2. 四川农业大学动物科技学院, 成都 611130
2. College of Animal Science and Technology, Sichuan Agricultural University, Chengdu 611130, China
提高饲粮内富含碳水化合物的饲料原料比例是反刍动物生产中提高生产效率的普遍手段,但瘤胃内微生物的发酵速率显著加快,挥发性脂肪酸(volatile fatty acid,VFA)的产生速率大于瘤胃上皮的吸收和代谢速率,导致瘤胃VFA蓄积、pH降低、菌群紊乱,诱发亚急性瘤胃酸中毒(subacute ruminal acidosis,SARA),现主要的SARA评定标准为瘤胃内低pH状态超过3 h/d,且维持在5.2~5.8[1]。SARA扰乱了瘤胃发酵,破坏了瘤胃上皮完整性,降低了饲料消化率和营养物质代谢率,还诱发腹泻、局部炎症、肝脓肿、蹄病以及乳脂下降综合征等疾病,进一步发展为酸中毒,还会导致休克或死亡,影响反刍动物的生存和生产[2-3]。丁酸占总挥发性脂肪酸(total volatile fatty acid,TVFA)的比例为10%~20%,参与反刍动物的能量代谢,其生理功能较为活跃[4]。研究发现,丁酸具有促进反刍动物胃肠道发育、改善瘤胃屏障功能、维持胃肠道微生态系统稳态、抵抗炎症等生理功能[5]。作为信号分子的丁酸还能通过细胞膜上的G蛋白偶联受体(G-protein-coupled receptors,GPRs)激活下游信号通路,调控细胞炎症因子、趋化因子和紧密连接蛋白等的表达,从而缓解炎症反应和改善屏障功能[6]。
1 丁酸的概述及在反刍动物体内的生成丁酸又称酪酸,是产丁酸菌利用碳水化合物发酵合成的一元羧酸,在瘤胃内主要通过被动转运、离子交换等多种转运方式被瘤胃壁吸收,进入瘤胃上皮细胞后参与能量代谢[7]。丁酸能以β-羟基丁酸(β-hydroxybutyrate,BHBA)形式为反刍动物供能,还是乳脂和体脂的前体物。在反刍动物瘤胃中,丁酸梭菌是主要产丁酸菌,饲料中的蔗糖、纤维二糖和乳糖等碳水化合物被其产生的胞外酶水解成小分子单糖,进入细胞胞浆后又在酶的作用下进行糖酵解,最终转化成丁酸[8-10]。丁酸的合成过程中,关键步骤是丁酰辅酶A的生成,限速酶是铁氧还原蛋白氧化还原酶,由于其对氧的敏感性决定了丁酸的产量与厌氧程度呈正相关。瘤、网胃壁吸收丁酸后,在上皮细胞中转化为BHBA,再被瘤胃上皮基底侧细胞膜上的转运蛋白转运入血液,最终被机体代谢产能。丁酸在瘤胃上皮细胞中经酰基辅酶A合成酶缩合生成乙酰辅酶A[11],且主要有2条去路:一是直接进入三羧酸循环(tricarboxylic acid cycle,TCA)代谢产能,为瘤胃上皮细胞ATP的产生提供了主要来源[9];二是在3-羟基3-甲基戊二酰辅酶A(3-hydroxy-3- methylglutaryl-CoA,HMG-CoA)合成酶、HMG-CoA裂解酶和BHBA脱氢酶的作用下进行生酮反应,最终将乙酰辅酶A转化为BHBA,后进入血液循环为机体供能[12]。丁酸梭菌产生丁酸的主要生化反应途径见图 1。
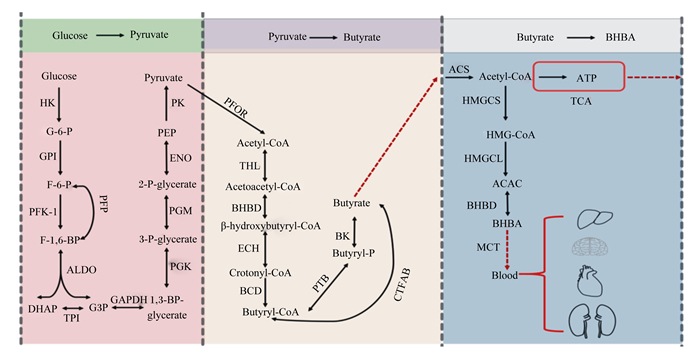
|
Glucose:葡萄糖;HK:己糖激酶hexokinase;G-6-P:葡萄糖-6-磷酸glucose-6-phosphatase;GPI:葡萄糖磷酸异构酶glucose phosphate isomerase;F-6-P:果糖-6-磷酸fructose-6-phosphate;PFK-1:磷酸果糖激酶-1 phosphofructokinase-1;F-1, 6-BP:果糖1, 6-二磷酸fructose 1, 6-diphosphate;ALDO:醛缩酶aldolase;DHAP:磷酸二羟丙酮dihydroxyacetone phosphate;TPI:磷酸丙糖异构酶triosephosphateisomerase;G3P:3-磷酸-甘油醛3-phosphoricacid-glyceraldehyde;GAPDH:磷酸甘油醛激酶phosphoglyceraldehyde kinase;1, 3-BP-glycerate:1, 3-二磷酸-甘油酸1, 3-diphosphate-glyceric acid;PGK:磷酸甘油酸激酶phosphoglyceric kinase;3-P-glycerate:3-磷酸-甘油酸glycericacid-3-phosphoric acid;PGM:磷酸甘油酸变位酶phosphoglyceromutase;2-P-glycerate:2-磷酸-甘油2-phosphate-glycerol;ENO:烯醇化酶enolase;PEP:磷酸烯醇式丙酮酸phosphoenolpyruvic acid;PK:丙酮酸激酶pyruvate kinase;Pyruvate:丙酮酸;PFOR:丙酮酸中还原酶pyruvate ferredoxinoxidoreductase;Acetyl-CoA:乙酰辅酶A acetyl coenzyme A;THL:硫解酶thiolase;Acetoacetyl-CoA:乙酰乙酰辅酶A acetoacetyl coenzyme A;BHBD:β-羟乙酰辅酶A脱氢酶β-hydroxylacetyl coenzyme A dehydrogenase;β-hydroxybutyryl-CoA:β-羟乙酰辅酶A β-hydroxyacetyl coenzyme A;ECH:烯酰辅酶A水化酶enyl coenzyme A hydration enzyme;Crotonyl-CoA:巴豆酰辅酶A crotonyl coenzyme A;Butyryl-CoA:丁酰辅酶A butyryl coenzyme A;PTB:丁酰磷酸转移酶butyryl phosphate transferase;Butyryl-P:丁酰磷酸butyryl phosphate;BK:丁酸激酶butyrate kinase;Butyrate:丁酸;ACS:酰基辅酶A合成酶acyl-coenzyme A synthetase;ATP:三磷酸腺苷酶adenosine triphosphatase;HMGCL:3-羟基-3-甲基戊二酸单酰辅酶A裂解酶3-hydroxy-3-methylglutarate monoacyl coenzyme A lyase;MCT:单羧酸转运蛋白体monocarboxylic acid transporter。 图 1 丁酸梭菌产生丁酸的主要生化反应途径 Fig. 1 Main biochemical pathway of Clostridium butyricum to produce butyric acid[8-9, 12] |
