2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
新生仔猪宫内发育迟缓(intrauterine growth retardation, IUGR)的发生率为6%~10%,早期死亡率高达75%,这给养猪业造成了较大的经济损失[1-2]。肠道屏障可通过机械、化学、免疫和生物屏障的互作,调节机体稳态,抵抗病原体和食源性抗原的入侵,对维持动物肠道健康至关重要[3]。因此,评价IUGR仔猪的小肠形态和屏障功能特征,可以为调控其肠道发育与健康提供依据。研究发现,IUGR新生仔猪的肠道发育和屏障功能受损严重[4]。与正常出生体重(normal birth weight,NBW)仔猪相比,IUGR仔猪的小肠绒毛变短且数量较少、隐窝细胞凋亡增加、刷状缘酶活性降低[5];IUGR仔猪的紧密连接发育不完善,导致肠道屏障的通透性增加[6];IUGR新生仔猪的小肠上皮杯状细胞和淋巴细胞数量减少[7],白细胞介素-8(IL-8)和肿瘤坏死因子-α(TNF-α)等促炎细胞因子的含量升高[8]。此外,IUGR仔猪肠黏膜上附着的总细菌数量增加[9]。笔者前期研究也发现,不同生长阶段的IUGR猪,其肠道微生物多样性显著降低,且更易富集一些病原菌和条件致病菌,其肠道微生物屏障受损[10-11]。小肠绒毛、紧密连接蛋白和黏膜免疫细胞分别在肠道营养物质吸收、通透性维持和炎症反应方面发挥着重要作用,是评价小肠健康的重要指标[12]。因此,本文比较了1~28日龄IUGR仔猪和NBW仔猪小肠形态、紧密连接蛋白和免疫细胞因子表达的差异,为IUGR仔猪肠道发育的调控提供依据。
1 材料与方法 1.1 试验设计动物试验在中国科学院亚热带农业生态研究所永安试验基地开展。试验选取“长白×大白”杂交新生仔猪24窝(第3~4胎次母猪生产,产仔数12头左右)。称取仔猪初生体重,统计产仔数,并计算窝重。根据Bauer等[13]的方法,从每窝初生重低于平均体重10%的仔猪中选取1头IUGR仔猪(IUGR组,平均体重为0.98 kg)、从大于平均体重10%的仔猪中选取1头NBW仔猪(NBW组,平均体重为1.68 kg)。所选仔猪均随母猪自然哺乳,不调栏。5~21日龄补充商品教槽饲粮,21日龄断奶后饲喂商品教槽饲粮至28日龄。饲喂、饮水、光照和免疫等饲养管理按养殖场规范操作。
1.2 样品采集分别于7、21和28日龄时选取IUGR仔猪和NBW仔猪各8头(每窝选取1头IUGR仔猪和1头NBW仔猪),空腹称重,7、21和28日龄选取的IUGR仔猪和NBW仔猪平均体重分别为1.77和2.79 kg、4.71和6.45 kg、5.06和7.96 kg[10]。麻醉放血处死,取中段部位的空肠和回肠组织,用10%中性福尔马林溶液固定,用于肠道形态学观察。刮取中段部位的空肠和回肠黏膜,液氮速冻后-80 ℃保存,用于mRNA表达分析。
1.3 小肠形态观察取固定的小肠组织,经修整、洗涤、脱水、透明、浸蜡和包埋等步骤后,再切片、苏木精-伊红(HE)染色和封片,并在显微镜下观察空肠和回肠组织形态,测定绒毛高度和隐窝深度,并计算绒毛高度/隐窝深度(V/C)值。
1.4 小肠黏膜屏障相关基因表达分析取-80 ℃冻存的空肠和回肠黏膜样品,液氮中研磨,并根据RNA TRIzol试剂盒说明书提取总RNA,采用Nanodrop 2000微量紫外分光光度计测定其浓度,用PrimeScript RT试剂盒将其反转为cDNA。以β-肌动蛋白(β-actin)为内参基因、cDNA为模板,利用ABI-7900HT RT PCR仪(Applied Biosystems,美国)测定小肠黏膜中紧密连接蛋白闭锁小带蛋白-1(ZO-1)、封闭蛋白-1(claudin-1)和闭合蛋白(occludin),抗炎细胞因子白细胞介素-4(IL-4),以及促炎细胞因子IL-8、白细胞介素-1β(IL-1β)和TNF-α等基因的mRNA相对表达量。所用引物由生工生物工程(上海)股份有限公司合成,引物序列见表 1,根据2-ΔΔCt法对数据进行处理分析。
|
|
表 1 引物序列 Table 1 Primer sequences |
试验数据用Excel 2019初步整理后,采用SPSS 22.0软件进行统计分析,试验结果以“平均值±标准误”表示,小肠形态测量和黏膜基因mRNA相对表达量比较使用独立样本t检验,P<0.05表示差异显著。
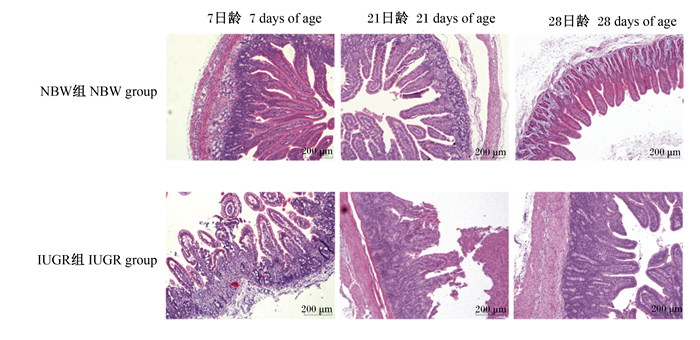
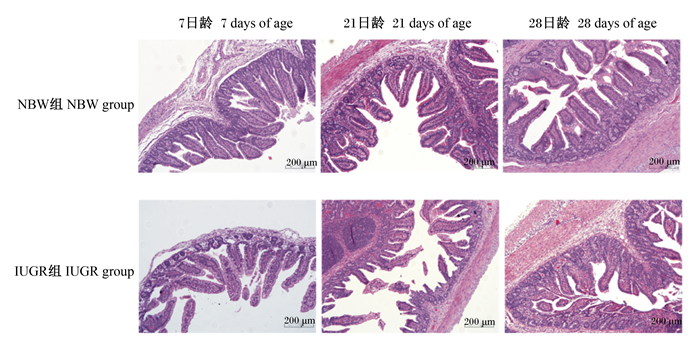
2 结果与分析 2.1 IUGR和NBW仔猪小肠形态的差异由表 2可知,与NBW仔猪相比,IUGR仔猪7日龄时空肠绒毛高度、21日龄时空肠和回肠绒毛高度显著降低(P<0.05),28日龄时空肠隐窝深度显著提高(P<0.05),其他指标差异不显著(P>0.05)。由图 1和图 2可知,IUGR仔猪空肠和回肠黏膜出现不同程度的损伤。
|
|
表 2 IUGR和NBW仔猪小肠形态的差异 Table 2 Differences in small intestinal morphology between IUGR and NBW piglets (n=6) |
