2. 广西大学动物科学技术学院, 南宁 530004
2. College of Animal Science and Technology, Guangxi University, Nanning 530004, China
细胞外基质(extracellular matrix, ECM)广泛存在于动物全身组织中,是一种由细胞分泌组装的复杂三维矩阵网络。ECM网络主要由胶原蛋白(collagen)、弹性蛋白、蛋白聚糖/糖胺聚糖、纤连蛋白、层黏连蛋白和其他几种糖蛋白组成,其中胶原蛋白是ECM网络的结构基础和主要组成成分,约占脊椎动物总蛋白的1/3[1]。透明质酸是多糖中最重要的一部分,其合成与降解可调节ECM的化学和物理特性[2]。ECM组件分子与细胞黏附受体相互结合,形成一个复杂的网络,所有组织和器官中的细胞都位于该网络中。嵌入ECM网络的细胞通过整合素、蛋白聚糖和透明质酸受体等与ECM互作来发挥调节作用。以往较多关注了ECM网络的信号传递对细胞增殖、迁移、生长、分化和维持动物机体正常稳态的重要作用[3]。然而,ECM网络对营养物质的生理屏障功能及调控作用关注较少。近年报道,ECM网络是一种高度动态的结构网络,在正常和病理条件下持续由几种基质降解酶介导降解和重塑[4]。此外,胶原蛋白等ECM分子存在昼夜节律和胞内胞外等动态调控机制[5-6],ECM的过度沉积造成炎症性肠病从而导致肠道纤维化[7]。研究表明,罗伊氏乳杆菌LR1促进断奶仔猪肠-肝轴氨基酸代谢和提高生长性能与肠黏膜中胶原蛋白分子[Ⅰ型胶原蛋白α2链(COL1A2)、Ⅳ型胶原蛋白α1链(COL4A1)和Ⅵ型胶原蛋白α2链(COL6A2)等]的表达量的下降有关,且肠黏膜的差异蛋白质主要富集在细胞外基质-受体互作(ECM-receptor interaction)等通路[8-9]。肌肉、肝脏和脂肪组织中ECM沉积和重塑的增加会导致胰岛素和葡萄糖转运的物理障碍增加[10]。由此可见,ECM网络可能是动物营养物质转运代谢的重要调控靶点。
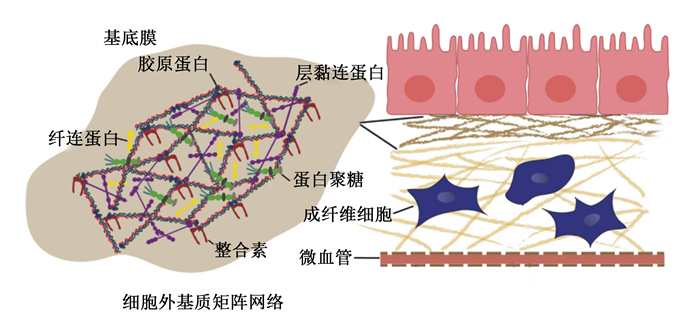
1 ECM网络的生理屏障功能及动态变化 1.1 ECM网络的生理屏障功能ECM组件分子与细胞黏附受体相互结合,形成一个复杂的网络,所有组织和器官中的细胞都位于该网络中。ECM的物理性质包括基质刚度、孔隙率、不溶性、空间排布和取向(拓扑结构)等,这些特性共同维持着组织结构的完整性,并能直接影响细胞的生物学行为[11]。ECM的物理特性取决于胶原蛋白、弹性蛋白、糖胺聚糖和相关蛋白聚糖[12]。胶原蛋白网络结构是由特征性重复序列(Gly-X-Y)n(X常为脯氨酸,Y常为羟脯氨酸)构成的三聚体纤维束,其是ECM网络结构稳定和力学性质的基础[13]。此外,不同组织中的ECM也有所差异。在健康的动物肝脏中,ECM的含量与其大小相关,约占总体积的10%,其中主要的ECM蛋白是胶原蛋白,且丰富的Ⅰ、Ⅲ、Ⅳ型胶原蛋白局限分布于门静脉、窦壁和中央静脉,并且Ⅳ型胶原蛋白与层黏连蛋白和巢蛋白参与基底膜的形成[14-15]。肠黏膜中围绕微血管和基底层含有丰富的ECM,且ECM的各种成分由不同类型的肠细胞组成。基底膜的成分由上皮细胞产生,而间质基质的成分主要由以成纤维细胞、肌成纤维细胞和平滑肌细胞为代表的间充质细胞产生。肠黏膜中ECM主要以Ⅰ、Ⅲ、Ⅳ、Ⅵ型胶原蛋白为主,且小肠黏膜下层有种天然的ECM,可广泛应用于组织工程中的组织修复和再生[16-18]。脊椎动物骨骼肌的ECM则由几个形态不同的层组成:肌内膜、肌周膜和肌外膜,分别围绕肌肉纤维束和整个肌肉;多层ECM是脊椎动物肌肉的一个共同特征[19]。血管壁中除了主动脉中鉴定的103种不同的ECM蛋白外还含有非结构性基质细胞蛋白,其中Ⅰ、Ⅲ型胶原蛋白是血管壁中含量最丰富的胶原蛋白,主要有助于调节血管稳态和重塑[20-21]。对于乳腺上皮来说,最重要的则是层黏连蛋白[22]。正常情况下,ECM网络孔径大小为60~100 nm,这对直径较大的营养物质能直接有效阻断其转运。研究表明,纤维状胶原蛋白囊和基底膜共同阻碍着癌细胞的侵袭[23]。研究发现,胶原溶液的浓度越大,制成的胶原蛋白纤维密度越大,网格孔径越小[24]。因此,ECM网络是氨基酸等营养物质逐级转运至门静脉并汇集到肝脏中进行代谢的生理屏障。ECM网络的结构和生理屏障功能如图 1所示。
