2. 扬州双扬生物科技有限公司, 扬州 225124
2. Yangzhou Shuangyang Biotechnology Co., Ltd., Yangzhou 225124, China
近年来,随着人们对食品安全的重视,饲料中的镉(cadmium,Cd)污染也日渐引起人们的关注。Cd是一种毒性较强的重金属,易在土壤、水体等环境中蓄积,并向植物迁移,最终通过食物链迁移至动物和人体内蓄积[1]。Cd的半衰期可长达25~30年,对机体的肝脏、肾脏和骨骼等组织系统造成严重损害,并具有强烈的致癌作用,被国际癌症研究所(IARC)列为Ⅰ级致癌物[2-3]。
鉴于Cd的严重危害性,《饲料卫生标准》(GB 13078—2017)对配合饲料中Cd的含量规定了限量:Cd≤0.5 mg/kg(水产配合饲料除外)。有调查研究发现,饲料中Cd元素存在超标现象和风险,如湖北省2012—2016年饲料Cd含量最高为1.2 mg/kg[4],而四川省、河北省蛋鸡配合饲粮中Cd含量虽未超标但有超标风险[5]。针对饲料中存在Cd超标的状况和风险,可采用吸附剂如麦饭石、沸石等减少动物体内Cd的吸收[6-7],或者补加维生素[8]、抗氧化剂[9]等降低Cd对机体造成的氧化损害,但目前还没有专一、安全有效地降低Cd毒性的方法[10]。近年研究发现,可调节动物肠道发育、促进营养物质吸收的乳酸菌可通过在肠道吸附Cd,减少Cd经肠道吸收进入血液和组织,还可通过调控肠肝循环促进动物体内Cd的排泄,减轻Cd对动物机体的损害和毒性[10-11]。乳酸菌是目前公认的安全微生物[12],在食品工业中应用广泛,而在《饲料添加剂品种目录》(中华人民共和国农业部公告第2045号)[13],允许使用的34种微生物添加剂中有16种为乳酸菌。目前具有耐Cd性质的菌株多是从土壤或粪便[14]、污泥[15]、食品[16]等筛选获得,且在动物上的应用研究多集中在鼠类和鱼类上[17-18],在家禽上的应用研究仅见于本团队在鸡上开展的试验[19-21]。而来源于动物体内的具有耐重金属乳酸菌的筛选研究仅见于耐铅[22-23]和耐汞[24],耐Cd的乳酸菌筛选还未见有报道。本研究的目的即是从鸡体内分离筛选出具有Cd吸附能力的耐Cd乳酸菌株,并对其进行生物学鉴定,为将其开发应用于家禽饲料中提供理论基础,同时对防控家禽饲料及动物养殖中Cd及其他重金属污染具有重要意义。
1 材料与方法 1.1 主要试剂与仪器试剂:MRS肉汤培养基(Soarbio,M8540)、MRS琼脂培养基(Soarbio,M8330)、乙酸镉(国药,33061)、牛胆盐(Soarbio,G8210)、DNA提取试剂盒(TaKaRa,9763)、磷酸盐缓冲液(PBS,Soarbio,P1032)、LB培养基。
仪器:生化培养箱(HPS-150,太仓市华美生化仪器厂)、生物安全柜(ESCO AC2,新加坡艺思高科技有限公司)、高压灭菌锅(MLS-3750,日本SANYO公司)、分光光度计(V-5000,上海元析仪器有限公司)、全温振荡培养箱(ZQTY-90S,知楚仪器公司)、原子吸收分光光度计(AA800,铂金埃尔默公司)。
1.2 耐Cd乳酸菌株的分离鉴定 1.2.1 耐Cd乳酸菌株的分离取鸡肠道内容物1 g,用无菌PBS逐级稀释至10-4、10-5、10-6,用接种环取1环接种于含50 mg/L Cd(取0.238 g C4H6CdO4·2H2O,溶解于10 mL超纯水中,配制成10 g/L的Cd溶液,按1 ∶ 200比例加入MRS培养基中,使Cd2+浓度为50 mg/L)的MRS固体培养基上,37 ℃厌氧培养48 h后,挑取单菌落,接种于含100 mg/L Cd(取10 g/L的Cd溶液,按1 ∶ 100比例加入MRS培养基中,使Cd2+浓度为100 mg/L)的MRS固体培养基上,同条件培养48 h后,挑选单菌落,重复接种于含100 mg/L Cd的MRS固体培养基上纯化后,挑选单菌落进行革兰氏染色。
1.2.2 耐Cd乳酸菌株生物学鉴定提取乳酸菌株基因组DNA,利用16S rRNA基因通用上游引物27F(5′-AGAGTTTGA TCCTGGCTCAG-3′)和下游引物1492R(5′-TACGGCTACCTTGTTACGACTT-3′),PCR扩增16S rRNA基因,PCR扩增条件为:95 ℃ 5 min, 95 ℃ 30 s, 55 ℃ 30 s, 72 ℃ 60 s,30个循环,72 ℃ 5 min,16 ℃。PCR扩增产物取4 μL用1%琼脂糖凝胶电泳,剩余PCR扩增产物送山东青岛英赛特生物科技有限公司测序,测序结果与NCBI数据库比对,确定乳酸菌种属。