|
图 1 IUGR和NBW仔猪空肠形态的差异 Fig. 1 Differences in jejunum morphology between IUGR and NBW piglets (100×, n=6) |
|
图 2 IUGR和NBW仔猪回肠形态的差异 Fig. 2 Differences in ileum morphology between IUGR and NBW piglets (100×, n=6) |
由表 3可知,与NBW仔猪相比,IUGR仔猪7日龄时空肠黏膜ZO-1、claudin-1、occludin、IL-4和IL-8以及21日龄时空肠黏膜ZO-1和occludin、回肠黏膜ZO-1的mRNA相对表达量显著下调(P<0.05);28日龄时空肠黏膜TNF-α、回肠黏膜IL-1β的mRNA相对表达量显著上调(P<0.05),回肠黏膜IL-4的mRNA相对表达量显著下调(P<0.05)。
|
|
表 3 IUGR和NBW仔猪小肠黏膜屏障功能相关基因mRNA表达的差异 Table 3 Differences in mRNA expression of genes related to small intestinal mucosal barrier function between IUGR and NBW piglets (n=8) |
肠道形态和功能的完整性对营养物质的吸收至关重要。小肠绒毛高度和隐窝深度是评定小肠形态的重要指标。绒毛高度越高则小肠吸收表面积就越大,其吸收功能就越强;隐窝深度越小则小肠上皮细胞就越成熟,其分泌功能就越强[14]。本研究中,与NBW仔猪相比,IUGR仔猪7日龄时空肠绒毛高度、21日龄时空肠和回肠绒毛高度显著降低,28日龄时空肠隐窝深度显著提高,提示IUGR仔猪小肠的吸收和分泌功能减弱。同样,Su等[15]研究发现,IUGR断奶仔猪小肠绒毛高度和绒毛表面积显著降低,隐窝深度显著提高;Li等[16]报道,IUGR断奶仔猪小肠绒毛高度显著降低。这可能与IUGR胎儿优先保障脑等重要器官的发育,肠道等相对次要器官的血流量供给减少,从而导致肠道隐窝-绒毛轴发育异常[17]。
肠黏膜的紧密连接是机体防御病原菌入侵的重要屏障[18]。当紧密连接功能受损时,有害物质进入血液循环引发机体炎症甚至疾病[19]。ZO-1、封闭蛋白(claudin)和occludin等紧密连接蛋白是决定肠黏膜通透性的重要分子[20]。本研究中,与NBW仔猪相比,IUGR仔猪7日龄时空肠黏膜ZO-1、claudin-1和occludin以及21日龄时空肠黏膜ZO-1和occludin、回肠黏膜ZO-1的mRNA相对表达量显著下调,提示IUGR哺乳仔猪的肠黏膜完整性受损。研究发现,大肠杆菌感染时仔猪肠黏膜ZO-1的表达量显著降低[21];7~28日龄IUGR仔猪肠道菌群的多样性降低,病原菌和条件致病菌丰度升高[10]。这可能与肠黏膜的紧密连接结构受损、肠黏膜通透性提高有关。
细胞因子在维持机体免疫反应和肠道屏障功能方面发挥着重要作用[22]。炎性细胞因子与紧密连接蛋白的改变密切相关[23]。本研究中,与NBW仔猪相比,IUGR仔猪7日龄时空肠黏膜IL-4、28日龄时回肠黏膜IL-4的mRNA相对表达量显著下调,28日龄时空肠黏膜TNF-α、回肠黏膜IL-1β的mRNA相对表达量显著上调,提示IUGR仔猪肠道中存在炎症反应。Wang等[24]也报道,与同窝NBW仔猪相比,IUGR新生仔猪血液炎症细胞因子和免疫球蛋白G含量显著降低,7和14日龄IUGR仔猪的空肠上皮内白细胞数量显著增加。这可能与肠道屏障受损有关;此外,本研究中,与NBW仔猪相比,IUGR仔猪7日龄时空肠黏膜IL-8的mRNA相对表达量显著下调,其原因仍有待进一步研究。
4 结论IUGR仔猪的空肠和回肠形态受损,紧密连接蛋白基因表达下调,免疫细胞因子表达异常,进而影响了小肠屏障功能。
| [1] |
FERENC K, PIETRZAK P, GODLEWSKI M M, et al. Intrauterine growth retarded piglet as a model for humans-studies on the perinatal development of the gut structure and function[J]. Reproductive Biology, 2014, 14(1): 51-60. DOI:10.1016/j.repbio.2014.01.005 |
| [2] |
孔祥峰, 伍国耀, 印遇龙. 猪宫内生长迟缓及其防治研究进展[J]. 畜牧与兽医, 2009, 41(10): 96-101. KONG X F, WU G Y, YIN Y L. Advances of intrauterine growth retardation in pigs[J]. Animal Husbandry & Veterinary Medicine, 2009, 41(10): 96-101 (in Chinese). |
| [3] |
