断奶应激会导致仔猪出现腹泻,影响其肠道健康和生长性能,严重的甚至会导致仔猪死亡,对我国养猪业的发展有着重大影响[1]。仔猪断奶应激发生后常伴随着肠道炎症反应和氧化应激,进而引起肠道损伤[2-4],而氧化平衡状态对于维持肠道黏膜完整性、调节肠黏膜的再生修复有着重要作用[5]。研究显示,核因子-κB(NF-κB)信号通络的激活与炎症反应密不可分[6],核因子-E2相关因子2(Nrf2)信号通路的激活可通过促进机体抗氧化酶的表达发挥抗氧化作用[7]。因此,维持肠道结构的完整性、降低肠道炎症反应和氧化应激,对防治仔猪断奶应激和保护机体健康起到了关键作用。
黄芩苷(baicalin,C21H18O11)是从黄芩的干燥根中提取的一种黄酮类化合物,其在治疗鸡的肝脏、肺脏损伤,小鼠的肺脏炎症和输卵管氧化应激损伤等方面具有积极作用[8-9]。已知黄芩苷可通过抑制NF-κB信号通路对急性胰腺炎模型大鼠发挥抗炎作用[10],通过激活Nrf2信号通路对胆汁淤积小鼠发挥抗氧化作用[11]。黄芩水提取物对于小鼠空肠损伤具有治疗效果[12],但黄芩苷对肠炎模型小鼠空肠的调节作用及分子机制尚不明确。因此,本研究通过给空肠炎症模型小鼠灌胃黄芩苷,从空肠组织形态、炎性因子表达、抗氧化酶活性、炎症和氧化应激相关信号通路关键蛋白表达等方面,探讨黄芩苷对于小鼠空肠炎症的修复作用机制及黄芩苷的最佳剂量,为黄芩苷治疗仔猪断奶应激提供相应的理论依据。
1 材料与方法 1.1 试验材料黄芩苷购于湖北某生物科技有限公司,纯度为85%;试验用小鼠为C57BL/6小鼠,购于斯贝福(北京)生物技术有限公司;脂多糖(LPS)购于Sigma公司(L2880)。
1.2 主要试剂试验所用主要试剂见表 1。
|
|
表 1 主要试剂 Table 1 Main reagents |
将36只C57BL/6雄性小鼠随机分为6组,即对照组、阴性对照组、模型组以及黄芩苷低、中、高剂量组,每组6只。适应性生长7 d后开始试验。对照组小鼠不作处理,阴性对照组和模型组小鼠每天灌胃0.2 mL磷酸盐缓冲液(PBS),黄芩苷组小鼠每天灌胃黄芩苷药液0.2 mL(根据小鼠体重计算用药量,低剂量组为100 mg/kg BW,中剂量组为200 mg/kg BW,高剂量组为400 mg/kg BW),连续灌胃7 d,第7天对照组和阴性对照组小鼠不做其他处理,模型组、黄芩苷组小鼠腹腔注射0.2 mL LPS(3.5 mg/kg BW)。注射LPS 24 h后脱臼处死小鼠并剖检,取空肠组织,分为3段,用生理盐水洗净后其中2段冻于液氮中,30 min后转移至-80 ℃冰箱中保存备用,另一段空肠置于10 mL福尔马林溶液中保存备用。
1.4 组织切片及苏木精-伊红(HE)染色将置于福尔马林溶液中的空肠固定72 h后,制作组织切片,HE染色,中性树胶封片。光镜下观察各组小鼠空肠结构形态的变化情况,观察后用Olympus Image Analysis System图像分析系统拍照,用Image-pro plus 6.0软件测量视野内空肠绒毛高度和隐窝深度,计算绒毛高度与隐窝深度的比值。
1.5 酶联免疫吸附试验(ELISA)每组空肠样本切取相同重量的组织样本,加入一定量的PBS(pH 7.4)用匀浆器将样本充分匀浆,3 000 r/min离心20 min后收集上清。根据试剂盒厂商说明书使用白细胞介素-6(IL-6)、白细胞介素-1β(IL-1β)、肿瘤坏死因子-α(TNF-α)、超氧化物歧化酶(SOD)、活性氧(ROS)、丙二醛(MDA)ELISA试剂盒进行相应指标的测定。
1.6 RNA提取、反转录及实时荧光定量PCR每组空肠样本切取相同重量的组织样本,根据厂商的说明书使用Trizol试剂从肠道组织中提取RNA。使用EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix将分离出的RNA反转录为cDNA,使用EasyScript Green qPCR SuperMix和特异性引物扩增cDNA。使用实时荧光定量PCR仪通过其特异性引物扩增目的基因mRNA(实时定量PCR引物序列如表 2所示)。每个目的基因mRNA样本利用β-肌动蛋白(β-actin)作为其平行对照进行数据分析。