瘤胃上皮结构的完整、细胞吸收功能的高度选择性和紧密连接蛋白将细胞层紧密连接构成了瘤胃上皮的生理屏障,阻断了瘤胃内的微生物和瘤胃异常代谢产物通过跨细胞途径随营养物质或旁细胞途径进入血液[13]。其中,瘤胃上皮细胞间紧密连接因具有维持细胞极性、调控小分子物质的跨上皮转运、阻碍有害物质跨上皮入体的功能,决定了瘤胃上皮的通透性[14]。SARA能够导致瘤胃上皮角质层脱落和损伤,基底层、颗粒层、棘突层变薄,破坏细胞间紧密连接和上皮完整性,增加上皮的通透性[15-16]。生产中常饲喂高精料饲粮引起瘤胃内pH降低和产生高浓度脂多糖(lipopolysaccharide,LPS),进而诱发SARA,导致瘤胃上皮的通透性和屏障功能受损。LPS主要能够提高细胞外信号调节蛋白激酶(extracellular signals regulate protein kinases,ERK)1/2磷酸化比例,激活丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)/ERK1/2信号通路,抑制紧密连接蛋白转录后机制,降低紧密连接蛋白表达[17]。此外,LPS还能够激活LPS/Toll样受体-4(Toll-like receptor-4,TLR-4)-核因子-κB(nuclear factor kappa B,NF-κB)/MAPK信号通路,抑制核因子E2相关因子2(nuclear factor E2-related factor 2,Nrf2)基因的表达,抑制细胞的抗氧化应激系统[18],引起细胞的氧化应激,增加细胞的凋亡比例[18-20]。
2.2 诱发炎症反应瘤胃内持续的的低pH状态导致瘤胃内的革兰氏阴性菌大量裂解和死亡以及促进某些细菌释放组氨酸脱羧酶,增加瘤胃中LPS和组胺的浓度[21-22]。LPS和组胺通过细胞旁途径易位进入血液和淋巴液参与体循环,激活系统炎症并引起保护性免疫反应[23-24]。LPS和组胺等毒素诱导的急性期反应就是这种免疫的一部分,如LPS诱导脂多糖结合蛋白(lipopolysaccharide binding protein,LBP)浓度增加[25]。LPS和LBP形成复合物和组胺又通过上调信号通路中关键蛋白的磷酸化水平,激活炎症相关的信号通路,促进炎症因子mRNA和蛋白的表达,诱导炎症反应的发生[26-28]。首先,LPS-LBP复合物催化转移至CD14,并与TLR-4和髓样分化蛋白-2(myeloid differential protein-2,MD-2)结合成复合物,再被巨噬细胞膜内受体识别,活化下游的炎症因子如肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-6(interleukin-6,IL-6)等[26]。活化后的炎症因子和LPS诱导核因子-κB抑制蛋白(inhibitor of NF-κB,IκB)家族的抑制剂蛋白(包括IκBa、IκBb、IκBg和IκBe)和p38迅速磷酸化,激活NF-κB/MAPK,致使NF-κB p50/p65二聚体与IκB分离易位至细胞核内与DNA结合,p38发生核转位,诱导靶基因的转录,促进炎症因子mRNA的表达[27, 29]。SARA还能够增加机体中组胺的浓度,上调组胺特异性受体mRNA的表达,激活蛋白激酶C(protein kinase C,PKC)/NF-κB或蛋白激酶A(protein kinase A,PKA)/NF-κB信号通路促发炎症反应。机体中的组胺除了从瘤胃中易位的外,炎症因子和LPS还分别诱导嗜碱性粒细胞和巨噬细胞合成并释放组胺,增加机体内组胺的浓度[30]。LPS与LBP形成的复合物活性的增强和组胺浓度的增加进一步加剧了局部和全身的炎症反应。
3 丁酸缓解SARA的影响机制 3.1 改善瘤胃上皮的屏障功能 3.1.1 加强瘤胃上皮的紧密连接丁酸除了作为瘤胃上皮细胞的能量底物,还可以作为信号分子和酶的抑制剂,促进反刍动物胃肠道的发育进而改善胃肠道的屏障功能。丁酸作为机体的信号分子识别细胞膜上的GPRs,随后调控下游的信号通路,如抑制MAPK、PKC等信号通路,进而促进紧密连接相关蛋白的表达[31]。在高精料饲粮诱导山羊瘤胃上皮的损伤试验中,丁酸钠盐就通过抑制PKC和MAPK信号通路逆转了瘤胃上皮的紧密连接损伤,减轻了高精料饲粮对瘤胃上皮的损伤[32]。在断奶羔羊饲粮中添加丁酸钠还降低了NF-κB蛋白表达量,抑制炎症因子的表达;同时,促进了闭合蛋白(Occludin)、封闭蛋白(Claudin)和闭锁小带蛋白-1(zonula occludens protein 1,ZO-1)蛋白的表达,增强了细胞间的紧密连接[31]。
3.1.2 促进瘤胃上皮细胞的生长瘤胃上皮屏障功能的完善需要瘤胃上皮间的紧密连接和具有高度选择性的瘤胃上皮细胞共同完成,因此瘤胃上皮细胞的增殖和凋亡至关重要[13]。已有研究发现,丁酸钠能够通过调控犊牛和山羊瘤胃上皮细胞的增殖、分化和凋亡,调控瘤胃上皮的生理功能[33-34]。哺乳动物细胞(包括瘤胃上皮细胞)的增殖主要由细胞周期决定,而细胞周期又分为4个阶段:G1期、S期、G2期、M期,缩短或延长任何一个阶段都会对细胞周期产生影响。通过对山羊屠宰前瘤胃灌注丁酸(0.3 g/kg BW)发现,丁酸显著上调了参与细胞周期调控的调控因子:细胞周期蛋白D1、细胞周期蛋白E1、细胞周期蛋白A、细胞周期蛋白B以及细胞周期蛋白依赖激酶(cyclin-dependent kinases,CDK)1、CDK2、CDK4和CDK6 mRNA的表达,缩短了细胞周期,增加了瘤胃上皮的细胞层数和瘤胃乳头的长度、宽度[35]。其中,细胞周期蛋白D1、CDK2、CDK4和CDK6参与调控G0/G1期;细胞周期蛋白E1参与调控G1/S期;细胞周期蛋白A、细胞周期蛋白B和CDK1参与调控S/M期。细胞周期蛋白D1与CDK4/6形成的复合物,主要促进细胞从G1早期到中期[36],而细胞周期蛋白E1/CDK2形成的复合物,只要是使细胞周期进入至G1/S晚期[37]。Malhi等[34]研究也发现,丁酸通过上调细胞周期蛋白D1,缩短了G1期,促进了瘤胃上皮细胞的生长。同时,丁酸在促进细胞快速增殖的过程中也促进了细胞的凋亡[35]。研究发现,丁酸上调了瘤胃组织中p21的mRNA的表达水平,p21具有抗增殖的作用,通过与CDK结合,在G0/G2期的任何阶段阻止细胞周期的发展,但值得注意的是,丁酸诱导的增殖的比例大于凋亡,最终促进细胞的增殖[35, 38]。Kowalski等[39]在奶牛饲粮中添加丁酸还发现,丁酸显著增加了生长期奶牛瘤胃乳头长度和瘤胃肌肉层的厚度,并且增强了产犊后泌乳奶牛的瘤胃适应产后饲料的潜力且不影响泌乳性能。同时,丁酸通过调节激素和生长因子[34, 40]、影响组蛋白乙酰化基因的表达[41]、刺激微生物发酵产物的吸收和代谢[42],也间接促进了瘤胃上皮细胞的生长。