|
图 1 ECM的网络结构和生理屏障功能 Fig. 1 Network structure and physiological barrier function of ECM[1] |
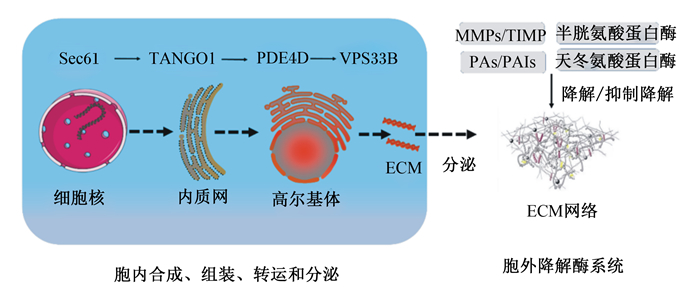
ECM网络是一种稳定的支撑结构,在细胞微环境中的发挥重要作用。近年来研究发现,ECM网络在动物生命过程中不断被更新,存在一种动态变化机制。ECM分子在成纤维细胞、上皮细胞和免疫细胞等受到多种信号的控制下合成和分泌,进而参与构成ECM网络[1]。成纤维细胞是大多数组织(肠道、肝脏等)的合成和分泌细胞,同时也能分泌基质蛋白酶家族等蛋白酶[12],其在运动后的机械刺激下会合成胶原蛋白[25]。在胞内,胶原蛋白的合成、组装、转运和分泌受到一些关键基因的调控。内质网通道蛋白Sec61负责调控胶原蛋白分子的基因表达,运输和高尔基体组织1(the transport and golgi organization 1, TANGO1)负责内质网中蛋白分子的加工组装,磷酸二酯酶4D(phosphodiesterase 4D, PDE4D)将蛋白分子转运至高尔基体,而囊泡分选蛋白33同源物B(vacuolar protein sorting 33 homolog B, VPS33B)对胶原翻译后进行修饰[26]。研究表明,Sec23同源物A(SEC23A)基因突变会导致内质网中的胶原蛋白积累[27],敲除Sec24同源物D(SEC24D)会影响ECM网络中Ⅱ型胶原蛋白的分泌[28]。另外,在培养的肾成纤维细胞(NRK-49F)中敲除羟甲基戊二酰辅酶A还原酶降解蛋白1基因(HMG-CoA reductase degradation protein 1, Hrd1)可减少约60%的Ⅰ型胶原蛋白分泌,而过度表达则会导致Ⅰ型胶原蛋白分泌量增加1.5倍[29]。此外,丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)可刺激核转录因子与纤连蛋白的启动子区域结合,引起纤连蛋白表达升高[30]。
在胞外,ECM的降解重塑受到基质金属蛋白酶(MMPs)/基质金属蛋白酶组织抑制物(TIMPs)、纤溶酶原激活物(PAs)/纤溶酶原激活物抑制物(PAIs)、半胱氨酸蛋白酶和天冬氨酸蛋白酶等的调控。MMPs/TIMPs和PAs/PAIs是调控ECM网络动态变化的主要酶系。MMP-2和MMP-9在胶原原纤维上可随机横向扩散,并在3/4和1/4位点内展开和裂解胶原蛋白[31]。TIMPs是MMPs的抑制剂,PAs主要参与ECM网络的降解和重塑,PAIs则通过抑制PAs而降低MMPs和纤维蛋白溶解酶活性,减少ECM网络蛋白的降解。另外,一些细胞因子和营养素对ECM网络也具有调控作用。研究表明,转化生长因子-β1(TGF-β1)持续过表达通过调节MMPs/TIMPs平衡增加肠黏膜ECM网络的合成[32]。白细胞介素-34(IL-34)、白细胞介素-36R(IL-36R)等可激活p38 MAPK等通路增强成纤维细胞胶原蛋白Ⅰ型胶原蛋白α1链(COL1A1)和Ⅲ型胶原蛋白α1链(COL3A1)的分泌[33-34]。白蔾芦醇和维生素D3等营养素可以通过降低炎症因子[白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)],通过过表达沉默信息调节因子1(silence information regulator 1, SIRT1)、胰岛素样生长因子-1(IGF-1)等通路调控ECM网络的动态变化[35-36]。