|
|
表 2 实时荧光定量PCR引物 Table 2 Primers used for real-time qPCR |
空肠样本切取相同重量的组织样本,加入RIPA裂解液,根据厂商的说明书提取组织全蛋白或组织胞浆胞核蛋白。蛋白提取后使用BCA试剂盒测定蛋白浓度,通过十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离蛋白,经转膜仪转移至0.22 μm聚偏氟乙烯(PVDF)膜上后用快速封闭液封闭。然后使用1 ∶ 1 000稀释的一抗与膜孵育。TBST中洗涤,使用1 ∶ 1 000稀释的辣根过氧化物酶(HRP)标记二抗与膜孵育,使用ECL检测系统显现印迹,并使用ChemiDoc XRS+图像分析仪定量目的蛋白。
1.8 统计分析使用SPSS 12.0软件采用单因素方差分析(one-way ANOVA)程序对数据进行分析,采用Tukey法进行多重比较检验,结果以平均值±标准误表示,P<0.05表示差异显著。
2 结果 2.1 空肠形态学观察正常情况下,小鼠空肠绒毛结构完整,排列有序,隐窝结构正常(图 1-A和图 1-B)。与对照组相比,模型组小鼠空肠绒毛结构受到严重破坏,绒毛从底部断裂(图 1-C),绒毛高度显著降低(图 1-G,P<0.05),绒毛高度与隐窝深度的比值显著降低(图 1-I,P<0.05),小鼠空肠固有层损伤明显,提示炎症模型构建成功。与模型组相比,黄芩苷低剂量组小鼠空肠绒毛结构仍然受到严重破坏(图 1-D),绒毛高度与隐窝深度的比值并没有显著变化(图 1-I,P>0.05);黄芩苷中剂量组小鼠空肠绒毛结构相比于模型组有所修复,但仍有部分绒毛结构受到明显破坏(图 1-E),而绒毛高度与隐窝深度的比值已经有了显著提高(图 1-I,P<0.05);黄芩苷高剂量组小鼠空肠绒毛分布较为整齐,有绒毛包裹成团脱落至肠腔,绒毛和隐窝结构相比于模型组有明显改善(图 1-E),与模型组相比黄芩苷高剂量组的绒毛长度与隐窝深度的比值显著升高(图 1-G,P<0.05),且未出现明显病理损伤。
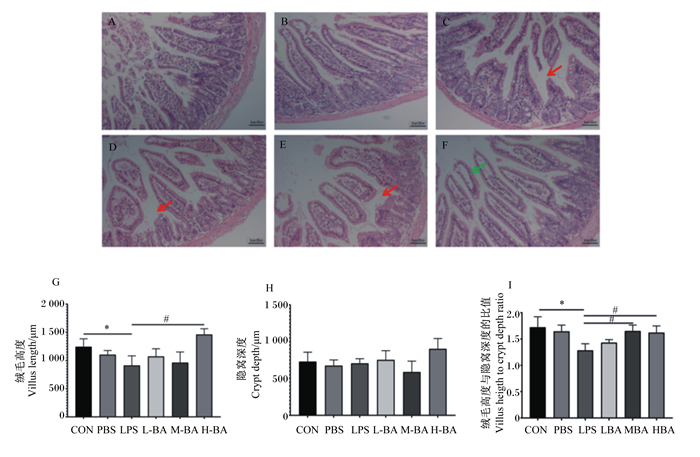
|
A~F分别为对照组、阴性对照组、模型组、黄芩苷低剂量组、黄芩苷中剂量组、黄芩苷高剂量组小鼠空肠形态。红色箭头为绒毛断裂处,绿色箭头为绒毛成团脱落处。 CON:对照组;PBS:阴性对照组;LPS:模型组;L-BA:低剂量黄芩苷组;M-BA:中剂量黄芩苷组;H-BA:高剂量黄芩苷组。*表示与对照组相比差异显著(P<0.05),#表示与模型组相比差异显著(P<0.05)。下图同。 Figures A to F were the jejunal morphology of mice in control group, negative control group, model group, low-dose baicalin group, medium-dose baicalin group and high-dose baicalin group, respectively. The red arrow was the aera of broken villi, and the green arrow was the aera of deciduous microvilli. CON: control group; PBS: negative control group; LPS: model group; L-BA: low-dose baicalin group; M-BA: medium-dose baicalin group; H-BA: high-dose baicalin group. * mean significant difference compared with control group (P < 0.05), and # mean significant difference compared with model group (P < 0.05). The same as below. 图 1 小鼠空肠形态及空肠绒毛高度与隐窝深度的比值 Fig. 1 Morphology of jejunum and villus height to crypt depth ratio of jejunum in mice |