1.2.3 耐Cd乳酸菌株的生长特性乳酸菌株按1%的接种量接种至MRS液体培养基中,37 ℃厌氧培养,分别在2、4、8、12、18和24 h时,取1 mL用分光光度计测量菌液在600 nm处的吸光度(OD600 nm)值,绘制生长曲线图。
1.3 耐Cd乳酸菌株最大Cd耐受量乳酸菌株按1%的接种量接种至MRS液体培养基中厌氧培养18 h,调OD600 nm为1,分别取1环划线接种至含100、200、400、800、1 000 mg/L Cd的培养基上,37 ℃厌氧培养72 h观察结果。
1.4 耐Cd乳酸菌株在不同pH条件下对Cd的吸附性乳酸菌株按1%的接种量接种至MRS液体培养基厌氧培养18 h后,10 000 r/min离心10 min,去上清,超纯水清洗沉淀后,调吸光度为1,取0.5 mL溶液10 000 r/min,离心10 min,去除上清,分别加入pH为2.6、4.0、6.0和7.0的50 mg/L Cd溶液0.5 mL悬浮沉淀,每株菌作3个重复。37 ℃中速振荡2 h,10 000 r/min离心10 min,取上清液稀释后,用石墨炉法测定溶液中的Cd含量,利用以下公式计算乳酸菌Cd吸附率:
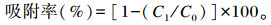
|
式中:C0为吸附前溶液Cd浓度;C1为吸附后上清液中Cd浓度。
1.5 耐Cd乳酸菌株胆盐耐受性参考文献[15, 22]报道的方法,稍做调整。耐Cd乳酸菌以1%的接种量接种至MRS液体培养基中培养18 h,调OD600 nm为1,取1 mL乳酸菌液分别加入9 mL含0.1%、0.3%、0.5%牛胆盐的MRS液体培养基中,37 ℃中速振摇培养1 h后取1环接种至MRS固体培养基上,37 ℃厌氧培养48 h观察结果。
1.6 耐Cd乳酸菌抑菌性耐Cd乳酸菌按1%的接种量接种至MRS液体培养基中培养18 h后,调菌液OD600 nm为1,取1 mL菌液10 000 r/min离心10 min,取上清液于无菌管中,将直径6 mm的无菌滤纸片分别浸入菌液和菌液上清液中。将金黄色葡萄球菌(本实验室分离)、大肠杆菌(ATCC 25922)、沙门氏菌(ATCC 14028)用LB液体培养基37 ℃培养18 h后,调三者菌液OD600 nm至0.08~0.10,各取100 μL菌液分别铺于LB培养皿,将浸液滤纸片分别铺在细菌培养基上,37 ℃培养24 h后观察结果。
1.7 数据统计分析试验数据采用SPSS 16.0软件进行统计分析,采用one-way ANOVA进行单因素方差分析,LSD进行均值间的多重比较,以P<0.05作为差异显著性判断标准,采用GraphPad Prism 5软件绘图。
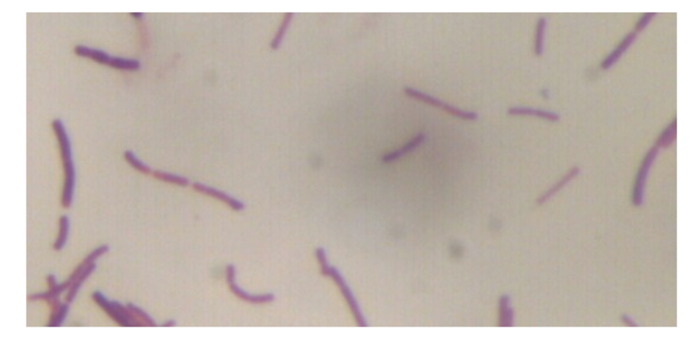
2 结果与分析 2.1 耐Cd乳酸菌的革兰氏染色结果样品经含50和100 mg/L Cd的MRS的培养基纯化后,在100 mg/L的培养基上形成单个小而突出的菌落。挑取单菌落经革兰氏染色,在显微镜下呈紫色杆状,表明菌株为革兰氏阳性菌(图 1)。
|
图 1 耐Cd乳酸菌株的革兰氏染色 Fig. 1 Gram staining of Cd-tolerant Lactobacillus (1 000×) |
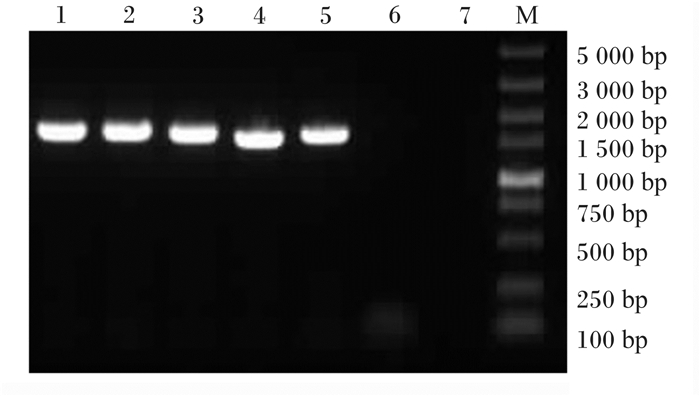
从筛选出的耐Cd乳酸菌株中选取5株菌,提取菌株的DNA经PCR扩增后,1%琼脂糖凝胶电泳显示目的条带约1 500 bp,与PCR产物大小相符(图 2),测序结果与NCBI数据库比对,确定5株耐Cd乳酸菌分别为:约氏乳杆菌(Lactobacillus johnsonii)1株,命名为Cd3-1,卷曲乳杆菌(Lactobacillus curella)4株,分别命名为Cd1-1、Cd5-2、Cd4-5、Cd8-2。
|
1~5为耐Cd乳酸菌株Cd1-1、Cd3-1、Cd5-2、Cd4-5、Cd8-2;6、7为阴性对照;M为Marker。 1 to 5 were Cd-tolerant Lactobacillus strains Cd1-1, Cd3-1, Cd5-2, Cd4-5 and Cd8-2; 6, 7 were negative control; M was Marker. 图 2 耐Cd乳酸菌16S rDNA凝胶电泳条带图 Fig. 2 Cd-tolerant Lactobacillus 16S rDNA gel electrophoresis bands |
