎症、消化道疾病的金黄色葡萄球菌、链球菌以及幽门螺杆菌等常见病原菌有着很强的抑制作用[11]。L-苹果酸对大肠杆菌和鼠伤寒沙门氏菌有很强的抑制作用[12],二者是引起犊牛腹泻的主要致病菌。与母带犊牛相比,离母犊牛血清抑菌功能物质的含量显著降低,在一定程度上降低离母犊牛抵御病原菌的能力,增加了犊牛腹泻及患其他疾病风险。
3.2 离母犊牛抗氧化能力弱于母带犊牛与母带犊牛相比,离母犊牛血清中γ-谷氨酰半胱氨酸、L-谷氨酸含量显著降低。差异代谢物通路分析结果显示,谷胱甘肽代谢途径受到影响。γ-谷氨酰半胱氨酸是合成谷胱甘肽的前体,可以通过绕过谷胱甘肽生物合成调节并提供限制底物,在氧化应激条件下补充减少的谷胱甘肽[13]。谷胱甘肽是一种常见的抗氧化物,有助于机体清除有害氧自由基,缓解各种炎症损伤[14]。本试验结果表明,离母犊牛血清中L-谷氨酸含量显著低于母带犊牛。谷氨酸作为谷胱甘肽合成的前体物质,在清除氧化基和调节免疫反应方面发挥着关键作用[15]。谷氨酸衍生物谷氨酰胺能够改善幼龄哺乳动物的肠道生理功能[16],增强免疫机能,提高机体抗氧化能力[17]。本试验室前期研究表明,90日龄时,离母犊牛血清中超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)活性和总抗氧化能力(T-AOC)显著低于母带犊牛[18],血清抗氧化指标与代谢组学测定结果相一致。
此外,离母犊牛血清中牛磺酸、橙皮素和根皮素等一系列具有抗氧化功能物质的含量显著低于母带犊牛。牛磺酸具有抗氧化功能,对肠道的氧化损伤有缓解作用[19],并可抑制肠道炎症反应,促进肠道的正常发育和功能稳定[20-21]。Barua等[22]研究发现,牛磺酸对肠黏膜核因子-κB(NF-κB)的表达和活化有很强的抑制性,可抑制炎症介质的产生,阻断炎症及其次级炎症的发生。橙皮素具有抗炎、抗氧化和抗病毒等多种功能[23],可调节脂代谢和抗氧化功能,并能够很好的保护肝功能。根皮素是一种抗氧化物质,能够抑制脂质过氧化,并有效清除过氧亚硝酸盐[24]。研究发现,根皮素可以通过抑制前列腺素E2(PGE2)、白细胞介素-8(IL-8)以及晚期糖基化终末产物(AGEs)的形成从而缓解肠道炎症[25]。离母犊牛遭遇早期断奶和饲粮变化,易产生应激反应,机体内自由基累积过多、氧化物过剩,致使机体发生氧化损伤[26]。氧化应激是造成犊牛机体免疫功能失调及炎症反应的一个重要因素[27]。离母犊牛抗氧化能力较弱,也将增加犊牛对各种疾患的易感性。
3.3 离母犊牛脂肪代谢能力强于母带犊牛本试验中,离母犊牛血清中长链饱和脂肪酸含量显著高于母带犊牛,包括硬脂酸、二十酸和二十一烷酸,这可能是离母犊牛机体脂肪分解代谢能力较强,而合成代谢能力较弱的综合结果。脂肪组织分解产生大量非酯化脂肪酸(NEFAs),有研究表明,NEFAs可通过激活肝细胞腺苷酸活化蛋白激酶(AMPK)信号通路,促进脂质氧化,抑制脂类合成[28]。Chatelain等[29]研究表明,肝细胞中长链脂肪酸能够促进肝脏脂肪酸氧化的限速酶肉毒碱棕榈酰基转移酶1α(CPT1α)的表达,促进脂肪酸氧化。另有研究表明,饱和脂肪酸(SFA)可能与动物机体炎症相关,SFA可激活Toll样受体(Toll-like receptor,TLR)中的TLR2和TLR4及其下游信号,导致机体无菌炎症[30]。离母犊牛血清中饱和脂肪酸含量的升高,可能是造成其机体炎症反应的一个潜在因素。离母犊牛血清中甲羟戊酸含量显著低于母带犊牛。甲羟戊酸是胆固醇和萜类化合物合成的重要中间产物,在调节细胞增殖和胶原合成中起重要作用[31]。甲羟戊酸含量与胆固醇的合成呈正相关[32]。在动物中,甲羟戊酸途径产生异戊二烯类物质,包括胆固醇、多萜醇、血红素A和泛醌,它们参与细胞膜生物合成、糖蛋白合成和电子传递[33]。因此,离母犊牛血清中长链脂肪酸含量高于母带犊牛,其健康状况弱于母带犊牛。
4 结论饲喂代乳粉的离母犊牛的抗菌、抗氧化能力弱于母带犊牛,脂肪代谢分解能力强于母带犊牛,脂肪合成代谢能力弱于母带犊牛。
| [1] |
HERNÁNDEZ-CASTELLANO L E, ALMEIDA A M, CASTRO N, et al. The colostrum proteome, ruminant nutrition and immunity: a review[J]. Current Protein & Peptide Science, 2014, 15(1): 64-74. |
| [2] |
KING A, CHIGERWE M, BARRY J, et al. Short communication: effect of feeding pooled and nonpooled high-quality colostrum on passive transfer of immunity, morbidity, and mortality in dairy calves[J]. Journal of Dairy Science, 2020, 103(2): 1894-1899. DOI:10.3168/jds.2019-17019 |
| [3] |
GODDEN S M, LOMBARD J E, WOOLUMS A R. Colostrum management for dairy calves[J]. Veterinary Clinics of North America: Food Animal Practice, 2019, 35(3): 535-556. DOI:10.1016/j.cvfa.2019.07.005 |
| [4] |
HU R, WANG Z S, PENG Q H, et al. Effects of GHRP-2 and cysteamine administration on growth performance, somatotropic axis hormone and muscle protein deposition in yaks (Bos grunniens) with growth retardation[J]. PLoS One, 2016, 11(2): e0149461. DOI:10.1371/journal.pone.0149461 |
| [5] |
王美美, 李秋凤, 高艳霞, 等. 代乳粉营养水平对荷斯坦犊牛养分消化率和血清生化指标的影响[J]. 畜牧与兽医, 2020, 52(11): 24-29. WANG M M, LI Q F, GAO Y X, et al. Effects of milk replacer at different nutrition levels on nutrient digestibility and blood biochemical indexes of Holstein calves[J]. Animal Husbandry & Veterinary Medicine, 2020, 52(11): 24-29 (in Chinese). |
| [6] |
鲍宇红, 宋天增, 冯柯, 等. 不同蛋白质水平代乳粉对牦犊牛体重及血清生化指标的影响[J]. 中国农业大学学报, 2020, 25(5): 78-85. BAO Y H, SONG T Z, FENG K, et al. Effects of different protein levels of milk replacer on the body weight and serum biochemical indexes of yak calves[J]. Journal of China Agricultural University, 2020, 25(5): 78-85 (in Chinese). |