与对照组相比,模型组空肠中IL-6、IL-1β、TNF-α的mRNA表达量显著提高(图 2-B、图 2-C、图 2-D,P<0.05)。与模型组相比,低、中、高剂量的黄芩苷可以显著抑制空肠中因LPS刺激提高的IL-6和TNF-α的mRNA表达量(图 2-B和图 2-C,P<0.05);与模型组相比,中和高剂量的黄芩苷可以显著抑制空肠中因LPS刺激提高的IL-1β的mRNA表达量(图 2-D,P<0.05)。Toll样受体4(TLR4)是细胞膜表面的重要受体,与炎症反应有着密切的关系。与对照组相比,模型组空肠中TLR4的mRNA表达量显著升高(图 2-A,P<0.05),而黄芩苷处理会逆转这一趋势,显著抑制空肠中TLR4 mRNA的表达(P<0.05),且呈现剂量依赖性(图 2-A)。
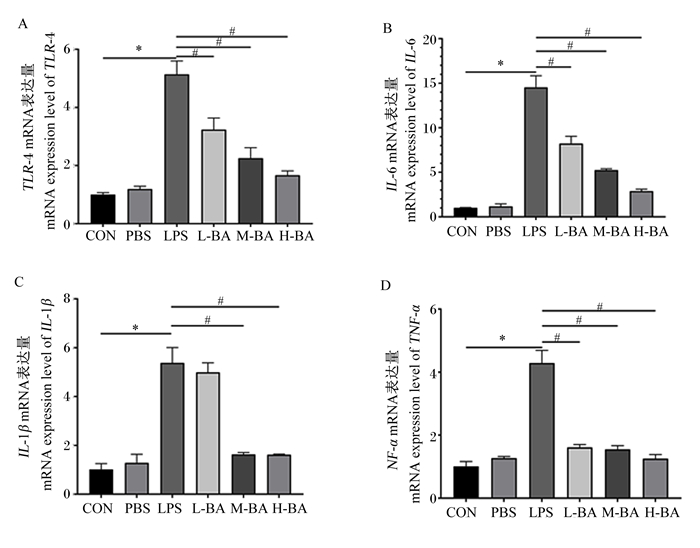
|
图 2 黄芩苷对LPS诱导小鼠空肠炎症相关因子mRNA表达量的影响 Fig. 2 Effects of baicalin on mRNA expression levels of inflammation related factors in jejunum of mice induced by LPS |
与对照组相比,模型组空肠中IL-6、IL-1β、TNF-α的含量显著提高(图 3-A、图 3-B、图 3-C,P<0.05)。与模型组相比,中和高剂量的黄芩苷可以显著降低空肠中因LPS诱导升高的IL-6和IL-1β含量(图 3-A和图 3-B,P<0.05)。与模型组相比,黄芩苷可以显著降低空肠中因LPS诱导升高的TNF-α含量(P<0.05),并呈现剂量依赖性(图 3-C)。
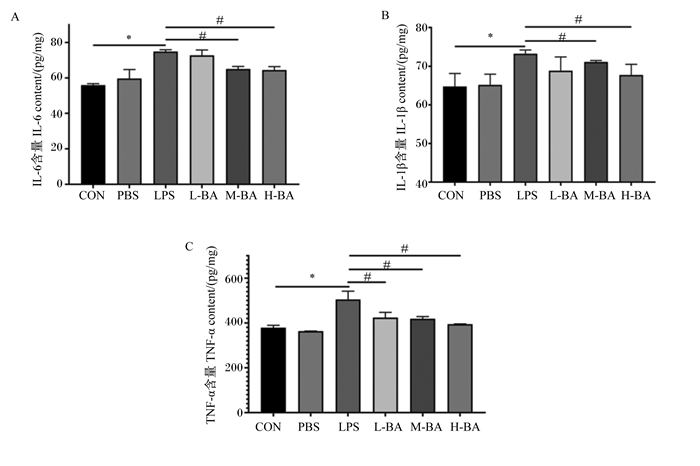
|
图 3 黄芩苷对LPS诱导小鼠空肠炎性因子含量的影响 Fig. 3 Effects of baicalin on inflammatory factor contents in jejunum of mice induced by LPS |
与对照组相比,模型组空肠中ROS和MDA的含量显著提高(图 4-A和图 4-B,P<0.05)。与模型组相比,黄芩苷可以显著抑制空肠中因LPS刺激而升高的ROS和MDA含量(P<0.05),并呈现剂量依赖性(图 4-A和图 4-B)。与对照组相比,模型组空肠中SOD的活性显著降低(图 4-C,P<0.05)。与模型组相比,高剂量的黄芩苷可以显著升高空肠中因LPS刺激而降低的SOD活性(图 4-C,P<0.05)。
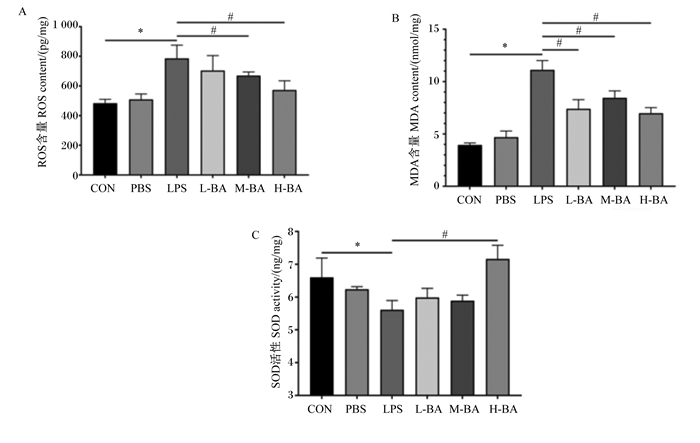
|
图 4 黄芩苷对LPS诱导小鼠空肠抗氧化指标的影响 Fig. 4 Effects of baicalin on antioxidant indexes in jejunum of mice induced by LPS |
与对照组相比,模型组空肠中HO-1和醌氧化还原酶-1(NQO-1)的mRNA表达量无显著变化(图 5-A和图 5-B,P>0.05)。与模型组相比,中和高剂量的黄芩苷可以显著增加空肠中HO-1的mRNA表达量(P<0.05),同时黄芩苷还可以显著增加空肠中NQO-1的mRNA表达量(P<0.05),且呈现剂量依赖性(图 5-A和图 5-B)。
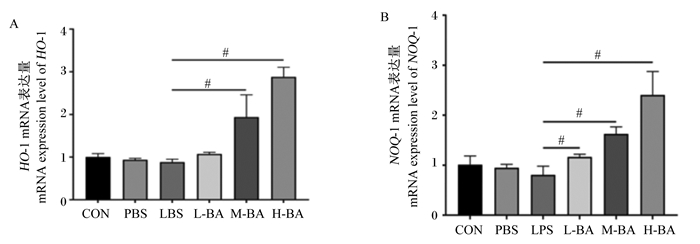
|
图 5 黄芩苷对LPS诱导小鼠空肠Nrf2信号通路关键因子mRNA表达量的影响 Fig. 5 Effects of baicalin on mRNA expression levels of key factors in Nrf2 signaling pathway in jejunum of mice induced by LPS |