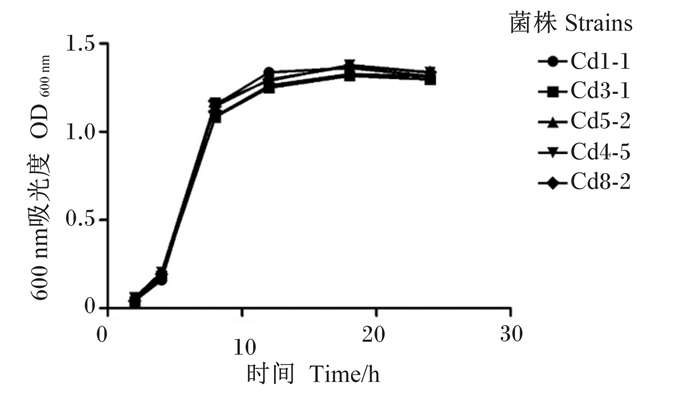
耐Cd乳酸菌的24 h生长曲线(图 3)显示,耐Cd乳酸菌的生长增殖符合一般微生物的生长特性,在0~2 h生长较为缓慢,在4~12 h处于对数生长期,在12~18 h处于缓慢生长期,而在18 h后数量开始减少。
|
图 3 5株耐Cd乳酸菌生长曲线 Fig. 3 Growth curves of five Cd-tolerant Lactobacillus strains |
表 1显示,菌株Cd3-1、Cd5-2和Cd8-2可耐受的最大Cd浓度为1 000 mg/L,而Cd1-1和Cd4-5可耐受的最大Cd浓度为400 mg/L,表明Cd3-1、Cd5-2和Cd8-2菌株的Cd耐受量高于Cd1-1和Cd4-5。
|
|
表 1 耐Cd乳酸菌耐受最大Cd浓度 Table 1 Maximum tolerant Cd concentration of Cd-tolerant Lactobacillus |
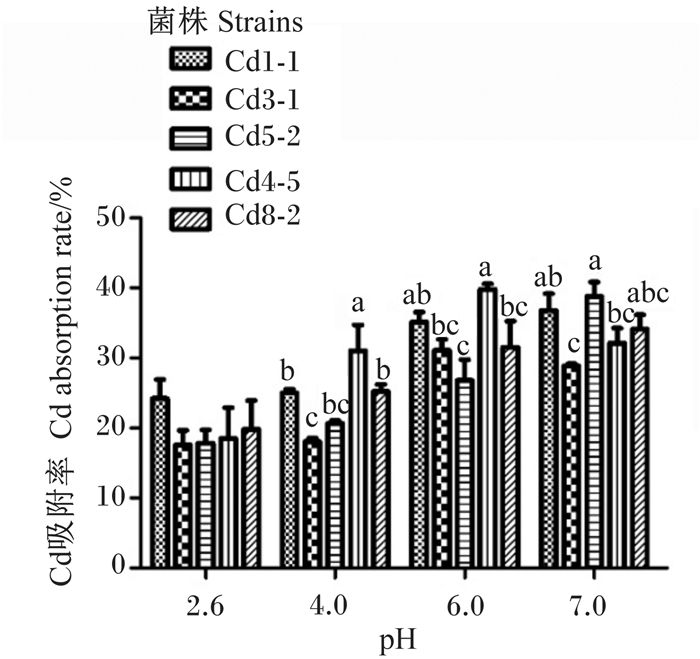
图 4显示,pH对菌株吸附Cd的影响较大。在pH为2.6时,各菌株对50 mg/L Cd溶液Cd的吸附率在20%左右,菌株间的Cd吸附率无显著差异(P>0.05)。随着pH的升高,Cd的吸附率上升,pH为6.0时,除Cd5-2外,其余菌株Cd的吸附率均超过30%,其中,Cd4-5的Cd吸附率达到最高,接近40%,显著高于Cd3-1、Cd5-2和Cd8-2(P<0.05)。在pH 7.0时除Cd3-1的Cd吸附率略低于30%外,其余菌株的Cd吸附率均高于30%,其中Cd5-2的Cd吸附率最高,为38.8%,显著高于Cd3-1和Cd4-5(P<0.05)。各菌株在pH 6.0和7.0时Cd吸附率均显著高于其在pH 2.6时的Cd吸附率(P<0.05)。
|
同一pH的数据柱形标注不同小写字母表示差异显著(P<0.05)。 Value columns of the same pH with different small letters mean significant difference (P < 0.05). 图 4 不同pH条件下耐Cd乳酸菌对Cd的吸附率 Fig. 4 Absorption rate of Cd by Cd-tolerant Lactobacillus under different pH |
选取的5株耐Cd乳酸菌株Cd1-1、Cd3-1、Cd5-2、Cd4-5、Cd8-2经0.3%胆盐作用1 h后均可在MRS培养基上生长,其中Cd3-1和Cd8-2经0.5%胆盐作用1 h后在MRS培养基上长出单菌落,结果表明这5株菌可耐受0.3%的胆盐,且Cd3-1和Cd8-2可耐受0.5%的胆盐。
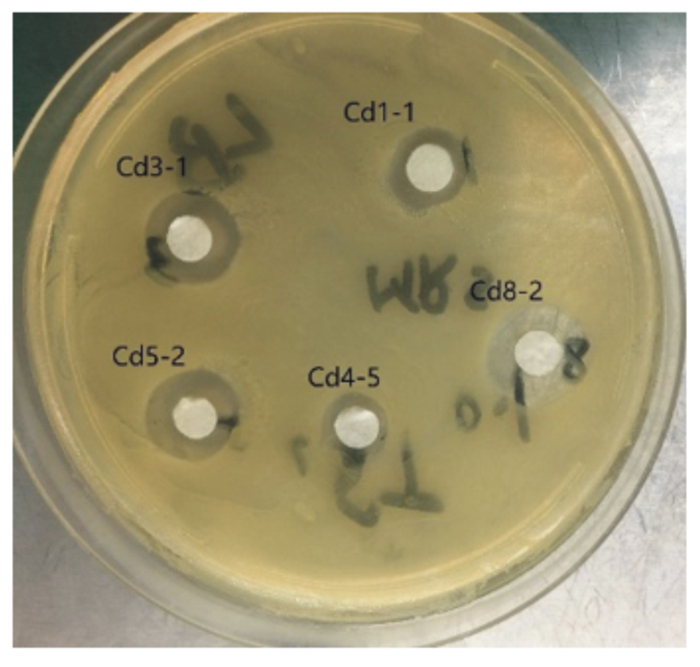
2.7 耐Cd乳酸菌抑菌结果5株耐Cd乳酸菌的抑菌试验结果表明,不含耐Cd乳酸菌的上清培养液对金黄色葡萄球菌、大肠杆菌和沙门氏菌均无抑菌效果,而含耐Cd乳酸菌的培养液均对金黄色葡萄球菌表现出可抑制其生长,其中以Cd3-1、Cd5-2和Cd8-2菌株抑菌圈较大,Cd1-1和Cd4-5抑菌圈较小,表明前3株菌的抑制金黄色葡萄球菌的效果强于后2株菌(图 5)。含耐Cd乳酸菌的培养液对大肠杆菌和沙门氏菌未表现出抑菌作用。
|
图 5 耐Cd乳酸菌对金黄色葡萄球菌的抑制作用 Fig. 5 Inhibition of Cd-tolerant Lactobacillus on Staphylococcus aureus |
乳酸菌是一类能够发酵碳水化合物产生大量乳酸的革兰氏阳性菌的统称,属于兼性厌氧菌。乳酸菌因其细胞壁的结构特征,可耐受一定浓度的Cd[25-26]。利用乳酸菌的这种吸附特性可筛选出高耐受Cd的乳酸菌。熊婧[14]通过最小抑菌浓度(minimum inhibitory concentration,MIC)测定方法,从土壤、粪便及标准乳酸杆菌中筛选出11株对Cd具有高耐受性的乳酸杆菌(MIC≥4.0 g/L)。Matyar等[27]以菌株能耐受Cd的MIC超过100 mg/L则认为其对Cd有耐受性。不同的菌株对Cd的耐受性相差较大,Zhai等[28]筛选的高耐受植物乳杆菌CCFM8610耐受超过1 000 mg/L的Cd,而低耐受性的植物乳杆菌CCFM191仅耐受10 mg/L的Cd。本研究以能耐受100 mg/L的Cd为筛选标准,从鸡肠道中分离获得耐Cd乳酸菌5株,其中3株菌最高可耐受1 000 mg/L的Cd。