| [7] |
李宁, 袁梅, 王之盛, 等. 活性干酵母对犊牛生长性能及血清生化、抗氧化和免疫指标的影响[J]. 动物营养学报, 2020, 32(11): 5448-5457. LI N, YUAN M, WANG Z S, et al. Effects of active dry yeast on growth performance and serum biochemical, antioxidant and immune indexes of calves[J]. Chinese Journal of Animal Nutrition, 2020, 32(11): 5448-5457 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.11.052 |
| [8] |
DIEULEVEUX V, LEMARINIER S, GUÉGUEN M. Antimicrobial spectrum and target site of D-3-phenyllactic acid[J]. International Journal of Food Microbiology, 1998, 40(3): 177-183. DOI:10.1016/S0168-1605(98)00031-2 |
| [9] |
LAVERMICOCCA P, VALERIO F, VISCONTI A. Antifungal activity of phenyllactic acid against molds isolated from bakery products[J]. Applied and Environmental Microbiology, 2003, 69(1): 634-640. DOI:10.1128/AEM.69.1.634-640.2003 |
| [10] |
SKRIVANOVÁ E, MAROUNEK M, DLOUHÁG, et al. Susceptibility of Clostridium perfringens to C-C fatty acids[J]. Letters in Applied Microbiology, 2005, 41(1): 77-81. DOI:10.1111/j.1472-765X.2005.01709.x |
| [11] |
SCHLIEVERT P M, PETERSON M L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures[J]. PLoS One, 2012, 7(7): e40350. DOI:10.1371/journal.pone.0040350 |
| [12] |
KIM J H, KWON K H, OH S W. Effects of malic acid or/and grapefruit seed extract for the inactivation of common food pathogens on fresh-cut lettuce[J]. Food Science and Biotechnology, 2016, 25(6): 1801-1804. DOI:10.1007/s10068-016-0274-5 |
| [13] |
BRAIDY N, ZARKA M, JUGDER B E, et al. The precursor to glutathione (GSH), γ-glutamylcysteine (GGC), can ameliorate oxidative damage and neuroinflammation induced by Aβ40 oligomers in human astrocytes[J]. Frontiers in Aging Neuroscience, 2019, 11: 177. DOI:10.3389/fnagi.2019.00177 |
| [14] |
SHEN H M, LIU Z G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species[J]. Free Radical Biology & Medicine, 2006, 40(6): 928-939. |
| [15] |
LI P, YIN Y L, LI D F, et al. Amino acids and immune function[J]. The British Journal of Nutrition, 2007, 98(2): 237-252. DOI:10.1017/S000711450769936X |
| [16] |
MARC RHOADS J, WU G Y. Glutamine, arginine, and leucine signaling in the intestine[J]. Amino Acids, 2009, 37(1): 111-122. DOI:10.1007/s00726-008-0225-4 |
| [17] |
CRUZAT V, MACEDO ROGERO M, NOEL KEANE K, et al. Glutamine: metabolism and immune function, supplementation and clinical translation[J]. Nutrients, 2018, 10(11): 1564. DOI:10.3390/nu10111564 |
| [18] |
李梦雅, 袁梅, 王之盛, 等. 母带犊牛与离母犊牛生长性能和血清生化、抗氧化、免疫指标的比较研究[J]. 动物营养学报, 2019, 31(12): 5571-5581. LI M Y, YUAN M, WANG Z S, et al. Comparative study on growth performance and serum biochemical, antioxidant and immune indexes between calves with dam and calves without dam[J]. Chinese Journal of Animal Nutrition, 2019, 31(12): 5571-5581 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.12.023 |
| [19] |
SON M W, KO J I, DOH H M, et al. Protective effect of taurine on TNBS-induced inflammatory bowel disease in rats[J]. Archives of Pharmacal Research, 1998, 21(5): 531-536. DOI:10.1007/BF02975370 |
| [20] |
MOCHIZUKI T, SATSU H, NAKANO T, et al. Regulation of the human taurine transporter by TNF-alpha and an anti-inflammatory function of taurine in human intestinal Caco-2 cells[J]. BioFactors, 2004, 21(1/4): 141-144. |