一些激动剂的研究发现,核激素受体——核受体亚家族1D组成员1(NR1D1)激动剂SR9009[37]或隐花色素1/2(CRY1/2)激动剂KL001[38]处理成纤维细胞可以减少胶原纤维的数量。哺乳动物雷帕霉素靶蛋白(mTOR)和瘦素可激活肌成纤维细胞导致ECM异常沉积,并由纤维化发展;此外成纤维细胞生长因子21(FGF21)可以通过SIRT1-mTOR信号通路抑制ECM分解代谢且成纤维细胞分泌的营养因子可以通过上调MMPs从而有助于ECM重塑来维持骨组织的稳态[39-41]。综上所述,ECM网络动态变化主要依赖于细胞内关键基因与胞外降解酶系统调控作用,同时肠黏膜免疫微环境和外源营养物质也能调控ECM网络的动态变化(图 2)。
|
ECM:细胞外基质extracellular matrix;TANGO1:运输和高尔基体组织1 the transport and golgi organization 1;PDE4D:磷酸二酯酶4D phosphodiesterase 4D;VPS33B:囊泡分选蛋白33同源物B vacuolar protein sorting 33 homolog B;MMPs:基质金属蛋白酶matrix metalloproteinases;TIMPs:基质金属蛋白酶组织抑制物tissue inhibitors of matrix metalloproteinase;PAs:纤溶酶原激活物plasminogen activators;PAIs:纤溶酶原激活物抑制物plasminogen activator inhibitors。 图 2 ECM网络的动态变化 Fig. 2 Dynamic changes of ECM network |
动物生长发育主要依赖于蛋白质沉积,而蛋白质沉积效率很大程度上取决于氨基酸的转运代谢。肠腔中氨基酸经肠黏膜上层(肠上皮细胞)吸收后,经肠黏膜逐级转运至微血管并通过门静脉循环到达肝脏,再分配到机体各组织[42]。研究表明,30%~60%的必需氨基酸被门静脉回流组织(胃、小肠、结肠、胰脏和脾脏等实体组织集合的总称)截取,其中60%的苏氨酸、39%的蛋氨酸、35%的苯丙氨酸和35%的赖氨酸在仔猪肠黏膜中被截留[43-44]。肠黏膜截留的氨基酸部分用于合成分泌蛋白、其他氨基酸以及谷胱甘肽等活性物质并参与动物免疫调控。然而,过剩的氨基酸在肠黏膜被当作代谢燃料氧化分解,导致门静脉血氨升高和肝脏尿素合成增加,造成氮排放升高和营养资源浪费[45]。当仔猪肠道黏膜感染病原体或者发生炎症时,肠成纤维细胞胶原蛋白的分泌增加,血液中丝氨酸、甘氨酸、精氨酸和天冬酰胺等氨基酸含量下降[46]。研究表明,纤维状的ECM通常增加了大多数氨基酸的丰度,当ECM网络结合整合素β1作用时,可机械地调节肝细胞中的氨基酸水平[47]。此外,ECM与细胞的互作可以调节膜蛋白内参——钠钾ATP酶(Na+/K+-ATPase)和谷氨酸转运蛋白-1(GLT-1)和天冬氨酸转运蛋白(GLAST)的表达,从而调节谷氨酸转运[48]。此外,ECM网络刚度的增加会通过激活谷氨酰胺代谢调节非必需氨基酸通量来重新编程氨基酸代谢[49]。ECM网络刚度升高会引起大分子细胞外囊泡的运输波动,易于其携带氨基酸通过ECM网格孔径[50-51]。细胞外基质与氨基酸等营养物质会形成氢键阻碍其转运,极性高、酸度系数(pKa)值低的氨基酸分子不易通过人工细胞外基质[52]。综上所述,ECM网络的动态变化影响氨基酸的截留,从而导致氨基酸进入门静脉运输至肝脏的过程受阻,影响其转运代谢。
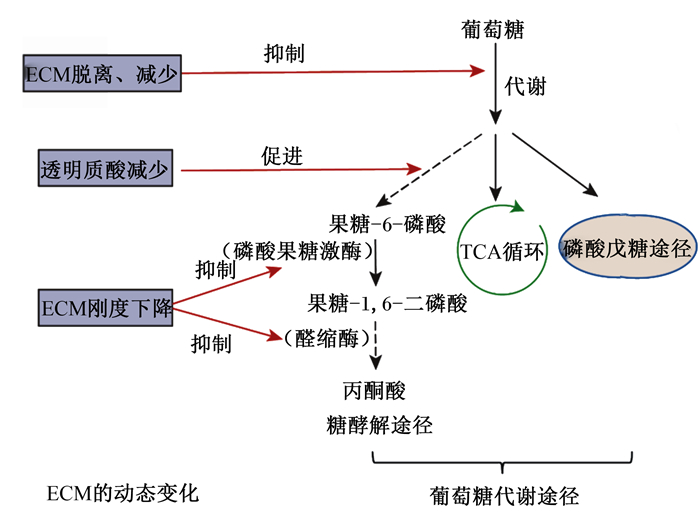