3.2 耐Cd乳酸菌对Cd的吸附能力耐Cd乳酸菌对Cd的吸附能力除与其自身结构相关外还与吸附条件包括菌量、浓度、温度、时间、pH等相关[29],而菌体吸附Cd受pH影响较大[30]。Ameen等[31]发现植物乳杆菌(Lactobacillus plantarum)MF042018在pH为2.0,温度为22 ℃时,1 h内对Cd2+和铅离子(Pb2+)的吸附达到最大容量。而Topco等[32]报道屎肠球菌(Enterococcus faecium)在pH低于3时对Cd和铅(Pb)的移除率最低,但随着pH的升高,其对Cd和Pb的移除率直线增加。本研究的结果表明,5株耐Cd乳酸菌的Cd吸附率随Cd溶液的pH条件的不同而变化,pH越低,耐Cd乳酸菌对Cd的吸附率越低。在pH中性和接近中性时对Cd的吸附率可达30%以上,均显著高于pH为2.6时的Cd吸附率。上述研究结果表明,不同的微生物对Cd等重金属的吸附有不同的最适pH,确定其适宜的pH有助于微生物发挥出最佳的吸附能力。
3.3 耐Cd乳酸菌的益生性对耐Cd乳酸菌的筛选除考察其对Cd的耐受性和吸附性外,其自身的益生性也是考虑的重要因素。对胆盐的抗性是益生菌的一个重要判断标准,它确保菌株在小肠内的定植和代谢活性[33]。Ojekunle等[34]筛选到耐Cd的魏斯菌属(Weissella cibaria)WD2菌株对0.3%和1.0%的胆盐有高耐受性。本研究中筛选的乳酸菌株可耐受0.3%的胆盐,且可抑制金黄色葡萄球菌的生长,在发挥其吸附Cd的特性同时可发挥其益生菌的作用。
4 结论本试验从鸡肠道中分离筛选鉴定出5株耐Cd乳酸菌,包括1株约氏乳杆菌和4株卷曲乳杆菌。在pH接近中性的条件下,上述乳酸菌株对Cd的吸附率均可达30%。
| [1] |
WANG P, CHEN H P, KOPITTKE P M, et al. Cadmium contamination in agricultural soils of China and the impact on food safety[J]. Environmental Pollution, 2019, 249: 1038-1048. DOI:10.1016/j.envpol.2019.03.063 |
| [2] |
GENCHI G, SINICROPI M S, LAURIA G, et al. The effects of cadmium toxicity[J]. International Journal of Environmental Research and Public Health, 2020, 17(11): 3782. DOI:10.3390/ijerph17113782 |
| [3] |
VAINIO H, HESELTINE E. Meeting of the IARC working group on beryllium, cadmium, mercury and exposures in the glass manufacturing industry[J]. Scandinavian Journal of Work, Environment & Health, 1993, 19(5): 360-363. |
| [4] |
TAO C, WEI X T, ZHANG B Y, et al. Heavy metal content in feedstuffs and feeds in Hubei Province, China[J]. Journal of Food Protection, 2020, 83(5): 762-766. DOI:10.4315/0362-028X.JFP-18-539 |
| [5] |