| [21] |
HAN H L, ZHANG J F, CHEN Y N, et al. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age[J]. Journal of Animal Science, 2020, 98(10): skaa311. DOI:10.1093/jas/skaa311 |
| [22] |
BARUA M, LIU Y, QUINN M R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: decreased NF-kappaB activation and IkappaB kinase activity[J]. Journal of Immunology, 2001, 167(4): 2275-2281. DOI:10.4049/jimmunol.167.4.2275 |
| [23] |
JO S H, KIM M E, CHO J H, et al. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways[J]. Archives of Pharmacal Research, 2019, 42(8): 695-703. DOI:10.1007/s12272-019-01174-5 |
| [24] |
REZK B M, HAENEN G R M M, VAN DER VIJGH W J F, et al. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids[J]. Biochemical and Biophysical Research Communications, 2002, 295(1): 9-13. DOI:10.1016/S0006-291X(02)00618-6 |
| [25] |
ZIELINSKA D, LAPARRA-LLOPIS J M, ZIELINSKI H, et al. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation[J]. Nutrients, 2019, 11(5): 1173. DOI:10.3390/nu11051173 |
| [26] |
赵永伟, 牛玉, 何进田, 等. 双氢青蒿素对脂多糖诱导的断奶仔猪肝氧化应激的影响[J]. 畜牧兽医学报, 2019, 50(10): 2139-2146. ZHAO Y W, NIU Y, HE J T, et al. Effects of dihydroartemisinin on oxidative stress in liver of weaned piglets induced by lipopolysaccharide[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(10): 2139-2146 (in Chinese). DOI:10.11843/j.issn.0366-6964.2019.10.021 |
| [27] |
魏筱诗. 烟酰胺对围产期奶畜糖脂代谢及其子代肠道发育的影响和机制[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2019. WEI X S. Nicotinamide regulates glucose and lipid metabolism of transition dairy and intestinal development of offspring: effects and potential mechanisms[D]. Ph. D. Thesis. Yangling: Northwest A & F University, 2019. (in Chinese) |
| [28] |
李心慰. 乙酸、非酯化脂肪酸、生长激素和催乳素调控奶牛肝细胞脂代谢的信号机制[D]. 博士学位论文. 长春: 吉林大学, 2013. LI X W. The signaling mechanism of acetic acid, non-esterified fatty acids, growth hormone and prolactin on the regulation of lipid metabolism in the hepatocytes of dairy cows[D]. Ph. D. Thesis. Changchun: Jilin University, 2013. (in Chinese) |
| [29] |
CHATELAIN F, KOHL C, ESSER V, et al. Cyclic AMP and fatty acids increase carnitine palmitoyltransferase Ⅰ gene transcription in cultured fetal rat hepatocytes[J]. European Journal of Biochemistry, 1996, 235(3): 789-798. DOI:10.1111/j.1432-1033.1996.00789.x |
| [30] |
HWANG D H, KIM J A, LEE J Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid[J]. European Journal of Pharmacology, 2016, 785: 24-35. DOI:10.1016/j.ejphar.2016.04.024 |
| [31] |
LIU X M, YOU W, CHENG H J, et al. Effect of mevalonic acid on cholesterol synthesis in bovine intramuscular and subcutaneous adipocytes[J]. Journal of Applied Genetics, 2016, 57(1): 113-118. DOI:10.1007/s13353-015-0300-y |
| [32] |
PARKER T S, MCNAMARA D J, BROWN C, et al. Mevalonic acid in human plasma: relationship of concentration and circadian rhythm to cholesterol synthesis rates in man[J]. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(9): 3037-3041. DOI:10.1073/pnas.79.9.3037 |
| [33] |
LIAO P, HEMMERLIN A, BACH T J, et al. The potential of the mevalonate pathway for enhanced isoprenoid production[J]. Biotechnology Advances, 2016, 34(5): 697-713. DOI:10.1016/j.biotechadv.2016.03.005 |