3 ECM网络对碳水化合物代谢的调控作用碳水化合物是动物机体内提供能量的主要来源,其中葡萄糖是有效供给动物代谢活动快速应变所需能量的营养素。碳水化合物在小肠内消化成单糖(主要是葡萄糖)后,经无氧酵解和有氧条件下经三羧酸循环(TCA)彻底氧化供能。研究发现,ECM网络的透明质酸介导的运动因子受体(hyaluronan-mediated motility receptor, HMMR)与葡萄糖高速代谢紧密相关[53]。透明质酸的减少可通过激活锌指蛋白36-硫氧还蛋白互作蛋白-葡萄糖转运蛋白1(ZFP36-TXNIP-GLUT1)信号路径大幅促进糖酵解反应[53]。当ECM网络脱离细胞后,细胞外环境营养吸收能力受损,摄取葡萄糖的能力丧失且通过磷酸戊糖途径的通量不足从而导致代谢途径发生改变[54]。乳腺上皮细胞的ECM网络脱离会降低磷脂酰肌醇3激酶/蛋白激酶B(PI3K/Akt)信号通路的活性,且通过糖酵解、磷酸戊糖途径和TCA的流量减少,从而抑制糖酵解反应,导致葡萄糖和谷氨酰胺的摄取减少[55]。当Ⅵ型胶原蛋白缺失时,肥胖小鼠的体重下降且对胰岛素的敏感性增强[56]。小鼠机体缺乏特定的ⅩⅧ型胶原蛋白时,小鼠对葡萄糖耐受不良、胰岛素敏感性受损,导致葡萄糖代谢异常[57-58]。此外胶原蛋白脯氨酰4-羟化酶α-2亚基(P4HA2)的表达与参与糖酵解的基因[磷酸甘油酸激酶1(PGK1)和乳酸脱氢酶A(LDHA)等]表达特征呈正相关。研究发现,敲除P4HA2后细胞中葡萄糖的摄取和乳酸的产生减少,但P4HA2可以通过PGK1和LDHA促进宫颈癌细胞糖酵解,表明了P4HA2影响宫颈癌细胞的糖酵解[59]。相反地,胶原蛋白沉积过多时,乳腺癌细胞通过TCA的氧消耗含量和葡萄糖含量都大幅度降低,但是糖酵解中间体和乳酸的产生却没有差异[60]。
ECM网络通过调节糖酵解相关酶的表达来改变糖酵解反应。研究发现,僵硬的ECM导致细胞张力增加,促进高度成束的肌动蛋白应力纤维的形成,这些纤维会捕获E3泛素连接酶(TRIM21),使它们失去活性并增加糖酵解的速度[61]。当ECM网络刚度逐渐从硬转换为软时,通过限速糖酵解过程中的3个关键酶之一的磷酸果糖激酶(PFK)的蛋白酶体降解从而下调糖酵解反应[62],同时软ECM网络可以抑制醛缩酶与F-肌动蛋白结合,从而导致糖酵解减少[63]。此外,ECM网络的刚度可以激活转录共激活因子相关蛋白,以此促进肿瘤细胞中的葡萄糖摄取和糖酵解速率[64]。以上研究表明,ECM网络能够通过调节糖酵解反应的信号通路和糖酵解相关酶来影响葡萄糖代谢,其中胶原蛋白的动态变化可以直接调控糖酵解反应从而改善机体代谢。ECM网络的动态变化对葡萄糖代谢的影响如图 3所示。
|
图 3 ECM网络的动态变化对葡萄糖代谢的影响 Fig. 3 Effects of dynamic changes of ECM network on glucose metabolism |
脂质在动物体内的主要作用是氧化供能,维持脂质代谢稳态对机体的健康尤其重要。脂质在小肠内消化吸收,由淋巴系统进入血循环,经肝脏转化后储存于脂肪组织中,需要时被组织利用。研究发现,中性脂质的合成被认为是对ECM黏附传递的机械信号的一般反应。ECM的信号可以影响高尔基体的机械特性从而调节脂素基因和胆固醇调节元件结合蛋白(SREBP)驱动脂质的合成和积累进而调控脂质代谢[65]。此外,Ⅰ和Ⅲ型胶原蛋白为小鼠胚胎前脂肪细胞(3T3-L1)的生长和迁移提供了良好的基质,通过刺激转录共激活因子相关蛋白的核易位来增强3T3-L1细胞迁移[66-67]。肥胖小鼠中的脂肪组织和成熟脂肪细胞中的ⅩⅤ型胶原蛋白高度增加,进一步加速了肥胖小鼠的脂质沉积。同时,ⅩⅤ型胶原蛋白可通过负环磷腺苷效应元件结合蛋白(CREB)转录、抑制DNA甲基化和环腺苷3′,5′-单磷酸/激酶A(cAMP/PKA)信号通路促进脂肪细胞分化并减弱脂肪细胞中的脂肪分解[68]。这些研究提示,ECM网络的动态平衡对脂肪细胞的结构完整性和脂肪细胞的迁移分化至关重要。
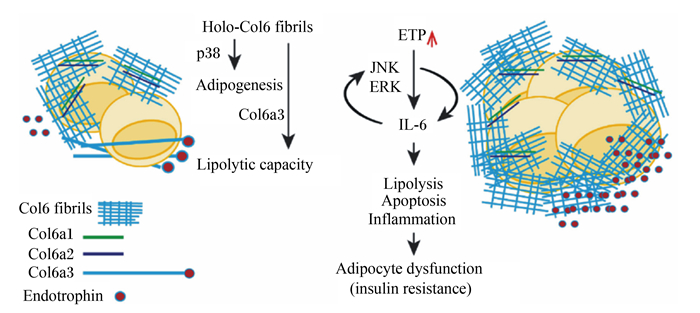
