2. 扬州大学农业科技发展研究院, 扬州 225009
2. Institutes of Agricultural Science and Technology Development, Yangzhou University, Yangzhou 225009, China
霉菌毒素在农产品生产的各个环节普遍存在,严重危害食品和饲料安全,威胁人类和动物健康[1]。据联合国粮农组织资料,每年全世界约有25%的农作物不同程度地受到霉菌毒素污染,由此造成的经济损失高达数千亿美元。黄曲霉毒素(aflatoxins,AF)属于二氢呋喃香豆素衍生物,主要是寄生曲霉和黄曲霉的次级代谢产物。AF主要有4种类型,分别为AFB1、AFB2、AFG1、AFG2,其中AFB1毒性最强,被世界卫生组织列为Ⅰ级致癌物[2]。周建川等[3]对国内一些省份采集到的1 304个饲料及原料样品调查发现,玉米、玉米副产物、小麦及麸皮、粕类及全价料中AFB1的检出率在66%以上,最高可达97.53%;超标率平均在30%以上,最高可达81.48%。霉菌毒素很难被消除,尽可能降低其对人类和动物健康的危害,是生产的需要也是研究的热点。
目前,处理霉变饲料通常采用的方法是霉菌毒素吸附剂,然而吸附剂类产品可能对饲料中营养物质也存在一定的吸附作用,从而影响饲料营养价值[4]。从动物自身对霉菌毒素的代谢和解毒过程入手,从生理调控的角度增强机体抗应激能力来缓解霉菌毒素的危害,也是动物生产中处理霉菌毒素的有效方法[5-6]。AFB1是肝毒性化学物质,在细胞色素P450(cytochrome P450,CYP450)酶的作用下活化为AFB1环氧化物而诱发癌症、产生血液毒性等;同时,产生活性氧自由基(reactive oxygen species,ROS)中间产物,诱导氧化应激和线粒体功能紊乱,也是AFB1发挥毒性作用的重要途径[7]。近年来,国内外学者对AFB1诱导的动物氧化损伤以及从抗氧化防御的角度缓解AFB1的毒性作用方面开展了诸多研究。本文对AFB1诱导的氧化损伤及不同生物活性物质的抗氧化防御机制做一综述,以期为动物生产中AFB1污染的防控提供参考。
1 AFB1诱导动物氧化应激的机制 1.1 AFB1诱导ROS的产生,消耗还原型谷胱甘肽(reduced glutathione,GSH),进而造成机体氧化应激AFB1的代谢主要在肝脏进行,在微粒体细胞色素P450(CYP450)酶(主要包括CYP1A1、CYP1A2、CYP2A6、CYP3A4)催化的Ⅰ相反应中形成AFB1-8, 9-环氧化物(AFB1-8, 9-epoxides,AFBO),同时产生ROS中间产物。因此,AFB1能够诱导ROS的蓄积而造成氧化应激[8-9],增加组织中丙二醛(malondialdehyde,MDA)、4-羟壬烯醛、蛋白质羰基和8-羟基脱氧鸟苷(8-hydroxydeoxyguanosine,8-OHdG)等氧化损伤产物的含量[7]。在Ⅱ相解毒反应中,在谷胱甘肽硫转移酶(glutathione S-transferase,GST)的作用下,AFB1环氧化物与GSH结合形成AFB1-GSH结合物,这一过程是主要的解毒途径,同时减少了细胞内GSH的含量[10]。GSH是衡量机体氧化还原状态的重要指标,在谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)和谷胱甘肽还原酶(glutathione reductase,GR)的作用下,机体内的GSH和氧化型谷胱甘肽(oxidized glutathione,GSSG)保持平衡。Yilmaz等[11]研究发现,大鼠口服AFB1[0.5 mg/(kg BW·d) 7 d或1.5 mg/(kg BW·d) 3 d]可使肾脏和心脏组织中GSH-Px的活性下降并消耗GSH。因此,AFB1在代谢过程中诱导ROS的产生,在Ⅱ相解毒反应中消耗GSH,进而诱导机体氧化应激。
1.2 AFB1通过影响线粒体ROS稳态诱导线粒体氧化应激线粒体是细胞内发生氧化磷酸化、合成三磷酸腺苷(adenosine triphosphate,ATP)提供能量的细胞器,同时也是细胞内产生ROS的主要场所,当ROS的平衡被扰乱发生氧化应激时,线粒体首先受到破坏[12]。研究表明,AFB1应激状态下ROS稳态和抗氧化防御系统失调,引起氧化应激,造成DNA和线粒体损伤,诱导细胞凋亡[13]。1或2 μmol/L的AFB1可使肉鸡原代肝细胞线粒体氧化磷酸化过程解偶联,线粒体膜电位下降,ROS大量产生,抗氧化酶活性降低,细胞凋亡,抗氧化酶相关基因mRNA表达水平下降[14]。肉鸡(0.2 mg/kg BW)和肉鸭(0.1 mg/kg BW)摄入AFB1会造成肝脏线粒体抗氧化酶活性下降、MDA含量升高,线粒体肿胀、嵴结构被破坏、膜通透性增加[15-16]。Huang等[17]报道,0.375~1.500 mg/kg BW的AFB1灌胃降低了小鼠睾丸中ATP水平,降低了线粒体电子传递链(electron transport chain,ETC)复合物Ⅰ~Ⅳ的活性;下调了小鼠睾丸中线粒体生物发生基因,如过氧化物酶体增殖物激活受体辅激活因子1α、核呼吸因子1和线粒体转录因子A的mRNA表达水平。因此,AFB1通过在代谢过程中产生ROS中间产物影响线粒体ROS稳态,破坏氧化还原平衡状态,诱导线粒体氧化损伤和功能紊乱。