3.2 缓解SARA引发的炎症反应 3.2.1 作为信号分子抑制炎症反应丁酸作为瘤胃中VFA的一种,具有显著的抗炎、抗氧化、调节细胞增殖/凋亡功能[43-45],并且作为GPRs的配体,能够被细胞膜上GPRs识别调节下游的信号通路[6]。Shen等[46]通过对山羊瘤胃上皮转录组分析发现,瘤胃上皮细胞中存在20个GPRs家族成员,并参与维持上皮细胞的完整性和调控动物的生长。Chang等[47]在奶山羊饲粮中添加丁酸钠(1% BW)也发现,丁酸钠显著降低了炎症因子及GPR41/GPR43 mRNA的表达,降低GPR41/43、ERK1/2和p38的蛋白表达水平,缓解了山羊盲肠黏膜的炎症损伤。丁酸钠还改变了GPR41/43基因启动子区域的DNA甲基化和染色质压实的比例[48],需要指出的是,DNA甲基化和染色质重塑也有助于丁酸钠的抗炎作用。此外,高精料饲粮添加丁酸钠还能够显著提高瘤胃中的pH,降低瘤胃中的LPS、γ-D-谷氨酰基-内消旋-二氨基庚二酸(γ-D-Glu-mDAP,IE-DAP)和炎症因子(TNF-α、IL-1β和IL-6)的浓度,显著下调核苷酸结合寡聚化域蛋白1(nucleotide-binding oligomerization domain-containing protein 1,NOD1)、pp65、p-IκBα、p-NF-κB/p-p65、p-ERK1/2、p-c-Jun氨基末端激酶(c-Jun amino-terminal kinase JNK)、p-p38蛋白表达以及髓过氧化物酶(myeloperoxidase,MPO)和基质金属蛋白酶(matrix metalloproteinase,MMP)-2, 9的活性,抑制LPS-NOD1/NF-κB炎症通路缓解反刍动物瘤胃上皮、乳腺、肝脏等局部炎症的发生[44, 48-50]。
3.2.2 作为组蛋白去乙酰化酶抑制剂(histone deacetylase inhibitors,HDACIs)抑制炎症反应丁酸还能够作为HDACIs抑制组蛋白去乙酰化酶(histone deacetylase, HDAC)的活性,抑制组蛋白乙酰基的去除,促进组蛋白的乙酰化水平,抑制基因转录[51],其中,DNA结合组蛋白的乙酰化,调控着染色质结构和转录因子的结合亲和力,影响基因的表达[52]。最新的研究发现,HDACIs通过促进HDAC的乙酰化,抑制LPS刺激而引发的磷酸化,促进了HDAC3的活性,上调NF-κB p65赖氨酸216处的乙酰化水平,抑制NF-κB信号通路的激活[53]。且在人体结肠中的研究已证实丁酸是一种强抑制剂,能够抑制80% HDAC1/2的活性[54]。Shen等[46]对山羊瘤胃上皮进行转录组典型相关分析(canonical correlation analysis,CCA)发现,瘤胃微生物衍生的丁酸摩尔比值与HDAC1的表达呈高度负相关,证明在反刍动物中丁酸也是HDAC1强有效的抑制因子。Sun等[55]进一步对永生化奶牛乳腺上皮细胞的研究发现,添加丁酸钠或组蛋白去乙酰化物酶抑制剂曲古抑菌素A(trichostatin A,TSA)对乳腺细胞预处理18 h后,丁酸钠除了能够下调LPS引起的组蛋白H3乙酰化蛋白丰度升高,还能够降低炎症因子转录水平、p-IκBα/IκBα和p-p65/p65蛋白丰度的比值以及NF-κB p65蛋白核定位水平,通过抑制NF-κB信号传导的翻译后机制减轻了乳腺细胞的促炎反应。同时,在奶山羊高精料饲粮中添加丁酸钠(1% BW)也发现,丁酸钠能够降低奶山羊肝脏和乳腺中的HDAC3蛋白的表达,缓解SARA引起的肝脏和乳腺炎症损伤[48, 56]。丁酸对屏障功能和抗炎作用的机制见图 2。
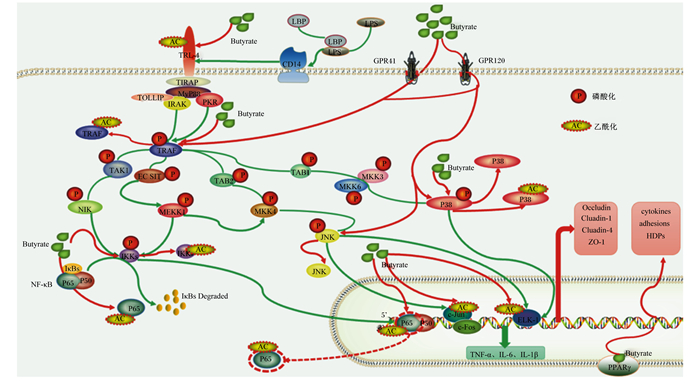
|
Butyrate:丁酸根离子;LPS:脂多糖lipopolysaccharide;TLR-4:Toll样受体-4 Toll-like receptor-4;CD14:LPS受体lipopolysaccharide receptor;LBP:脂多糖结合蛋白LPS binding protein;GPR:G蛋白偶联受体G protein-coupled receptor;TIRAP:Toll/白细胞介素-1受体域衔接因子蛋白Toll-interleukin-1 receptor domain containing adaptor protein;MyD88:髓样分化因子88 myeloid differentiation protein 88;TOLLIP:Toll样受体接头蛋白Toll-like receptor connector protein;IRAK:白细胞介素受体相关激酶interleukin receptor-associated kinase;PKR:双链RNA依赖的蛋白激酶double-stranded RNA-dependent protein kinase;TRAF:肿瘤坏死因子受体相关因子tumornecrosisfactor receptor associated factor;TAK:转化生长因子激酶transformed growth factor kinase;NIK:核因子-κB诱导激酶NF-κB induces kinase;MKK/MEK:丝裂原活化蛋白激酶激酶mitogen-activated protein kinase kinase;MEKK:丝裂原活化蛋白激酶激酶激酶mitogen-activated protein kinase kinase kinase;p38:p38丝裂原活化蛋白激酶p38 mitogen-activated protein kinase;JNK:c-Jun氨基末端激酶c-Jun amino-terminal kinase;IκB:核因子-κB抑制蛋白nuclear factor-κB inhibitor