LUISSINT A C, PARKOS C A, NUSRAT A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair[J]. Gastroenterology, 2016, 151(4): 616-632. DOI:10.1053/j.gastro.2016.07.008 |
| [4] |
WANG J J, CHEN L X, LI D F, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs[J]. The Journal of Nutrition, 2008, 138(1): 60-66. DOI:10.1093/jn/138.1.60 |
| [5] |
OLSZEWSKI J, ZABIELSKI R, SKRZYPEK T, et al. Differences in intestinal barrier development between intrauterine growth restricted and normal birth weight piglets[J]. Animals, 2021, 11(4): 990. DOI:10.3390/ani11040990 |
| [6] |
WANG W, DEGROOTE J, VAN GINNEKEN C, et al. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes[J]. FASEB Journal, 2016, 30(2): 863-873. DOI:10.1096/fj.15-274779 |
| [7] |
FERENC K, PIETRZAK P, WIERZBICKA M, et al. Alterations in the liver of intrauterine growth retarded piglets may predispose to development of insulin resistance and obesity in later life[J]. Journal of Physiology and Pharmacology, 2018, 69(2): 211-218. |
| [8] |
MCELRATH T F, ALLRED E N, VAN MARTER L, et al. Perinatal systemic inflammatory responses of growth-restricted preterm newborns[J]. Acta Paediatrica, 2013, 102(10): e439-e442. DOI:10.1111/apa.12339 |
| [9] |
D'INCA R, KLOAREG M, GRAS-LE GUEN C, et al. Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs[J]. The Journal of Nutrition, 2010, 140(5): 925-931. DOI:10.3945/jn.109.116822 |
| [10] |
ZHANG W, MA C, XIE P, et al. Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances[J]. Journal of Applied Microbiology, 2019, 127(2): 354-369. DOI:10.1111/jam.14304 |
| [11] |
XIONG L, YOU J M, ZHANG W H, et al. Intrauterine growth restriction alters growth performance, plasma hormones, and small intestinal microbial communities in growing-finishing pigs[J]. Journal of Animal Science and Biotechnology, 2020, 11(1): 86. DOI:10.1186/s40104-020-00490-x |
| [12] |
TURNER J R. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application[J]. The American Journal of Pathology, 2006, 169(6): 1901-1909. DOI:10.2353/ajpath.2006.060681 |
| [13] |
BAUER R, WALTER B, HOPPE A, et al. Body weight distribution and organ size in newborn swine (sus scrofa domestica)-a study describing an animal model for asymmetrical intrauterine growth retardation[J]. Experimental and Toxicologic Pathology, 1998, 50(1): 59-65. DOI:10.1016/S0940-2993(98)80071-7 |