1.3 核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)介导的Ⅱ相解毒和抗氧化系统与AFB1的关系Nrf2是机体解毒和抗氧化防御系统的重要转录因子,通过调控抗氧化酶、抗炎因子、解毒酶等基因的表达而发挥缓解应激的作用[18-20]。Nrf2靶基因对线粒体结构完整性、ROS的产生、ATP合成等功能发挥重要的调节作用[21]。Kovac等[22]研究表明,Kelch样环氧氯丙胺相关蛋白1(Kelch-like ECH-associated protein 1,Keap1)-Nrf2信号通路通过线粒体和烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate,NADPH)氧化酶调控ROS的产生,从而对细胞内氧化还原平衡起重要的调节作用。分离线粒体和细胞培养试验证明,Nrf2缺失导致线粒体脂肪酸氧化、呼吸链功能和ATP合成功能障碍[23]。在非诱导状态下,Nrf2与Keap1结合成非活性复合物;Keap1含有5个活性半胱氨酸残基,在诱导状态下,诱导物与其中任何一个半胱氨酸巯基发生反应即可使Nrf2与Keap1分离,然后Nrf2进入细胞核与抗氧化反应元件结合,进而诱导GST、醌氧化还原酶1[NAD(P)H quinone oxidoreductase 1,NQO1]、GR、GSH-Px、过氧化氢酶(catalase,CAT)、超氧化物歧化酶(superoxide dismutase,SOD)等Ⅱ相解毒酶和抗氧化酶基因的表达以及GSH的合成[18-19]。Thimmulappa等[24]应用微阵列试验技术研究发现,Nrf2选择性优先调控的基因有环氧化物水解酶、黄曲霉毒素醛还原酶和GST等。Taguchi等[25]研究发现,Nrf2敲除的大鼠肝脏解毒酶活性下调,对AFB1的敏感性增强。Ilic等[26]研究表明,GSTA3敲除小鼠对AFB1的毒性作用更敏感。由此可见,Nrf2介导的Ⅱ相解毒和抗氧化系统以及对线粒体结构和功能的调控可对AFB1解毒发挥重要作用。
因此,AFB1在动物体内诱导氧化应激的机制主要是在代谢过程中产生ROS,造成组织形态结构损伤和功能紊乱,同时破坏机体的酶类和非酶类抗氧化防御系统。
2 生物活性物质对AFB1诱导的氧化应激的抗氧化防御机制 2.1 天然植物及其提取物天然植物及其提取物因其含有的生物活性物质而具有多种生理调节作用,在霉菌毒素诱导的氧化应激中也发挥了良好的抗氧化防御功能。近年来,国内外学者研究发现,姜黄素、白藜芦醇、番茄红素、槲皮素、根皮素、表没食子儿茶素、没食子酸酯、葡萄籽原花青素、伞序臭黄荆乙醇提取物等天然植物及其提取物能够缓解AFB1诱导的动物氧化损伤。天然植物及其提取物的抗氧化活性主要是能够清除自由基,通过抑制Ⅰ相反应酶的活性提高Nrf2介导的Ⅱ相解毒和抗氧化防御系统的功能缓解AFB1诱导的氧化应激。不同的天然植物及其提取物对AFB1诱导的动物氧化损伤的缓解作用见表 1。
|
|
表 1 天然植物及其提取物对AFB1诱导的动物氧化损伤的缓解作用 Table 1 Alleviating effects of natural plants and their extracts on AFB1 induced oxidative damage in animals |
硒和锌是2种研究较多的微量元素,有研究表明,硒和锌对AFB1诱导的氧化应激和其他不良反应具有保护作用[6]。硒和锌是机体酶类和非酶类抗氧化系统的重要组成部分,可以调节抗氧化酶基因的表达,是潜在的抗氧化剂,对人和动物的各种生理功能具有调节作用,能够有效缓解AFB1诱导的氧化应激。Liao等[46]研究发现,灌胃0.1 mg/kg BW AFB1通过干扰组织酶活性和促进细胞凋亡而导致雏鸭肝脏功能障碍,但硒能够保护肝脏组织免受AFB1诱导的氧化损伤。饲粮添加硒能够降低线粒体膜通透性,提高线粒体抗氧化酶和ETC复合物Ⅰ~Ⅳ的活性,改善线粒体结构,从而提高AFB1(0.1 mg/kg BW)暴露的雏鸭肝脏抗氧化能力和线粒体功能[16, 47]。硒对AFB1(0.3 mg/kg饲粮)引起的肉鸡脾脏抗氧化酶活性降低和MDA含量升高具有抑制作用[48]。在含有100 μg/kg的AFB1肉鸡饲粮中添加硒,提高了GSH-Px、硫氧还蛋白还原酶(thioredoxin reductase,Txnrd)和CAT的活性以及GSH的含量,降低了MDA、8-OHdG和AFBO-DNA的含量,对CYP1A1、CYP1A2、CYP2A6和CYP3A4的活性及其mRNA的表达水平有抑制作用,而且提高了6种硒蛋白基因(GSH-Px3、Txnrd1、Txnrd2、Txnrd3、碘甲状腺原氨酸脱碘酶2和硒蛋白N)的表达水平[49]。硒能够缓解100 μg/kg AFB1污染饲粮诱导的肉鸡肝脏细胞坏死和萎缩、胆管增生以及GSH-Px和总超氧化物歧化酶(total superoxide dismutase,T-SOD)活性降低[50]。富硒酵母通过提高GSH-Px1的mRNA表达水平和总抗氧化能力,缓解饲喂含AFB1饲粮(250 μg/kg)诱导的小鼠肝脏损伤[51]。锌可以上调过氧化物氧化还原酶1、过氧化物氧化还原酶5和过氧化物氧化还原酶6的蛋白表达水平,缓解10 μg/mL AFB1诱导的人肝细胞氧化应激[52]。