protein;IKK:核因子-κB抑制蛋白激酶nuclear factor-κB inhibitor protein kinase;p65/p50:核因子-κB1二聚体蛋白nuclear factor-κB1 dimer protein;ELK-1:转录激活因子ETS样蛋白1 transcriptional activator ETS like protein-1;PPARγ:过氧化物酶体增殖物激活受体peroxisome proliferator activated receptor;Occludin:闭合蛋白;Claudin-1:封闭蛋白;ZO-1:闭锁小带蛋白-1 zonula occludens protein 1;cytokines:细胞因子;adhesions:黏附因子;HDPs:宿主防御肽host defense peptides;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;IL-6:白细胞介素-6 interleukin-6;IL-1β:白细胞介素-1β interleukin-1β。 图 2 丁酸改善屏障功能和抗炎作用机制示意图 Fig. 2 Schematic diagram of improving barrier function and anti-inflammatory effect of butyric acid |
SARA引起了瘤胃发酵紊乱、瘤胃上皮屏障功能受损以及炎症反应。丁酸作为瘤胃产生的代谢物之一,能促进组织生长发育修补已损伤的瘤胃壁和增加瘤胃壁的抵抗力、调控瘤胃上皮细胞的增殖周期;丁酸作为信号分子调控了瘤胃上皮中紧密连接蛋白的表达,改善了瘤胃上皮的屏障功能。另外,丁酸作为信号分子和HDACIs通过NF-κB和MAPK等信号通路直接或间接抑制炎症因子的表达,缓解局部性和全身性炎症反应。
| [1] |
GOZHO G N, PLAIZIER J C, KRAUSE D O, et al. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response[J]. Journal of Dairy Science, 2005, 88(4): 1399-1403. DOI:10.3168/jds.S0022-0302(05)72807-1 |
| [2] |
GOZHO G N, KRAUSE D O, PLAIZIER J C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows[J]. Journal of Dairy Science, 2007, 90(2): 856-866. DOI:10.3168/jds.S0022-0302(07)71569-2 |
| [3] |
KHAFIPOUR E, KRAUSE D O, PLAIZIER J C. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation[J]. Journal of Dairy Science, 2009, 92(4): 1712-1724. DOI:10.3168/jds.2008-1656 |
| [4] |
BERGMAN E N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species[J]. Physiological Reviews, 1990, 70(2): 567-590. DOI:10.1152/physrev.1990.70.2.567 |
| [5] |
GUILLOTEAU P, MARTIN L, EECKHAUT V, et al. From the gut to the peripheral tissues: the multiple effects of butyrate[J]. Nutrition Research Reviews, 2010, 23(2): 366-384. DOI:10.1017/S0954422410000247 |
| [6] |
冉舒文, 慕春龙, 朱伟云. 丁酸抑制溃疡性结肠炎分子机制的研究进展[J]. 世界华人消化杂志, 2018, 26(14): 856-861. RAN S W, MU C L, ZHU W Y. Mechanisms for butyrate to inhibit ulcerative colitis[J]. World Chinese Journal of Digestology, 2018, 26(14): 856-861 (in Chinese). |
| [7] |
姜茂成, 詹康, 贡笑笑, 等. 不同pH和SCFAs对奶牛瘤胃上皮细胞SCFAs转运蛋白和GPR41表达的影响[J]. 中国农业大学学报, 2018, 23(10): 63-70. JIANG M C, ZHAN K, GONG X X, et al. Effects of different pH and SCFAs on the expressions of SCFAs transport proteins and GPR41 in the rumen epithelial cells of dairy cow[J]. Journal of China Agricultural University, 2018, 23(10): 63-70 (in Chinese). DOI:10.11841/j.issn.1007-4333.2018.10.08 |
| [8] |
刘颖. 丁酸梭状芽孢杆菌补料发酵的优化[D]. 硕士学位论文. 武汉: 湖北工业大学, 2018. LIU Y. Fed-batch culture optimazations for Clostridium butyricum[D]. Master's Thesis. Wuhan: Hubei University of Technology, 2018. (in Chinese) |
| [9] |
PUCHALSKA P, CRAWFORD P A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics[J]. Cell Metabolism, 2017, 25(2): 262-284. |
| [10] |