罗成, 张军民, 赵青余, 等. 我国四川、山东、河北省蛋鸡配合饲料中镉、铬、铅污染程度评估[J]. 动物营养学报, 2017, 29(8): 2851-2866. LUO C, ZHANG J M, ZHAO Q Y, et al. Contamination of cadmium, chromium and lead in compound feed of laying hens in Sichuan, Shandong and Hebei provinces[J]. Chinese Journal of Animal Nutrition, 2017, 29(8): 2851-2866 (in Chinese). DOI:10.3969/j.issn.1006-267x.2017.08.030 |
| [6] |
杜鹃, 石宝明, 单安山. 麦饭石对重金属镉吸附效果的研究[J]. 中国畜牧兽医, 2012, 39(12): 70-72. DU J, SHI B M, SHAN A S. Research on the absorption of cadmium by medical stone under different conditions in vitro[J]. China Animal Husbandry & Veterinary Medicine, 2012, 39(12): 70-72 (in Chinese). DOI:10.3969/j.issn.1671-7236.2012.12.017 |
| [7] |
王菲. 吸附剂调控镉胁迫肉鸡生长与其在体内分布及累消的研究[D]. 硕士学位论文. 贵阳: 贵州大学, 2020: 38-63. WANG F. Effects of adsorbent on growth, distribution, accumulation and disappearance in cadmium-stressed broilers[D]. Master's Thesis. Guiyang: Guizhou University, 2020: 38-63. (in Chinese) |
| [8] |
蒋哲舜, 巴乾. 食源性镉暴露的肠道菌群毒性及维生素D对镉毒性保护作用研究进展[J]. 中国公共卫生, 2020, 36(11): 1661-1664. JIANG Z S, BA Q. Toxicity of foodborne cadmium exposure to gut microbiota and protective effect of vitamin D against the toxicity: a review of research progress[J]. Chinese Journal of Public Health, 2020, 36(11): 1661-1664 (in Chinese). DOI:10.11847/zgggws1123346 |
| [9] |
陈大伟, 刘茵茵, 王倩倩, 等. 硒镉同时暴露对鸡蛋和蛋鸡组织中硒镉含量的影响[J]. 江苏农业科学, 2019, 47(20): 203-205. CHEN D W, LIU Y Y, WANG Q Q, et al. Effects of simultaneous exposure of selenium and cadmium on the content of selenium and cadmium in eggs and laying hens[J]. Jiangsu Agricultural Sciences, 2019, 47(20): 203-205 (in Chinese). |
| [10] |
BHATTACHARYA S. The role of probiotics in the amelioration of cadmium toxicity[J]. Biological Trace Element Research, 2020, 197(2): 440-444. DOI:10.1007/s12011-020-02025-x |
| [11] |
ZHAI Q X, LIU Y, WANG C, et al. Increased cadmium excretion due to oral administration of Lactobacillus plantarum strains by regulating enterohepatic circulation in mice[J]. Journal of Agricultural and Food Chemistry, 2019, 67(14): 3956-3965. DOI:10.1021/acs.jafc.9b01004 |
| [12] |