2.3 益生菌枯草芽孢杆菌、解淀粉芽孢杆菌、植物乳杆菌等益生菌能够调节动物的生理功能,改善动物健康状态,起到缓解AFB1毒性损伤的作用。枯草芽孢杆菌能够提高采食AFB1污染饲粮(用含70 μg/kg AFB1的玉米代替20%、40%和60%的正常玉米)的蛋鸡血清抗氧化酶SOD和GSH-Px的活性[53]。在AFB1污染的肉鸡饲粮[AFB1、AFB2、AFG1和AFG2检测值分别为(70.7±1.3) μg/kg、(11.0±1.5) μg/kg、(6.5±0.8) μg/kg和(2.0±0.3) μg/kg]中添加枯草芽孢杆菌能够提高肉鸡的生长性能、肉品质和抗氧化能力[54-55]。在AF污染的饲粮[AFB1、AFB2、AFG1和AFG2检测值分别为(22.44±2.46) μg/kg、(6.69±1.32) μg/kg、(1.65±0.65) μg/kg和0.00 μg/kg]中添加枯草芽孢杆菌显著提高了鸭血清抗氧化酶SOD和GSH-Px的活性,降低了肝脏中MDA的含量[56]。在AFB1(450 μg/kg BW)诱导的小鼠肝脏损伤模型中,解淀粉芽孢杆菌能够降低ROS和MDA的含量,提高SOD、GSH-Px和CAT的活性,从而缓解AFB1诱导的肝脏氧化损伤[57];在AFB1(450 μg/kg BW)诱导的小鼠肾脏损伤模型中,解淀粉芽孢杆菌能够降低MDA含量,提高SOD、GSH-Px和CAT活性,提高Nrf2和血红素加氧酶-1(heme oxygenase-1,HO-1)的蛋白表达水平,降低Keap1的蛋白表达水平,从而缓解AFB1诱导的肾脏氧化损伤[58]。饮水中添加植物乳杆菌能够提高饲喂AFB1污染饲粮(低剂量组200 μg/kg和高剂量组2 000 μg/kg)的肉鸡血清SOD和GSH-Px活性[59]。
除了天然植物及其提取物、微量元素、益生菌以外,国内外学者在维生素、氨基酸、寡糖、有机酸等对AFB1诱导的动物氧化应激的影响也进行了研究。含维生素C、维生素E、酵母硒等成分的抗氧化复合添加剂与含丁酸钠、乳酸杆菌、黄芪多糖等成分的肠道健康复合添加剂能够提高饲喂含280 μg/kg AFB1饲粮的生长肥育猪的血浆SOD活性,降低MDA含量,提高机体抗氧化能力,缓解肝脏氧化损伤[60]。被74 μg/kg AFB1污染的肉鸡饲粮中添加α-硫辛酸能够提高肉鸡肝脏T-SOD、GR、GSH-Px活性以及GSH含量,降低MDA含量,下调CYP1A1和CYP2H1 mRNA表达水平,有上调GSTα mRNA表达水平的趋势[61]。胚蛋注射蛋氨酸能够提高初生雏鸡肝脏、肾脏、胸肌和十二指肠SOD、GSH-Px和CAT的活性以及GSH的含量,降低MDA的含量,提高GSH-Px、GST-α和SOD的mRNA表达水平,降低Bcl-2相关X蛋白基因(Bcl-2 associated X protein, Bax)、半胱氨酸蛋白酶-3(Caspase-3)、半胱氨酸蛋白酶-7(Caspase-7)、半胱氨酸蛋白酶-9(Caspase-9)、P53、CYP1A1和CYP2H1的mRNA表达水平,从而缓解AFB1(每枚胚蛋注射20 μL含36 ng/L AFB1的溶液)诱导的胚胎毒性[62]。三萜烯化合物通过诱导Nrf2介导的Ⅱ相抗氧化基因GSTA2和GSTA5等的表达水平缓解AFB1(25 μg/只)引起的大鼠肝脏损伤[63]。在大鼠急性AFB1(2.0 mg/kg BW)肝脏损伤模型中,用富氢水灌胃可降低肝脏组织中MDA含量,提高GSH含量,有效减轻肝脏损伤[64]。壳寡糖能够降低大鼠肝细胞内ROS和MDA的含量,增强SOD和GST的活性,提高Nrf2、Keap1、HO-1、NQO1的mRNA表达水平,还可能通过调控CYP450对外源物质的代谢作用、药物代谢CYP450和P53信号通路缓解AFB1(0.4~250.0 μmol/L)引起的大鼠肝脏细胞毒性损伤[65]。牛磺酸通过提高SOD、CAT、GSH-Px活性和GSH含量,降低MDA含量,提高Nrf2信号通路关键因子Nrf2、NQO1、HO-1、谷氨酰半胱氨酸连接酶催化亚基(glutamate-cysteine ligase catalytic subunit,GCLC)、GSH-Px和SOD的mRNA表达水平,对AFB1(250 μg/kg BW)诱导的大鼠肝脏、肾脏和脾脏损伤具有保护作用,并通过提高肝脏线粒体膜电位、线粒体解偶联蛋白2、肉毒碱棕榈酰转移酶1、细胞色素C氧化酶、NADPH细胞色素C还原酶的含量或活性保护线粒体的结构与功能[66]。油酸通过提高HO-1的表达水平、降低ROS含量对AFB1(5~20 μg/mL)引发的人肝细胞损伤具有保护作用[67]。
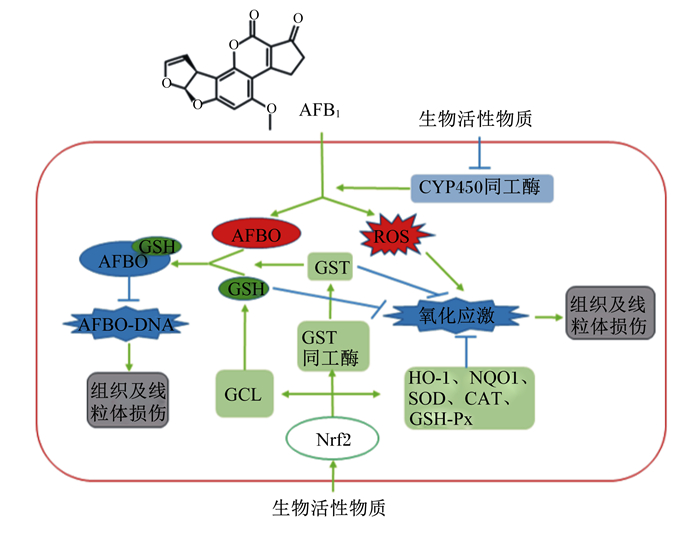
3 小结综上所述,AFB1在代谢过程中产生ROS,并通过影响线粒体ROS稳态和氧化还原平衡状态,引起线粒体氧化损伤和功能紊乱,进而造成机体氧化应激。外源生物活性物质能够通过调控Ⅰ相和Ⅱ相代谢酶的活性,改善线粒体功能,缓解AFB1诱导的氧化应激,其作用机制与核转录因子Nrf2介导的解毒和抗氧化防御系统有关(图 1)。天然植物及其提取物、微量元素、益生菌等是具有抗氧化作用的生物活性物质,在动物生产中缓解霉菌毒素对动物造成的毒性损伤具有重要的应用价值。但此类研究多集中于单一的生物活性物质,对不同生物活性物质联合使用的研究较少。另外,结合物理、化学、生物等不同的霉菌毒素脱毒方法,开发兼具脱毒与解毒的经济、有效的新产品,有望在饲料霉菌毒素的防控工作中成为新的可行的研究方向。
|