与对照组相比,LPS刺激对于空肠中IκBα和P65的蛋白表达量并没有显著影响(图 6-B和图 6-E,P>0.05)。与对照组相比,模型组空肠中P-IκBα和P-P65的蛋白表达量显著提高(图 6-C和图 6-F,P<0.05);与模型组相比,低、中、高剂量的黄芩苷可以显著抑制空肠中因LPS刺激而升高的P-IκBα和P-P65蛋白表达量(图 6-C和图 6-F,P<0.05)。与对照组相比,模型组小鼠空肠中P-IκBα/IκBα比值显著升高(图 6-D,P<0.05);与模型组相比,低、中、高剂量的黄芩苷可以显著抑制这一比值的升高(图 6-D,P<0.05)。与对照组相比,模型组空肠中P-P65/P65比值显著升高(图 6-G,P<0.05);与模型组相比,黄芩苷显著降低这一比值(P<0.05),且呈现剂量依赖性(图 6-G)。
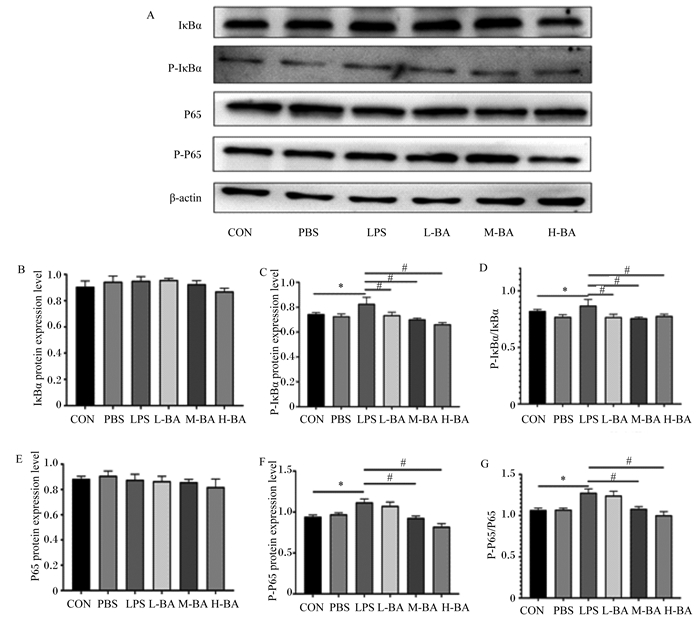
|
IκBα:核因子-κB抑制蛋白α inhibitor of nuclear factor-kappa Bα;P-IκBα:磷酸化核因子-κB抑制蛋白α phosphorylated inhibitor of nuclear factor-kappa Bα;P-P65:磷酸化P65 phosphorylated P65;β-actin:β-肌动蛋白;protein expression level:蛋白表达量。 图 6 黄芩苷对LPS诱导小鼠空肠NF-κB信号通路关键蛋白表达量的影响 Fig. 6 Effects of baicalin on expression levels of key proteins in NF-κB signaling pathway in jejunum of mice induced by LPS |
与对照组相比,模型组空肠中HO-1的蛋白表达量显著降低(图 7-B,P<0.05);与模型组相比,中、高剂量的黄芩苷会显著提高空肠中HO-1的蛋白表达量(图 7-B,P<0.05)。与对照组相比,模型组空肠中胞浆Nrf2的蛋白表达量无显著变化(图 7-C,P>0.05),空肠中胞核Nrf2的蛋白表达量也没有显著变化(图 7-D,P>0.05);但是与模型组相比,低、中、高剂量的黄芩苷会显著提高空肠中胞核Nrf2的蛋白表达量(图 7-D,P<0.05)。
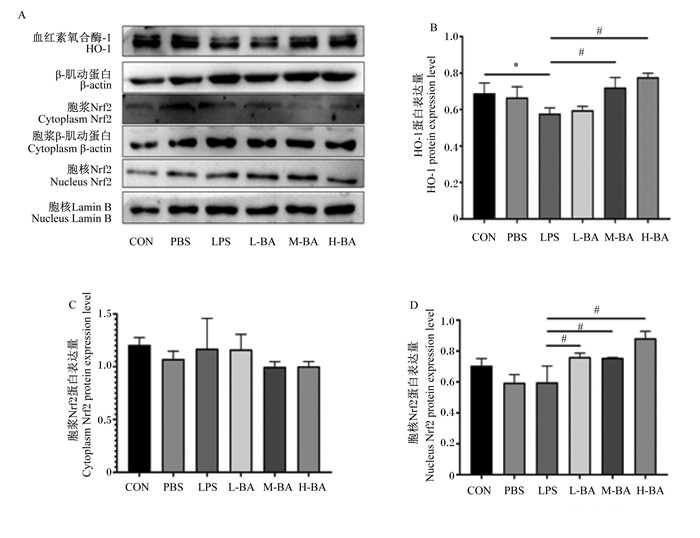
|
图 7 黄芩苷对LPS诱导小鼠空肠Nrf2/HO-1信号通路关键蛋白表达量的影响 Fig. 7 Effects of baicalin on expression levels of key proteins in Nrf2/HO-1 signaling pathway in jejunum of mice induced by LPS |
空肠是哺乳动物体内重要的营养物质吸收器官,肠道炎症和氧化应激的发生会导致肠道屏障功能恶化,可能会使得有害物质或病原进入血液,导致消化系统的疾病[13]。LPS是革兰氏阴性菌细胞壁的成分,可以诱导肠道的炎症反应[14],用LPS注射小鼠腹腔后还能诱导空肠发生损伤[15]。黄芩苷对于LPS诱导的多器官急性损伤、肝脏细胞和H9c2细胞的炎症反应有较好的治疗效果[16-18]。因此,本文采用腹腔注射LPS构建小鼠空肠炎症模型,使用黄芩苷预处理作为治疗组。本研究中阴性对照组与对照组基本无显著差异,故不单独讨论阴性对照组情况。与对照组相比,模型组小鼠空肠组织结构炎症受损,这与前人研究结果[19]一致,表明空肠炎症反应诱发了结构损伤。相对于模型组,低、中、高剂量黄芩苷组小鼠空肠结构得到改善,尤其是高剂量黄芩苷组的小鼠空肠绒毛结构趋于完整,表明黄芩苷对于LPS诱导的小鼠空肠组织损伤具有保护作用。黄芩苷可以促进猪肠上皮细胞的增殖[20-21],这可能是黄芩苷维持小鼠空肠结构完整性的潜在作用机制。