ZHANG R Y, LIU J H, JIANG L S, et al. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows[J]. Animal Feed Science and Technology, 2020, 269: 114619. DOI:10.1016/j.anifeedsci.2020.114619 |
| [11] |
ASH R, BAIRD G D. Activation of volatile fatty acids in bovine liver and rumen epithelium.Evidence for control by autoregulation[J]. Biochemical Journal, 1973, 136(2): 311-319. DOI:10.1042/bj1360311 |
| [12] |
汪水平, 王文娟, 谭支良. 离体瘤胃上皮细胞在瘤胃代谢中的研究进展[J]. 家畜生态学报, 2006, 27(2): 1-4. WANG S P, WANG W J, TAN Z L. Advance of isolated ruminal epithelial cells in the study of rumen metabolism[J]. Journal of Domestic Animal Ecology, 2006, 27(2): 1-4 (in Chinese). DOI:10.3969/j.issn.1673-1182.2006.02.001 |
| [13] |
ASCHENBACH J R, ZEBELI Q, PATRA A K, et al. Symposium review: the importance of the ruminal epithelial barrier for a healthy and productive cow[J]. Journal of Dairy Science, 2019, 102(2): 1866-1882. DOI:10.3168/jds.2018-15243 |
| [14] |
STEELE M A, CROOM J, KAHLER M, et al. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis[J]. American Journal of Physiology.Regulatory, Integrative and Comparative Physiology, 2011, 300(6): R1515-R1523. DOI:10.1152/ajpregu.00120.2010 |
| [15] |
KLEVENHUSEN F, HOLLMANN M, PODSTATZKY-LICHTENSTEIN L, et al. Feeding barley grain-rich diets altered electrophysiological properties and permeability of the ruminal wall in a goat model[J]. Journal of Dairy Science, 2013, 96(4): 2293-2302. DOI:10.3168/jds.2012-6187 |
| [16] |
程萌. 亚急性瘤胃酸中毒对奶山羊瘤胃上皮通透性及细胞连接蛋白表达的影响[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2016. CHENG M. Effect of subacute ruminal acidosis on rumen epithelium permeability and intercellula junction protein expression in dairy goats[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2016. (in Chinese) |
| [17] |
刘军花. 亚急性瘤胃酸中毒对山羊瘤胃上皮屏障功能的影响及其机制[D]. 博士学位论文. 南京: 南京农业大学, 2014. LIU J H. The effect of subacute ruminal acidosis on ruminal epithelial barrier function and its underlying mechanism in goats[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2014. (in Chinese) |
| [18] |
MEMON M A, WANG Y, XU T L, et al. Lipopolysaccharide induces oxidative stress by triggering MAPK and Nrf2 signalling pathways in mammary glands of dairy cows fed a high-concentrate diet[J]. Microbial Pathogenesis, 2019, 128: 268-275. DOI:10.1016/j.micpath.2019.01.005 |
| [19] |
ZHANG H, PENG A L, ZHAO F F, et al. Thiamine ameliorates inflammation of the ruminal epithelium of Saanen goats suffering from subacute ruminal acidosis[J]. Journal of Dairy Science, 2020, 103(2): 1931-1943. DOI:10.3168/jds.2019-16944 |
| [20] |
MA Y, ZHANG Y, ZHANG H, et al. Thiamine alleviates high-concentrate-diet-induced oxidative stress, apoptosis, and protects the rumen epithelial barrier function in goats[J]. Frontiers in Veterinary Science, 2021, 8: 663698. DOI:10.3389/fvets.2021.663698 |