AFB1:黄曲霉毒素B1 aflatoxin B1;AFBO:AFB1-8, 9-环氧化物AFB1-8, 9-epoxides;CAT:过氧化氢酶catalase;CYP450:细胞色素P450 cytochrome P450;GCL:谷胱甘肽半胱氨酸连接酶glutamylcysteine ligase;GSH:还原型谷胱甘肽reduced glutathione;GST:谷胱甘肽硫转移酶glutathione S-transferase;GSH-Px:谷胱甘肽过氧化物酶glutathione peroxidase;HO-1:血红素加氧酶-1 heme oxygenase-1;NQO1:醌氧化还原酶1 NAD(P)H quinone oxidoreductase 1;ROS:活性氧自由基reactive oxygen species;SOD:超氧化物歧化酶superoxide dismutase。 图 1 AFB1诱导的动物氧化应激及不同生物活性物质的抗氧化防御机制 Fig. 1 Oxidative damage induced by AFB1 and antioxidant defense mechanism of different bioactive substances in animals |
| [1] |
范楷, 祭芳, 徐剑宏, 等. 长三角地区市场常见农产品中40种真菌毒素的污染状况和特征分析[J]. 中国农业科学, 2021, 54(13): 2870-2884. FAN K, JI F, XU J H, et al. Natural occurrence and characteristic analysis of 40 mycotoxins in agro-products from Yangtze delta region[J]. Scientia Agricultura Sinica, 2021, 54(13): 2870-2884 (in Chinese). DOI:10.3864/j.issn.0578-1752.2021.13.015 |
| [2] |
SAINI S S, KAUR A. Aflatoxin B1: toxicity, characteristics and analysis: mini review[J]. Global Advanced Research Journal of Chemistry and Material Science, 2012, 1(4): 63-70. |
| [3] |
周建川, 郑文革, 赵丽红, 等. 2016年中国饲料和原料中霉菌毒素污染调查报告[J]. 中国猪业, 2017, 12(6): 22-26, 32. ZHOU J C, ZHENG W G, ZHAO L H, et al. Investigation report on mycotoxin contamination in feed and raw materials in China in 2016[J]. China Swine Industry, 2017, 12(6): 22-26, 32 (in Chinese). |
| [4] |
夏超笃, 艾琴, 湛穗璋, 等. 霉菌毒素吸附剂在动物饲料中应用的研究进展[J]. 畜牧与饲料科学, 2017, 38(4): 27-31. XIA C D, AI Q, ZHAN S Z, et al. Research progress on application of mycotoxin adsorbent in animal feed[J]. Animal Husbandry and Feed Science, 2017, 38(4): 27-31 (in Chinese). DOI:10.3969/j.issn.1672-5190.2017.04.010 |
| [5] |
LEE S E, CAMPBELL B C, MOLYNEUX R J, et al. Inhibitory effects of naturally occurring compounds on aflatoxin B1 biotransformation[J]. Journal of Agricultural and Food Chemistry, 2001, 49(11): 5171-5177. DOI:10.1021/jf010454v |
| [6] |
MUGHAL M J, PENG X, KAMBOH A A, et al. Aflatoxin B1 induced systemic toxicity in poultry and rescue effects of selenium and zinc[J]. Biological Trace Element Research, 2017, 178(2): 292-300. DOI:10.1007/s12011-016-0923-9 |
| [7] |
MARIN D E, TARANU I. Overview on aflatoxins and oxidative stress[J]. Toxin Reviews, 2012, 31(3/4): 32-43. |
| [8] |
DIAZ G J, MURCIA H W, CEPEDA S M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail[J]. Poultry Science, 2010, 89(11): 2461-2469. DOI:10.3382/ps.2010-00864 |
| [9] |
SHIMAMOTO N. A pathophysiological role of cytochrome p450 involved in production of reactive oxygen species[J]. Yakugaku Zasshi, 2013, 133(4): 435-450. DOI:10.1248/yakushi.12-00263 |