IL-6、IL-1β、TNF-α是重要的细胞炎性因子,在小鼠肠道炎症反应中起着关键作用。TLR4-NF-κB信号通路是重要的炎症反应通路[22],也是IL-6、IL-1β和TNF-α等炎性因子的上游通路,其中TLR4是细胞膜表面重要的识别受体[23]。一般情况下,非活化NF-κB复合物(包括功能性亚基P65和抑制性亚基IκBα)位于细胞质中[24],当TLR4受到外源刺激时,抑制性亚基IκBα发生磷酸化,与功能性亚基P65解离[25],解离的功能性亚基P65磷酸化后进入细胞核诱导炎性因子IL-6、IL-1β和TNF-α等分泌[26],进而导致组织的炎症反应。结果显示,相对于对照组,模型组小鼠空肠中炎性因子在mRNA和蛋白水平的表达量均显著升高,这表明LPS刺激使小鼠空肠发生炎症反应。相比于对照组,模型组小鼠空肠NF-κB信号通路关键蛋白的表达量显著升高;相比于模型组,中、高剂量的黄芩苷显著抑制了小鼠空肠NF-κB信号通路关键蛋白的表达量,与感染产肠毒素大肠杆菌(ETEC)的猪肠道上皮细胞的反应[27]相一致,以上结果表明黄芩苷通过阻断LPS激活的NF-κB信号通路降低炎症反应。王欢[28]研究发现,小鼠空肠NF-κB信号通路激活后会诱导炎症反应的产生,进而破坏空肠肠道黏膜屏障的完整性。因此,黄芩苷通过发挥抗炎作用有助于维持小鼠空肠黏膜屏障的完整性。
健康状态的组织处于ROS和抗氧化酶之间的平衡状态,而当这一平衡状态被打破时,过量的ROS及抗氧化酶活性或表达量降低会导致组织氧化应激的发生[29]。氧化应激会引起细胞发生死亡和不可修复的氧化损伤[30]。本研究中,LPS诱导小鼠空肠TLR4的mRNA表达量显著升高,并诱导空肠发生炎症反应。TLR4的激活可以诱导过量的ROS产生[31],LPS刺激鸡HD11巨噬细胞也能产生过量的ROS,发生氧化应激[32]。肾炎模型小鼠中因炎症反应产生的炎性因子会破坏细胞内氧化还原的平衡状态,诱导产生氧化应激[33],炎症反应活化中性粒细胞会持续产生过量的ROS。因此作者推测,LPS会诱导小鼠空肠产生过量的ROS,诱发氧化应激。SOD是一种重要的抗氧化酶,其对于维持氧化还原平衡有着重要的意义[34],当SOD活性异常降低时组织可能会发生氧化应激。本研究中,LPS抑制了抗氧化酶的表达量,提高了ROS和MDA的含量,表明LPS刺激引起小鼠空肠组织的氧化应激。正常情况下Kelch样环氧氯丙烷相关蛋白-1(Keap1)与Nrf2形成复合体处于细胞质中,当发生氧化应激时,Keap1与Nrf2解离,Nrf2进入细胞核内与DNA上相应靶位点相结合,诱导相应的解毒酶和抗氧化酶的合成[35],而HO-1和NQO-1是Nrf2信号通路所激活的重要抗氧化酶,HO-1和NQO-1的活性对于维持氧化平衡状态有着重要意义[36]。在鸡的支原体病毒感染过程中,黄芩苷也可以激活脾脏的Nrf2信号通路及其下游蛋白的表达[37]。本试验中,黄芩苷通过激活小鼠空肠的Nrf2信号通路促进抗氧化酶HO-1和NQO-1 mRNA的表达以抑制氧化应激,避免了氧化应激造成的结构损伤。
药物的治疗效果与剂量有关[38]。本试验中黄芩苷在反映抗炎、抗氧化效果的各项指标如炎性因子、抗氧化酶表达等上均表现出剂量依赖性。中和高剂量的黄芩苷都可提高空肠绒毛高度和隐窝深度的比值,但高剂量的黄芩苷提高的更为显著。P-P65蛋白与IL-6、IL-1β mRNA的表达量结果都显示中剂量黄芩苷组和高剂量黄芩苷组较模型组有显著降低,且2组间差异不显著。在氧化应激中空肠SOD的活性只有高剂量黄芩苷组相比于模型组有显著提高,其余氧化因子及Nrf2信号通路关键蛋白的表达量都呈现剂量依赖性。小鼠在灌胃200和400 mg/kg BW黄芩苷后炎症和氧化应激等各项指标得到显著改善。综合考虑作为饲料添加剂的成本问题,黄芩苷的推荐剂量是200 mg/kg BW。LPS刺激断奶仔猪后,血清中炎性因子IL-6、IL-8和ROS的含量升高,表明机体产生了较强的应激反应[39],小鼠腹腔注射LPS后其空肠也发生了炎症反应和氧化应激,所以小鼠空肠炎症模型可模拟仔猪的断奶应激反应。本研究证实黄芩苷对空肠炎症小鼠可以通过抑制NF-κB信号通路发挥抗炎症作用,通过激活Nrf2信号通路发挥抗氧化作用,因此,黄芩苷可作为断奶仔猪应激综合征的潜在治疗药物。
4 结论在腹腔注射LPS构建的小鼠空肠炎症模型中,黄芩苷在适宜剂量内可以通过抑制NF-κB信号通路发挥抗炎症作用,通过激活Nrf2信号通路发挥抗氧化作用,推荐剂量是200 mg/kg BW。
| [1] |