| [21] |
PLAIZIER J C, KRAUSE D O, GOZHO G N, et al. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences[J]. The Veterinary Journal, 2008, 176(1): 21-31. DOI:10.1016/j.tvjl.2007.12.016 |
| [22] |
王汉海, 隋美霞. 瘤胃异常代谢产物组胺影响反刍动物生产性能和瘤胃性能的研究现状[J]. 畜牧与兽医, 2016, 48(4): 126-128. WANG H H, SUI M X. Research status of rumen abnormal metabolite histamine affecting rumen performance and rumen performance of ruminants[J]. Animal Husbandry & Veterinary Medicine, 2016, 48(4): 126-128 (in Chinese). |
| [23] |
IQBAL S, ZEBELI Q, MAZZOLARI A, et al. Feeding rolled barley grain steeped in lactic acid modulated energy status and innate immunity in dairy cows[J]. Journal of Dairy Science, 2010, 93(11): 5147-5156. DOI:10.3168/jds.2010-3118 |
| [24] |
STEFANSKA B, CZŁAPA W, PRUSZYNSKA-OSZMAŁEK E, et al. Subacute ruminal acidosis affects fermentation and endotoxin concentration in the rumen and relative expression of the CD14/TLR4/MD2 genes involved in lipopolysaccharide systemic immune response in dairy cows[J]. Journal of Dairy Science, 2018, 101(2): 1297-1310. DOI:10.3168/jds.2017-12896 |
| [25] |
RODRÍGUEZ-LECOMPTE J C, KROEKER A D, CEBALLOS-MÁRQUEZ A, et al. Evaluation of the systemic innate immune response and metabolic alterations of nonlactating cows with diet-induced subacute ruminal acidosis[J]. Journal of Dairy Science, 2014, 97(12): 7777-7787. DOI:10.3168/jds.2014-8319 |
| [26] |
FAN W J, LI H P, ZHU H S, et al. NF-κB is involved in the LPS-mediated proliferation and apoptosis of MAC-T epithelial cells as part of the subacute ruminal acidosis response in cows[J]. Biotechnology Letters, 2016, 38(11): 1839-1849. DOI:10.1007/s10529-016-2178-0 |
| [27] |
SHI X X, LI D D, DENG Q H, et al. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2015, 145: 103-112. DOI:10.1016/j.jsbmb.2014.10.014 |
| [28] |
PARK J, MIN J S, KIM B, et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways[J]. Neuroscience Letters, 2015, 584: 191-196. DOI:10.1016/j.neulet.2014.10.016 |
| [29] |
赵娜, 方慧, 唐亚平, 等. MAPK信号转导通路与慢性牙周炎的相关研究进展[J]. 口腔医学研究, 2017, 33(9): 1012-1015. ZHAO N, FANG H, TANG Y P, et al. Advancement on correction of MAPK signal pathway and chronic periodontitis[J]. Journal of Oral Science Research, 2017, 33(9): 1012-1015 (in Chinese). |
| [30] |
冯小倩, 武曦, 谭颖徽. 组胺及组胺受体的研究进展[J]. 中华肺部疾病杂志(电子版), 2015, 8(2): 88-91. FENG X Q, WU X, TAN Y H. Research progress of histamine and histamine receptor[J]. Chinese Journal of Lung Disease(Electronic Edition), 2015, 8(2): 88-91 (in Chinese). DOI:10.3877/cma.j.issn.1674-6902.2015.02.023 |
| [31] |
邴新帅. 丁酸钠与沙蒿多糖对断奶羔羊肠道发育及胰高血糖素样肽-2的影响[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2018. BING X S. Effect of sodium butyrate and Artemisia seed polysaccharide on intestinal development and glucagon-like peptide-2 of weaning lambs[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2018. (in Chinese) |