| [10] |
RAWAL S, KIM J E, COULOMBE R, J r. Aflatoxin B1 in poultry: toxicology, metabolism and prevention[J]. Research in Veterinary Science, 2010, 89(3): 325-331. DOI:10.1016/j.rvsc.2010.04.011 |
| [11] |
YILMAZ S, KAYA E, KARACA A, et al. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene[J]. Research in Veterinary Science, 2018, 119: 268-275. DOI:10.1016/j.rvsc.2018.07.007 |
| [12] |
VAKIFAHMETOGLU-NORBERG H, OUCHIDA A T, NORBERG E. The role of mitochondria in metabolism and cell death[J]. Biochemical and Biophysical Research Communications, 2017, 482(3): 426-431. DOI:10.1016/j.bbrc.2016.11.088 |
| [13] |
CHEN J, CHEN K J, YUAN S B, et al. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers[J]. Toxicology and Industrial Health, 2016, 32(2): 278-284. DOI:10.1177/0748233713500819 |
| [14] |
余程, 刘妍, 王胜军, 等. 黄曲霉毒素B1致肉仔鸡肝损伤的氧化应激机制研究[J]. 中国畜牧杂志, 2017, 53(6): 92-97. YU C, LIU Y, WANG S J, et al. Oxidative stress mechanism of aflatoxin B1 induced liver injury in broilers[J]. Chinese Journal of Animal Science, 2017, 53(6): 92-97 (in Chinese). |
| [15] |
石达友, 李燕华, 黄墁玲, 等. 黄曲霉毒素B1对肉鸡肝线粒体自由基代谢的影响[J]. 中国兽医杂志, 2011, 47(3): 9-12. SHI D Y, LI Y H, HUANG M L, et al. Influences of aflatoxin B1 on hepatic mitochondrial free radical metabolism in broiler[J]. Chinese Journal of Veterinary Medicine, 2011, 47(3): 9-12 (in Chinese). DOI:10.3969/j.issn.0529-6005.2011.03.003 |
| [16] |
SHI D Y, LIAO S Q, GUO S N, et al. Protective effects of selenium on aflatoxin B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver[J]. Biological Trace Element Research, 2015, 163(1/2): 162-168. |
| [17] |
HUANG W Y, CAO Z, YAO Q C, et al. Mitochondrial damage are involved in aflatoxin B1-induced testicular damage and spermatogenesis disorder in mice[J]. The Science of the Total Environment, 2020, 701: 135077. DOI:10.1016/j.scitotenv.2019.135077 |
| [18] |
NITURE S K, KASPAR J W, SHEN J, et al. Nrf2 signaling and cell survival[J]. Toxicology and Applied Pharmacology, 2010, 244(1): 37-42. DOI:10.1016/j.taap.2009.06.009 |
| [19] |
PALL M L, LEVINE S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors[J]. Acta Physiologica Sinica, 2015, 67(1): 1-18. |
| [20] |
DINKOVA-KOSTOVA A T, ABRAMOV A Y. The emerging role of Nrf2 in mitochondrial function[J]. Free Radical Biology & Medicine, 2015, 88(Pt B): 179-188. |
| [21] |