PLAVEC T V, BERLEC A. Safety aspects of genetically modified lactic acid bacteria[J]. Microorganisms, 2020, 8(2): 297. DOI:10.3390/microorganisms8020297 |
| [13] |
农业部. 饲料添加剂品种目录(2013)[EB/OL]. (2013-12-30)[2021-08-20]. http://www.moa.gov.cn/govpublic/XMYS/201401/t20140103_3730193.htm. The Ministry of Agriculture of the People's Republic of China. Catalogue of feed additive varieties (2013)[EB/OL]. (2013-12-30)[2021-08-20]. http://www.moa.gov.cn/govpublic/XMYS/201401/t20140103_3730193.htm. |
| [14] |
熊婧. 乳酸菌对重金属镉的耐受性和吸附机制研究[D]. 硕士学位论文. 广州: 暨南大学, 2015: 31-41. XIONG J. Mechanism of cadmium resistance of lactic acid bacteria[D]. Master's Thesis. Guangzhou: Jinan University, 2015: 31-41. (in Chinese) |
| [15] |
BHAKTA J N, OHNISHI K, MUNEKAGE Y, et al. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents[J]. Journal of Applied Microbiology, 2012, 112(6): 1193-1206. DOI:10.1111/j.1365-2672.2012.05284.x |
| [16] |
翟齐啸. 乳酸菌减除镉危害的作用及机制研究[D]. 博士学位论文. 无锡: 江南大学, 2015: 23-33. ZHAI Q X. Effects of lactic acid bacteria against cadmium toxicity and the involved protective mechanisms[D]. Ph. D. Thesis. Wuxi: Jiangnan University, 2015: 23-33. (in Chinese) |
| [17] |
BANWO K, ALONGE Z, SANNI A I. Binding capacities and antioxidant activities of Lactobacillus plantarum and Pichia kudriavzevii against cadmium and lead toxicities[J]. Biological Trace Element Research, 2021, 199(2): 779-791. DOI:10.1007/s12011-020-02164-1 |
| [18] |
ZHAI Q X, YU L L, LI T Q, et al. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure[J]. Antonie Van Leeuwenhoek, 2017, 110(4): 501-513. DOI:10.1007/s10482-016-0819-x |
| [19] |
蒲俊华, 陈大伟, 陆俊贤, 等. 耐镉乳酸菌对蛋鸡组织和鸡蛋中镉残留及部分矿物元素沉积的影响[J]. 中国畜牧兽医, 2020, 47(1): 53-62. PU J H, CHEN D W, LU J X, et al. Effects of cadmium-tolerant Lactobacillus on Cd residue and some mineral elements accumulation in hens' tissues and eggs[J]. China Animal Husbandry & Veterinary Medicine, 2020, 47(1): 53-62 (in Chinese). |