| [32] |
ZHANG K, MENG M J, GAO L P, et al. Sodium butyrate improves high-concentrate-diet-induced impairment of ruminal epithelium barrier function in goats[J]. Journal of Agricultural and Food Chemistry, 2018, 66(33): 8729-8736. DOI:10.1021/acs.jafc.8b03108 |
| [33] |
KOCH C, GERBERT C, FRIETEN D, et al. Effects of ad libitum milk replacer feeding and butyrate supplementation on the epithelial growth and development of the gastrointestinal tract in Holstein calves[J]. Journal of Dairy Science, 2019, 102(9): 8513-8526. DOI:10.3168/jds.2019-16328 |
| [34] |
MALHI M, GUI H B, YAO L, et al. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion[J]. Journal of Dairy Science, 2013, 96(12): 7603-7616. DOI:10.3168/jds.2013-6700 |
| [35] |
SOOMRO J, LU Z Y, GUI H B, et al. Synchronous and time-dependent expression of cyclins, cyclin-dependant kinases, and apoptotic genes in the rumen epithelia of butyrate-infused goats[J]. Frontiers in Physiology, 2018, 9: 496. DOI:10.3389/fphys.2018.00496 |
| [36] |
MATHEW O P, RANGANNA K, YATSU F M. Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells[J]. Biomedicine & Pharmacotherapy, 2010, 64(10): 733-740. |
| [37] |
SHERR C J, ROBERTS J M. Living with or without cyclins and cyclin-dependent kinases[J]. Genes & Development, 2004, 18(22): 2699-2711. |
| [38] |
OGRYZKO V V, WONG P, HOWARD B H. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases[J]. Molecular and Cellular Biology, 1997, 17(8): 4877-4882. DOI:10.1128/MCB.17.8.4877 |
| [39] |
KOWALSKI Z M, GÓRKA P, FLAGA J, et al. Effect of microencapsulated sodium butyrate in the close-up diet on performance of dairy cows in the early lactation period[J]. Journal of Dairy Science, 2015, 98(5): 3284-3291. DOI:10.3168/jds.2014-8688 |
| [40] |
FUKUMORI R, OBA M, IZUMI K, et al. Effects of butyrate supplementation on blood glucagon-like peptide-2 concentration and gastrointestinal functions of lactating dairy cows fed diets differing in starch content[J]. Journal of Dairy Science, 2020, 103(4): 3656-3667. DOI:10.3168/jds.2019-17677 |
| [41] |
CANANI R B, COSTANZO M D, LEONE L, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases[J]. World Journal of Gastroenterology, 2011, 17(12): 1519-1528. DOI:10.3748/wjg.v17.i12.1519 |
| [42] |
AGARWAL U, HU Q, BALDWIN R L, et al. Role of rumen butyrate in regulation of nitrogen utilization and urea nitrogen kinetics in growing sheep[J]. Journal of Animal Science, 2015, 93(5): 2382-2390. DOI:10.2527/jas.2014-8738 |
| [43] |
MA N N, AHAMED A J, BILAL M S, et al. Sodium butyrate improves antioxidant stability in sub-acute ruminal acidosis in dairy goats[J]. BMC Veterinary Research, 2018, 14(1): 275-288. |