HOLMSTRÖM K M, KOSTOV R V, DINKOVA-KOSTOVA A T. The multifaceted role of Nrf2 in mitochondrial function[J]. Current Opinion in Toxicology, 2016, 1: 80-91. DOI:10.1016/j.cotox.2016.10.002 |
| [22] |
KOVAC S, ANGELOVA P R, HOLMSTRÖM K M, et al. Nrf2 regulates ROS production by mitochondria and NADPH oxidase[J]. Biochimica et Biophysica Acta, 2015, 1850(4): 794-801. DOI:10.1016/j.bbagen.2014.11.021 |
| [23] |
LUDTMANN M H R, ANGELOVA P R, ZHANG Y, et al. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation[J]. Biochemical Journal, 2014, 457(3): 415-424. DOI:10.1042/BJ20130863 |
| [24] |
THIMMULAPPA R K, MAI K H, SRISUMA S, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray[J]. Cancer Research, 2002, 62(18): 5196-5203. |
| [25] |
TAGUCHI K, TAKAKU M, EGNER P A, et al. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity[J]. Toxicological Sciences, 2016, 152(1): 40-52. DOI:10.1093/toxsci/kfw065 |
| [26] |
ILIC Z, CRAWFORD D, VAKHARIA D, et al. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1[J]. Toxicology and Applied Pharmacology, 2010, 242(3): 241-246. DOI:10.1016/j.taap.2009.10.008 |
| [27] |
GOWDA N K S, LEDOUX D R, ROTTINGHAUS G E, et al. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks[J]. Poultry Science, 2008, 87(6): 1125-1130. DOI:10.3382/ps.2007-00313 |
| [28] |
GOWDA N K S, LEDOUX D R, ROTTINGHAUS G E, et al. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1[J]. The British Journal of Nutrition, 2009, 102(11): 1629-1634. DOI:10.1017/S0007114509990869 |
| [29] |
ZHANG N Y, QI M, ZHAO L, et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver[J]. Toxins, 2016, 8(11): 327. DOI:10.3390/toxins8110327 |
| [30] |
EL-AGAMY D S. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats[J]. Archives of Toxicology, 2010, 84(5): 389-396. DOI:10.1007/s00204-010-0511-2 |
| [31] |
SRIDHAR M, SUGANTHI R U, THAMMIAHA V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds[J]. Journal of Animal Physiology and Animal Nutrition, 2015, 99(6): 1094-1104. DOI:10.1111/jpn.12260 |
| [32] |
ZHOU Y F, JIN Y C, YU H, et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway[J]. Toxicon, 2019, 164: 10-15. DOI:10.1016/j.toxicon.2019.03.022 |
| [33] |
YILMAZ S, KAYA E, KISACAM M A, et al. The effect on oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage[M]//ABDULRA'UF L B. Aflatoxin-control, analysis, detection and health risks. London: IntechOpen, 2017.