| [20] |
孔令武, 蒲俊华, 陈大伟, 等. 耐镉乳酸菌对蛋鸡生产性能及蛋品质的影响[J]. 中国饲料, 2019(7): 28-32. KONG L W, PU J H, CHEN D W, et al. Effects of cadmium-tolerant Lactobacillus on productivity of laying hens and egg quality[J]. China Feed, 2019(7): 28-32 (in Chinese). |
| [21] |
蒲俊华, 陈大伟, 刘茵茵, 等. 耐镉乳酸菌对镉暴露仔鸡体重、脏器系数和组织中矿物元素含量的影响[J]. 动物营养学报, 2021, 33(8): 4730-4739. PU J H, CHEN D W, LIU Y Y, et al. Effects of cadmium-tolerant lactobacillus on body weight, organ coefficients and contents of mineral elements in tissues of cadmium-exposed chickens[J]. Chinese Journal of Animal Nutrition, 2021, 33(8): 4730-4739 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.08.051 |
| [22] |
夏爽, 谷珊珊, 关乃瑜, 等. 动物肠道中具有益生特征的抗铅乳酸菌的分离与鉴定[J]. 中国兽医科学, 2015, 45(12): 1254-1259. XIA S, GU S S, GUAN N Y, et al. Isolation and identification of Pb-resistance lactic acid bacteria with probiotic characteristics in animal intestine[J]. Chinese Veterinary Science, 2015, 45(12): 1254-1259 (in Chinese). |
| [23] |
满兆红, 都启晶, 姜彦君, 等. 一株鸡源嗜铅乳酸菌的筛选鉴定[J]. 山东农业科学, 2014, 46(2): 72-76, 81. MAN Z H, DU Q J, JIANG Y J, et al. Isolation and identification of lead-resistant lactic acid bacteria from chicken[J]. Shandong Agricultural Sciences, 2014, 46(2): 72-76, 81 (in Chinese). DOI:10.3969/j.issn.1001-4942.2014.02.018 |
| [24] |
陈金泰, 谷珊珊, 翟羽飞, 等. 优良动物源抗汞益生乳酸菌的筛选及其抗性基因的克隆[J]. 黑龙江畜牧兽医, 2017(3): 138-141. CHEN J T, GU S S, ZHAI Y F, et al. Screening of good animal source mercury-resistant probiotic lactic acid bacteria and cloning of their resistance genes[J]. Heilongjiang Animal Science and Veterinary Medicine, 2017(3): 138-141 (in Chinese). |
| [25] |
KUMAR N, KUMAR V, PANWAR R, et al. Efficacy of indigenous probiotic Lactobacillus strains to reduce cadmium bioaccessibility-an in vitro digestion model[J]. Environmental Science and Pollution Research, 2017, 24(2): 1241-1250. DOI:10.1007/s11356-016-7779-6 |
| [26] |