CHAN G, FARZAN A, SOLTES G, et al. The epidemiology of Clostridium perfringens type A on Ontario swine farms, with special reference to cpb2-positive isolates[J]. BMC Veterinary Research, 2012, 8: 156. DOI:10.1186/1746-6148-8-156 |
| [2] |
范长友. 刺五加多糖对脂多糖刺激断奶仔猪肠粘膜损伤的影响研究[D]. 硕士学位论文. 沈阳: 沈阳农业大学, 2020. FAN C Y. Effects of Acanthopanax senticosus polysaccharides on intestinal mucosal injury in immune stress weaned piglets[D]. Master's Thesis. Shenyang: Shenyang Agricultural University, 2020. (in Chinese) |
| [3] |
易宗容, 冯堂超. 饲粮添加白藜芦醇对断奶仔猪生长性能和肠道健康的影响[J]. 中国饲料, 2020(10): 32-36. YI Z R, FENG T C. Effects of dietary resveratrol supplementation on growth performance and intestinal health of weaned piglets[J]. China Feed, 2020(10): 32-36 (in Chinese). |
| [4] |
ZENG Y D, WANG Z R, ZOU T D, et al. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets[J]. Frontiers in Veterinary Science, 2021, 8: 623899. DOI:10.3389/fvets.2021.623899 |
| [5] |
FANDY T E, JIEMJIT A, THAKAR M, et al. Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes[J]. Clinical Cancer Research, 2014, 20(5): 1249-1258. DOI:10.1158/1078-0432.CCR-13-1453 |
| [6] |
朱凌羽, 张子琪, 兰海楠, 等. 虾青素对脂多糖通过TLR4/MyD88/NF-κB信号通路诱导的IPEC-J2细胞炎症的影响[J]. 华南农业大学学报, 2018, 39(5): 53-58. ZHU L Y, ZHANG Z Q, LAN H N, et al. Effects of astaxanthin on IPEC-J2 cell inflammation induced by lipopolysaccharide via TLR4/MyD88/NF-κB signaling pathway[J]. Journal of South China Agricultural University, 2018, 39(5): 53-58 (in Chinese). |
| [7] |
ZHOU J Y, LIN H L, QIN Y C, et al. L-carnosine protects against deoxynivalenol-induced oxidative stress in intestinal stem cells by regulating the Keap1/Nrf2 signaling pathway[J/OL]. Molecular Nutrition & Food Research, 2021: e2100406. [2021-07-03]. https://pubmed.ncbi.nlm.nih.gov/34216418/. DOI: 10.1002/mnfr.202100406.