然而,ECM网络失衡会导致一系列脂质代谢障碍。当Ⅵ型胶原蛋白动态沉积较少时,脂肪生成基因过氧化物酶体增殖物激活受体γ(PPARγ)和脂肪酸结合蛋白4(FABP4)的mRNA表达显著增加,从而增强脂肪细胞的功能和提高甘油三酯含量[69]。同时,敲除小鼠Ⅵ型胶原蛋白基因诱导脂肪生成和脂肪分解缺陷,导致小鼠肠系膜脂肪组织中mRNA表达的减少和脂肪酸消耗率增加[56, 70],此外,Ⅵ型胶原蛋白能与其裂解产物内营养因子协同调节脂肪细胞的脂肪生成和脂肪分解能力,具体如图 4所示。当小鼠体内缺乏ⅩⅤⅢ型胶原蛋白时会导致肝脏异位脂质积累,并提高极低密度脂蛋白和甘油三酯含量。因此ⅩⅤⅢ型胶原蛋白被确定为ECM的导向机制,其可能有助于控制多步骤脂肪生成程序[58]。透明质酸的降低也会抑制体外和体内的脂肪生成,同时抑制脂滴的形成和甘油三酯的积累[71]。层黏连蛋白α4的减少会导致脂肪细胞脂肪生成受损[72]。纤连蛋白是前脂肪细胞分化的负调控因子,纤连蛋白降解时会促进猪前脂肪细胞分化[73]。由此可见,ECM网络分子的减少或缺失会降低脂质代谢,减少脂肪的生成。此外,研究发现胶原蛋白过度沉积会导致脂肪组织纤维化,进而抑制脂质代谢[74-75]。在小鼠模型中,ECM网络的异常积累使脂肪组织纤维化发展从而导致脂质代谢紊乱[76]。综上所述,ECM网络会影响脂肪细胞生成,其动态变化会影响脂质代谢,揭示了ECM网络是调控脂质代谢的幕后关键。
|
Holo-Col6 fibrils:全息图-Ⅵ型胶原蛋白原纤维hologram-type Ⅵ collagen fibrils;p38:p38丝裂原激活蛋白激酶p38 mitogen-activated protein kinases;Adipogenesis:脂肪生成;Lipolytic capacity:脂肪分解的能力;ETP:内营养因子endotrophin;JNK:c-Jun氨基末端激酶c-Jun N-terminal kinases;ERK:胞外信号调节激酶extracellular signal-regulated kinases;IL-6:白细胞介素-6 interleukin-6;Lipolysis:脂肪分解;Apoptosis:细胞凋亡;Inflammation:炎症;Adipocyte dysfunction (insulin resistance):脂肪细胞功能障碍(胰岛素抵抗);Col6 fibrils:Ⅵ型胶原蛋白原纤维type Ⅵ collagen fibrils;Col6a1:Ⅵ型胶原蛋白α1链type Ⅵ collagen alpha 1 chain;Col6a2:Ⅵ型胶原蛋白α2链type Ⅵ collagen alpha 2 chain;Col6a3:Ⅵ型胶原蛋白α3链type Ⅵ collagen alpha 3 chain;Endotrophin:内营养因子。 图 4 Ⅵ型胶原蛋白对脂肪细胞和脂肪分解能力的影响 Fig. 4 Effects of type Ⅵ collagen on adipocytes and lipolysis ability[70] |
综上所述,ECM具有独特的网络的结构和物理特性,是动物营养物质逐级转运代谢的重要生理屏障。ECM网络存在一种动态变化机制,Sec61、TANGO1、PDE4D和VPS33B是调控ECM网络分子胞内合成、组装、转运和分泌的关键基因;胞外的降解重塑主要与MMPs/TIMPs和PAs/PAIs降解酶系统有关。ECM网络对于营养物质的代谢意义重大,ECM网络的动态变化参与调控营养物质的转运代谢,例如氨基酸进入门静脉运输至肝脏的过程受ECM网络动态变化影响,从而导致氨基酸截留进而影响转运代谢。然而,在畜禽生理上有关ECM网络动态变化调控营养物质转运代谢及其机制的研究非常缺乏,其重要的营养调控功能还未得到重视。鉴于ECM网络的重要生理屏障和营养调控功能,后续研究应重点关注以下几个方面:1)对不同的动物,ECM网络动态变化调控营养物质转运代谢的平衡点;2)ECM网络动态变化对营养物质转运的调控作用机理。通过针对性地深入研究,以期为营养调控ECM网络动态变化、提高动物营养物质转运代谢提供新方向。