KIRILLOVA A V, DANILUSHKINA A A, IRSOV D S, et al. Assessment of resistance and bioremediation ability of Lactobacillus strains to lead and cadmium[J]. International Journal of Microbiology, 2017, 9869145. |
| [27] |
MATYAR F, KAYA A, DINÇER S. Antibacterial agents and heavy metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey[J]. Science of the Total Environment, 2008, 407(1): 279-285. DOI:10.1016/j.scitotenv.2008.08.014 |
| [28] |
ZHAI Q X, XIAO Y, ZHAO J X, et al. Identification of key proteins and pathways in cadmium tolerance of Lactobacillus plantarum strains by proteomic analysis[J]. Scientific Reports, 2017, 7(1): 1182. DOI:10.1038/s41598-017-01180-x |
| [29] |
MASSOUD R, KHOSRAVI-DARANI K, SHARIFAN A, et al. Lead and cadmium biosorption from milk by Lactobacillus acidophilus ATCC 4356[J]. Food Science & Nutrition, 2020, 8(10): 5284-5291. |
| [30] |
HALTTUNEN T, SALMINEN S, TAHVONEN R. Rapid removal of lead and cadmium from water by specific lactic acid bacteria[J]. International Journal of Food Microbiology, 2007, 114(1): 30-35. DOI:10.1016/j.ijfoodmicro.2006.10.040 |
| [31] |
AMEEN F A, HAMDAN A M, EL-NAGGAR M Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018[J]. Scientific Reports, 2020, 10(1): 314. DOI:10.1038/s41598-019-57210-3 |
| [32] |
TOPCU A, BULAT T. Removal of cadmium and lead from aqueous solution by Enterococcus faecium strains[J]. Journal of Food Science, 2010, 75(1): T13-T17. DOI:10.1111/j.1750-3841.2009.01429.x |
| [33] |
BANWO K, SANNI A, TAN H. Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk[J]. Journal of Applied Microbiology, 2013, 114(1): 229-241. DOI:10.1111/jam.12031 |
| [34] |
OJEKUNLE O, BANWO K, SANNI A I. In vitro and in vivo evaluation of Weissella cibaria and Lactobacillus plantarum for their protective effect against cadmium and lead toxicities[J]. Letters in Applied Microbiology, 2017, 64(5): 379-385. DOI:10.1111/lam.12731 |