|
| [8] |
MENG X L, HU L, LI W Q. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway[J]. Naunyn-Schmiedeberg's Archives of Pharmacology, 2019, 392(11): 1421-1433. DOI:10.1007/s00210-019-01680-9 |
| [9] |
WANG J, ISHFAQ M, LI J C. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism[J]. Food & Function, 2021, 12(9): 4092-4104. |
| [10] |
QIAN Y Z, CHEN Y, WANG L Y, et al. Effects of baicalin on inflammatory reaction, oxidative stress and PKDl and NF-κB protein expressions in rats with severe acute pancreatitis1[J]. Acta Cirurgica Brasileira, 2018, 33(7): 556-564. DOI:10.1590/s0102-865020180070000001 |
| [11] |
WANG X X, CHANG X H, ZHAN H B, et al. Curcumin and baicalin ameliorate ethanol-induced liver oxidative damage via the Nrf2/HO-1 pathway[J/OL]. Journal of Food Biochemistry, 2020: e13425. [2020-08-08]. https://pubmed.ncbi.nlm.nih.gov/32770697/. DOI: 10.1111/jfbc.13425.
|
| [12] |
刘晓曦, 董杰, 李敏霞, 等. 黄芩水提液对肠炎模型小鼠空肠损伤修复的作用[J]. 畜牧兽医学报, 2020, 51(2): 392-398. LIU X X, DONG J, LI M X, et al. Effect of the Scutellaria baicalensis water extraction on the regulation of jejunum damage and repair in the model of enteritis mice[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(2): 392-398 (in Chinese). |
| [13] |
YI Q Y, LIU J X, ZHANG Y F, et al. Anethole attenuates enterotoxigenic Escherichia coli-induced intestinal barrier disruption and intestinal inflammation via modification of TLR signaling and intestinal microbiota[J]. Frontiers in Microbiology, 2021, 12: 647242. DOI:10.3389/fmicb.2021.647242 |
| [14] |
杨志远, 苏洁, 俞静静, 等. 铁皮石斛超微粉对LPS致肠黏膜损伤小鼠的保护作用研究[J]. 中国中药杂志, 2021, 46(7): 1667-1673. YANG Z Y, SU J, YU J J, et al. Protective effects of Dendrobium officinale superfine powder against LPS-induced intestinal mucosal injury in mice[J]. China Journal of Chinese Materia Medica, 2021, 46(7): 1667-1673 (in Chinese). |
| [15] |
王梦竹. 京尼平苷对LPS诱导小鼠肠道损伤时NF-κB/IAP的影响[D]. 硕士学位论文. 大庆: 黑龙江八一农垦大学, 2020. WANG M Z. Effect of geniposide on NF-κB/IAP in LPS-induced intestinal damage in mice[D]. Master's Thesis. Daqing: Heilongjiang Bayi Agricultural University, 2020. (in Chinese) |
| [16] |
HUANG Y Q, SUN M Y, YANG X F, et al. Baicalin relieves inflammation stimulated by lipopolysaccharide via upregulating TUG1 in liver cells[J]. Journal of Physiology and Biochemistry, 2019, 75(4): 463-473. DOI:10.1007/s13105-019-00698-0 |
| [17] |
LIU X Y, WANG S L, ZHAO G A. Baicalin relieves lipopolysaccharide-evoked inflammatory injury through regulation of miR-21 in H9c2 cells[J]. Phytotherapy Research, 2020, 34(5): 1134-1141. DOI:10.1002/ptr.6583 |
| [18] |
汪旭, 张鑫, 马晓娟, 等. 黄芩苷在脂多糖(LPS)诱导的大鼠多器官急性损伤中的保护作用[J]. 河南科技大学学报(医学版), 2019, 37(2): 104-107, 123. WANG X, ZHANG X, MA X J, et al. Protective effect of baicalin on LPS-induced acute injury in multiple rat organs[J]. Journal of Henan University of Science & Technology (Medical Science), 2019, 37(2): 104-107, 123 (in Chinese). |
| [19] |
张莹. 诃子提取物对LPS致小鼠肠黏膜损伤的保护作用及机制研究[D]. 硕士学位论文. 哈尔滨: 东北农业大学, 2019. ZHANG Y. Protective effect and its mechanism of Chebulia extract on intestinal mucosal injury induced by LPS in mice[D]. Master's Thesis. Harbin: Northeast Agricultural University, 2019. (in Chinese) |
| [20] |
王忠清, 林春发, 钟文杰, 等. 术芩提取液对脂多糖损伤的IPEC-J2细胞增殖及炎症因子转录的影响[J]. 畜牧兽医学报, 2019, 50(7): 1500-1508. WANG Z Q, LIN C F, ZHONG W J, et al. Effect of Zhuqin extractive fluid on the proliferation and transcription of inflammatory factors in LPS-injured IPEC-J2 cells[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(7): 1500-1508 (in Chinese). |
| [21] |
刘晓曦, 马云飞, 李焕荣, 等. 加味葛根芩连汤对湿热泄泻仔猪肠道炎症和损伤修复的作用[J]. 畜牧兽医学报, 2021, 52(1): 238-247. LIU X X, MA Y F, LI H R, et al. Effect of Jiawei Gegen Qinlian decoction on the regulation of intestine damage and repair in the piglets of damp-heat diarrhea[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(1): 238-247 (in Chinese). |
| [22] |
WULLAERT A. Role of NF-kappaB activation in intestinal immune homeostasis[J]. International Journal of Medical Microbiology, 2010, 300(1): 49-56. DOI:10.1016/j.ijmm.2009.08.007 |
| [23] |