| [44] |
AABDIN Z U, BILAL M S, DAI H Y, et al. NOD1/NF-κB signaling pathway inhibited by sodium butyrate in the mammary gland of lactating goats during sub-acute ruminal acidosis[J]. Microbial Pathogenesis, 2018, 122: 58-62. |
| [45] |
CHANG G J, YAN J Y, MA N N, et al. Dietary sodium butyrate supplementation reduces high-concentrate diet feeding-induced apoptosis in mammary cells in dairy goats[J]. Journal of Agricultural and Food Chemistry, 2018, 66(9): 2101-2107. |
| [46] |
SHEN H, LU Z Y, XU Z H, et al. Associations among dietary non-fiber carbohydrate, ruminal microbiota and epithelium G-protein-coupled receptor, and histone deacetylase regulations in goats[J]. Microbiome, 2017, 5(1): 123-135. |
| [47] |
CHANG G J, MA N N, ZHANG H M, et al. Sodium butyrate modulates mucosal inflammation injury mediated by GPR41/43 in the cecum of goats fed a high concentration diet[J]. Frontiers in Physiology, 2019, 10: 1130-1142. |
| [48] |
WANG Y, LIU J, HUANG J, et al. Sodium butyrate attenuated iE-DAP induced inflammatory response in the mammary glands of dairy goats fed high-concentrate diet[J]. Journal of the Science of Food and Agriculture, 2021, 101(3): 1218-1227. |
| [49] |
DAI H Y, LIU X X, YAN J Y, et al. Sodium butyrate ameliorates high-concentrate diet-induced inflammation in the rumen epithelium of dairy goats[J]. Journal of Agricultural and Food Chemistry, 2017, 65(3): 596-604. |
| [50] |
CHANG G J, LIU X X, MA N N, et al. Dietary addition of sodium butyrate contributes to attenuated feeding-induced hepatocyte apoptosis in dairy goats[J]. Journal of Agricultural and Food Chemistry, 2018, 66(38): 9995-10002. |
| [51] |
SETO E, YOSHIDA M. Erasers of histone acetylation: the histone deacetylase enzymes[J]. Cold Spring Harbor Perspectives in Biology, 2014, 6(4): a018713. |
| [52] |
GARCIA-RAMIREZ M, ROCCHINI C, AUSIO J. Modulation of chromatin folding by histone acetylation[J]. Journal of Biological Chemistry, 1995, 270(30): 17923-17928. |
| [53] |
SUBRAMANIAN V S, TEAFATILLER T, MORADI H, et al. Histone deacetylase inhibitors regulate vitamin C transporter functional expression in intestinal epithelial cells[J]. Journal of Nutritional Biochemistry, 2021, 98: 108838. |
| [54] |
KASUBUCHI M, HASEGAWA S, HIRAMATSU T, et al. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation[J]. Nutrients, 2015, 7(4): 2839-2849. |
| [55] |
SUN X D, LUO S B, JIANG C H, et al. Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-κB signaling[J]. Journal of Dairy Science, 2020, 103(9): 8388-8397. |
| [56] |
CHANDRA ROY A, WANG Y, ZHANG H M, et al. Sodium butyrate mitigates iE-DAP induced inflammation caused by high-concentrate feeding in liver of dairy goats[J]. Journal of Agricultural and Food Chemistry, 2018, 66(34): 8999-9009. |