|
| [34] |
TANG L L, GUAN H X, DING X L, et al. Modulation of aflatoxin toxicity and biomarkers by lycopene in F344 rats[J]. Toxicology and Applied Pharmacology, 2007, 219(1): 10-17. DOI:10.1016/j.taap.2006.12.001 |
| [35] |
XU F B, YU K Y, YU H Y, et al. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation[J]. Journal of Functional Foods, 2017, 39: 215-224. DOI:10.1016/j.jff.2017.10.027 |
| [36] |
REDDY L, ODHAV B, BHOOLA K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: morphology, apoptosis and DNA damage[J]. Biological Chemistry, 2006, 387(1): 87-93. DOI:10.1515/BC.2006.012 |
| [37] |
CHOI K C, CHUNG W T, KWON J K, et al. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice[J]. Food and Chemical Toxicology, 2010, 48(10): 2747-2753. DOI:10.1016/j.fct.2010.07.001 |
| [38] |
李丽, 张根义. 槲皮素对黄曲霉毒素B1致大鼠肝细胞毒性的保护作用[J]. 食品工业科技, 2018, 39(13): 117-121, 127. LI L, ZHANG G Y. Protective effect of quercetin against the toxicity of aflatoxin B1 toward buffalo rat liver cells[J]. Science and Technology of Food Industry, 2018, 39(13): 117-121, 127 (in Chinese). |
| [39] |
GHADIRI S, SPALENZA V, DELLAFIORA L, et al. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line[J]. Toxicology in Vitro, 2019, 57: 174-183. DOI:10.1016/j.tiv.2019.03.002 |
| [40] |
GAO S S, CHEN X Y, ZHU R Z, et al. Dual effects of phloretin on aflatoxin B1 metabolism: activation and detoxification of aflatoxin B1[J]. BioFactors, 2012, 38(1): 34-43. DOI:10.1002/biof.190 |
| [41] |
赵静芳, 邓志杰, 肖德强, 等. 表没食子儿茶素没食子酸酯对黄曲霉毒素B1诱导大鼠急性肝损伤的抗氧化作用[J]. 实用医学杂志, 2018, 34(11): 1753-1756, 1761. ZHAO J F, DENG Z J, XIAO D Q, et al. Antioxidant effect of EGCG on AFB1 induced acute liver injury in rats[J]. The Journal of Practical Medicine, 2018, 34(11): 1753-1756, 1761 (in Chinese). DOI:10.3969/j.issn.1006-5725.2018.11.001 |
| [42] |
RAJPUT S A, SUN L H, ZHANG N Y, et al. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1[J]. Toxins, 2017, 9(11): 371. DOI:10.3390/toxins9110371 |
| [43] |
RAJPUT S A, SUN L H, ZHANG N Y, et al. Grape seed proanthocyanidin extract alleviates aflatoxin B1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers[J]. Toxins, 2019, 11(1): 23. DOI:10.3390/toxins11010023 |
| [44] |
RAJPUT S A, ZHANG C, FENG Y, et al. Proanthocyanidins alleviates aflatoxin B1-induced oxidative stress and apoptosis through mitochondrial pathway in the bursa of fabricius of broilers[J]. Toxins, 2019, 11(3): 157. DOI:10.3390/toxins11030157 |
| [45] |
SINGH C, PRAKASH C, MISHRA P, et al. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice[J]. Toxicon, 2019, 166: 88-100. DOI:10.1016/j.toxicon.2019.05.014 |
| [46] |
LIAO S Q, SHI D Y, CLEMONS-CHEVIS C L, et al. Protective role of selenium on aflatoxin B1-induced hepatic dysfunction and apoptosis of liver in ducklings[J]. Biological Trace Element Research, 2014, 162(1/2/3): 296-301. |
| [47] |
SHI D Y, GUO S, LIAO S Q, et al. Protection of selenium on hepatic mitochondrial respiratory control ratio and respiratory chain complex activities in ducklings intoxicated with aflatoxin B1[J]. Biological Trace Element Research, 2012, 145(3): 312-317. DOI:10.1007/s12011-011-9195-6 |
| [48] |
WANG F Y, SHU G, PENG X, et al. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen[J]. International Journal of Environmental Research and Public Health, 2013, 10(7): 2834-2844. DOI:10.3390/ijerph10072834 |
| [49] |
SUN L H, ZHANG N Y, ZHU M K, et al. Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of 6 selenoprotein genes in chick liver[J]. The Journal of Nutrition, 2015, 146(4): 655-661. DOI:10.3945/jn.115.224626 |
| [50] |
芮小丽, 陈思潭, 李春梅. 硒对黄曲霉毒素B1暴露肉鸡肝脏氧化损伤的保护作用[J]. 动物营养学报, 2014, 26(8): 2281-2288. RUI X L, CHEN S T, LI C M. Protective effects of selenium on aflatoxin B1 induced hepatic oxidative stress in broilers[J]. Chinese Journal of Animal Nutrition, 2014, 26(8): 2281-2288 (in Chinese). DOI:10.3969/j.issn.1006-267x.2014.08.032 |
| [51] |
LIU L N, CHEN F, QIN S Y, et al. Effects of selenium-enriched yeast improved aflatoxin B1-induced changes in growth performance, antioxidation capacity, IL-2 and IFN-γ contents, and gene expression in mice[J]. Biological Trace Element Research, 2019, 191(1): 183-188. DOI:10.1007/s12011-018-1607-4 |