BURKEY T E, SKJOLAAS K A, DRITZ S S, et al. Expression of Toll-like receptors, interleukin 8, macrophage migration inhibitory factor, and osteopontin in tissues from pigs challenged with Salmonella enterica serovar Typhimurium or serovar Choleraesuis[J]. Veterinary Immunology and Immunopathology, 2007, 115(3/4): 309-319. |
| [24] |
MAO X H, SU Z Y, MOOKHTIAR A K. Long non-coding RNA: a versatile regulator of the nuclear factor-κB signalling circuit[J]. Immunology, 2017, 150(4): 379-388. DOI:10.1111/imm.12698 |
| [25] |
王宇, 陈霞, 吴立斌, 等. 艾灸对腹泻型肠易激综合征模型大鼠海马与结肠组织中IKKβ/IKBα/NF-κB通路的影响[J]. 安徽中医药大学学报, 2020, 39(3): 32-36. WANG Y, CHEN X, WU L B, et al. Effect of moxibustion on the IKKβ/IKBα/NF-κB pathway in hippocampal and colonic tissue in a rat model of diarrhea-predominant irritable bowel syndrome[J]. Journal of Anhui University of Chinese Medicine, 2020, 39(3): 32-36 (in Chinese). DOI:10.3969/j.issn.2095-7246.2020.03.009 |
| [26] |
LIU M J, SONG S X, LI H R, et al. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide[J]. Journal of Dairy Science, 2014, 97(5): 2856-2865. DOI:10.3168/jds.2013-7600 |
| [27] |
LIU X X, LIU F H, MA Y F, et al. Effect of puerarin, baicalin and berberine hydrochloride on the regulation of IPEC-J2 cells infected with enterotoxigenic Escherichia coli[J]. Evidence-Based Complementary and Alternative Medicine, 2019, 2019: 7438593. |
| [28] |
王欢. 虾青素对赭曲霉毒素A引起小鼠空肠损伤的影响[D]. 硕士学位论文. 沈阳: 沈阳农业大学, 2020. WANG H. Effects of astaxanthin on ochratoxin A-induced jejunum damage in mice[D]. Master's Thesis. Shenyang: Shenyang Agricultural University, 2020. (in Chinese) |
| [29] |
SIES H. Oxidative stress: oxidants and antioxidants[J]. Experimental Physiology, 1997, 82(2): 291-295. DOI:10.1113/expphysiol.1997.sp004024 |
| [30] |
YIN J, WU M M, XIAO H, et al. Development of an antioxidant system after early weaning in piglets[J]. Journal of Animal Science, 2014, 92(2): 612-619. DOI:10.2527/jas.2013-6986 |
| [31] |
YUAN X, ZHOU Y, WANG W, et al. Activation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS production[J]. Cell Death & Disease, 2013, 4(9): e794. |
| [32] |
GOU Z Y, JIANG S Q, ZHENG C T, et al. Equol inhibits LPS-induced oxidative stress and enhances the immune response in chicken HD11 macrophages[J]. Cellular Physiology and Biochemistry, 2015, 36(2): 611-621. DOI:10.1159/000430124 |
| [33] |
FAN H, LE J W, SUN M, et al. Pretreatment with S-nitrosoglutathione attenuates septic acute kidney injury in rats by inhibiting inflammation, oxidation, and apoptosis[J]. BioMed Research International, 2021, 2021: 6678165. |
| [34] |
王思博, 崔红霞, 杨季, 等. 超氧化物歧化酶模拟物对氧化应激IPEC-J2细胞抗氧化性能的影响[J]. 中国畜牧兽医, 2021, 48(5): 1566-1573. WANG S B, CUI H X, YANG J, et al. Effect of superoxide dismutase mimetic on antioxidant capacity of oxidatively damaged IPEC-J2 cells[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(5): 1566-1573 (in Chinese). |
| [35] |
LI L R, DONG H, SONG E Q, et al. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling[J]. Chemico-Biological Interactions, 2014, 209: 56-67. DOI:10.1016/j.cbi.2013.12.005 |
| [36] |
HEO H S, HAN G E, WON J, et al. Pueraria montana var. lobata root extract inhibits photoaging on skin through Nrf2 pathway[J]. Journal of Microbiology and Biotechnology, 2019, 29(4): 518-526. DOI:10.4014/jmb.1812.12019 |
| [37] |
ISHFAQ M, CHEN C L, BAO J X, et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection[J]. Poultry Science, 2019, 98(12): 6296-6310. DOI:10.3382/ps/pez406 |
| [38] |
朱慧渊, 万海同, 王江, 等. 中医药防治缺血性脑卒中剂量-效应关系的探讨[J]. 中医药学报, 2021, 49(6): 9-11. ZHU H Y, WAN H T, WANG J, et al. Dose-effect relationship in preventing and treating cerebral ischemic stroke with traditional Chinese medicine[J]. Acta Chinese Medicine and Pharmacology, 2021, 49(6): 9-11 (in Chinese). |
| [39] |
刘显军, 陈静, 王丽, 等. 断奶仔猪试验中建立LPS应激模型的作用体现[J]. 黑龙江畜牧兽医, 2017(17): 141-143. LIU X J, CHEN J, WANG L, et al. Effects of LPS stress model in weaning piglet test[J]. Heilongjiang Animal Science and Veterinary Medicine, 2017(17): 141-143 (in Chinese). |