| [14] |
GEHART H, CLEVERS H. Tales from the crypt: new insights into intestinal stem cells[J]. Nature Reviews Gastroenterology & Hepatology, 2019, 16(1): 19-34. |
| [15] |
SU W P, ZHANG H, YING Z X, et al. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets[J]. European Journal of Nutrition, 2018, 57(8): 2735-2745. DOI:10.1007/s00394-017-1539-3 |
| [16] |
LI Y, ZHANG H, SU W P, et al. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets[J]. Journal of Animal Science and Biotechnology, 2018, 9: 22. DOI:10.1186/s40104-018-0236-2 |
| [17] |
TRAHAIR J F, SANGILD P T. Systemic and luminal influences on the perinatal development of the gut[J]. Equine Veterinary Journal, 1997, 29(S24): 40-50. |
| [18] |
ZHONG X, WANG T, ZHANG X H, et al. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets[J]. Cell Stress & Chaperones, 2010, 15(3): 335-342. |
| [19] |
FRANK D N, ST AMAND A L, FELDMAN R A, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(34): 13780-13785. DOI:10.1073/pnas.0706625104 |
| [20] |
宗秋芳, 钦伟云, 霍永久, 等. 猪肠上皮紧密连接蛋白研究进展[J]. 浙江农业学报, 2018, 30(8): 1435-1444. ZONG Q F, QIN W Y, HUO Y J, et al. Research progress of intestinal epithelial tight junction proteins in piglets[J]. Acta Agriculturae Zhejiangensis, 2018, 30(8): 1435-1444 (in Chinese). DOI:10.3969/j.issn.1004-1524.2018.08.23 |
| [21] |
YAN T, ZHANG F, HE Y, et al. Enterococcus faecium HDRsEf1 elevates the intestinal barrier defense against enterotoxigenic Escherichia coli and regulates occludin expression via activation of TLR-2 and PI3K signalling pathways[J]. Letters in Applied Microbiology, 2018, 67(5): 520-527. DOI:10.1111/lam.13067 |
| [22] |
ANDREWS C, MCLEAN M H, DURUM S K. Cytokine tuning of intestinal epithelial function[J]. Frontiers in Immunology, 2018, 9: 1270. DOI:10.3389/fimmu.2018.01270 |
| [23] |
AL-SADI R, BOIVIN M, MA T. Mechanism of cytokine modulation of epithelial tight junction barrier[J]. Frontiers in Bioscience (Landmark Edition), 2009, 14: 2765-2778. |
| [24] |
WANG X Q, WU W Z, LIN G, et al. Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction[J]. Journal of Proteome Research, 2010, 9(2): 924-935. DOI:10.1021/pr900747d |