| [52] |
ZHU L Y, HUANG C C, YANG X, et al. Proteomics reveals the alleviation of zinc towards aflatoxin B1-induced cytotoxicity in human hepatocyes (HepG2 cells)[J]. Ecotoxicology and Environmental Safety, 2020, 198: 110596. DOI:10.1016/j.ecoenv.2020.110596 |
| [53] |
MA Q G, GAO X, ZHOU T, et al. Protective effect of Bacillus subtilis ANSB060 on egg quality, biochemical and histopathological changes in layers exposed to aflatoxin B1[J]. Poultry Science, 2012, 91(11): 2852-2857. DOI:10.3382/ps.2012-02474 |
| [54] |
FAN Y, ZHAO L H, MA Q G, et al. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins[J]. Food and Chemical Toxicology, 2013, 59: 748-753. DOI:10.1016/j.fct.2013.07.010 |
| [55] |
FAN Y, ZHAO L H, JI C, et al. Protective effects of Bacillus subtilis ANSB060 on serum biochemistry, histopathological changes and antioxidant enzyme activities of broilers fed moldy peanut meal naturally contaminated with aflatoxins[J]. Toxins, 2015, 7(8): 3330-3343. DOI:10.3390/toxins7083330 |
| [56] |
ZHANG L Y, MA Q G, MA S S, et al. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins[J]. Toxins, 2016, 9(1): 1. DOI:10.3390/toxins9010001 |
| [57] |
LI X T, LV Z M, CHEN J, et al. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1[J]. Food and Chemical Toxicology, 2021, 151: 112124. DOI:10.1016/j.fct.2021.112124 |
| [58] |
ZHAO Y Q, WANG T C, LI P, et al. Bacillus amyloliquefaciens B10 can alleviate aflatoxin B1-induced kidney oxidative stress and apoptosis in mice[J]. Ecotoxicology and Environmental Safety, 2021, 218: 112286. DOI:10.1016/j.ecoenv.2021.112286 |
| [59] |
KHANIAN M, KARIMI-TORSHIZI M A, ALLAMEH A. Alleviation of aflatoxin-related oxidative damage to liver and improvement of growth performance in broiler chickens consumed Lactobacillus plantarum 299v for entire growth period[J]. Toxicon, 2019, 158: 57-62. DOI:10.1016/j.toxicon.2018.11.431 |
| [60] |
蒲俊宁, 原清会, 陈代文, 等. 营养性复合添加剂对饲喂含黄曲霉毒素B1饲粮生长育肥猪生长性能、抗氧化能力、肝脏功能和毒素残留的影响[J]. 动物营养学报, 2019, 31(10): 4691-4700. PU J N, YUAN Q H, CHEN D W, et al. Effects of nutritional compound additives on growth performance, antioxidant capacity, liver function and toxin residues of growing-finishing pigs fed diets contaminated with aflatoxin B1[J]. Chinese Journal of Animal Nutrition, 2019, 31(10): 4691-4700 (in Chinese). |
| [61] |
LI Y, MA Q G, ZHAO L H, et al. Protective efficacy of alpha-lipoic acid against aflatoxin B1-induced oxidative damage in the liver[J]. Asian-Australasian Journal of Animal Sciences, 2014, 27(6): 907-915. |
| [62] |
ELWAN H, XIE C, MIAO L P, et al. Methionine alleviates aflatoxin B1-induced broiler chicks embryotoxicity through inhibition of caspase-dependent apoptosis and enhancement of cellular antioxidant status[J]. Poultry Science, 2021, 100(8): 101103. |
| [63] |
YATES M S, KWAK M K, EGNER P A, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-, 12-dioxooleana-1, 9(11)-dien-28-oyl]imidazole[J]. Cancer Research, 2006, 66(4): 2488-2494. |
| [64] |
扈红蕾, 高健, 郭文君, 等. 富氢水在黄曲霉毒素B1致大鼠肝损伤模型中的抗损伤作用[J]. 生理学报, 2019, 71(5): 725-731. HU H L, GAO J, GUO W J, et al. Anti-injury effect of hydrogen-enriched water in a rat model of liver injury induced by aflatoxin B1[J]. Acta Physiologica Sinica, 2019, 71(5): 725-731 (in Chinese). |
| [65] |
谢佳雨, 张雯, 杨靖亚, 等. 壳寡糖对黄曲霉毒素B1诱导大鼠肝细胞毒性损伤的干预作用[J/OL]. 上海海洋大学学报: 1-14. (2021-02-05). http://kns.cnki.net/kcms/detail/31.2024.S.20210205.1346.006.html. XIE J Y, ZAHNG W, YANG J Y, et al. Intervention effect of chitooligosaccharides on aflatoxin B1 induced toxic damage of rat liver cells[J/OL]. Journal of Shanghai Ocean University: 1-14. (2021-02-05)]. http://kns.cnki.net/kcms/detail/31.2024.S.20210205.1346.006.html. (in Chinese) |
| [66] |
唐日益. 牛磺酸对大鼠黄曲霉毒素B1中毒的干预作用及机制研究[D]. 博士学位论文. 沈阳: 沈阳农业大学, 2019. TANG R Y. Protective effect and mechanism of taurine on AFB1 induced injury in rats[D]. Ph. D. Thesis. Shenyang: Shenyang Agricultural University, 2019. (in Chinese) |
| [67] |
师文文, 吕攀, 徐庆强, 等. 油酸在黄曲霉毒素诱导肝细胞损伤中的保护作用[J]. 中国油料作物学报, 2019, 41(2): 267-274. SHI W W, LYU P, XU Q Q, et al. Protective effect of oleic acid on aflatoxin-induced hepatocyte injury[J]. Chinese Journal of Oil Crop Sciences, 2019, 41(2): 267-274 (in Chinese). |