| [1] |
THEOCHARIS A D, SKANDALIS S S, GIALELI C, et al. Extracellular matrix structure[J]. Advanced Drug Delivery Reviews, 2016, 97: 4-27. |
| [2] |
AMORIM S, REIS C A, REIS R L, et al. Extracellular matrix mimics using hyaluronan-based biomaterials[J]. Trends in Biotechnology, 2021, 39(1): 90-104. DOI:10.1016/j.tibtech.2020.06.003 |
| [3] |
ENGIN A B, NIKITOVIC D, NEAGU M, et al. Mechanistic understanding of nanoparticles' interactions with extracellular matrix: the cell and immune system[J]. Particle and Fibre Toxicology, 2017, 14(1): 22. DOI:10.1186/s12989-017-0199-z |
| [4] |
KJAER M, LANGBERG H, HEINEMEIER K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon[J]. Scandinavian Journal of Medicine & Science in Sports, 2009, 19(4): 500-510. |
| [5] |
BURRIS T P. Clock regulation of protein secretion[J]. Nature Cell Biology, 2020, 22(1): 1-3. DOI:10.1038/s41556-019-0449-4 |
| [6] |
唐青松, 徐娥, 王丽, 等. 胶原蛋白动态平衡及其对动物肠道健康的作用[J]. 动物营养学报, 2020, 32(12): 5578-5586. TANG Q S, XU E, WANG L, et al. Collagen homeostasis and its effects on intestinal health of animal[J]. Chinese Journal of Animal Nutrition, 2020, 32(12): 5578-5586 (in Chinese). |
| [7] |
LIU B, YANG M Q, YU T Y, et al. Mast cell tryptase promotes inflammatory bowel disease-induced intestinal fibrosis[J]. Inflammatory Bowel Diseases, 2021, 27(2): 242-255. DOI:10.1093/ibd/izaa125 |
| [8] |
YI H B, WANG L, XIONG Y X, et al. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs[J]. Journal of Animal Science, 2018, 96(6): 2342-2351. DOI:10.1093/jas/sky129 |
| [9] |
YI H B, YANG G D, XIONG Y X, et al. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut-liver axis of weaned pigs[J]. Food & Function, 2019, 10(11): 7387-7396. |
| [10] |
WILLIAMS A S, KANG L, WASSERMAN D H. The extracellular matrix and insulin resistance[J]. Trends in Endocrinology and Metabolism, 2015, 26(7): 357-366. DOI:10.1016/j.tem.2015.05.006 |
| [11] |
李艺. 基质刚度在女性生殖系统和乳腺肿瘤中的研究进展[J]. 现代妇产科进展, 2018, 27(12): 954-956, 958. LI Y. Research progress of matrix stiffness in female reproductive system and breast tumors[J]. Progress in Obstetrics and Gynecology, 2018, 27(12): 954-956, 958 (in Chinese). |
| [12] |
HUMPHREY J D, DUFRESNE E R, SCHWARTZ M A. Mechanotransduction and extracellular matrix homeostasis[J]. Nature Reviews.Molecular Cell Biology, 2014, 15(12): 802-812. DOI:10.1038/nrm3896 |
| [13] |
SORUSHANOVA A, DELGADO L M, WU Z N, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development[J]. Advanced Materials, 2019, 31(1): e1801651. |
| [14] |
BEDOSSA P, PARADIS V. Liver extracellular matrix in health and disease[J]. The Journal of Pathology, 2003, 200(4): 504-515. DOI:10.1002/path.1397 |
| [15] |
NYSTRÖM H. Extracellular matrix proteins in metastases to the liver-composition, function and potential applications[J]. Seminars in Cancer Biology, 2021, 71: 134-142. DOI:10.1016/j.semcancer.2020.06.004 |
| [16] |
ITO S, NAGATA K. Biology of Hsp47(Serpin H1), a collagen-specific molecular chaperone[J]. Seminars in Cell & Developmental Biology, 2017, 62: 142-151. |
| [17] |
JI Y H, ZHOU J G, SUN T F, et al. Diverse preparation methods for small intestinal submucosa (SIS): decellularization, components, and structure[J]. Journal of Biomedical Materials Research.Part A, 2019, 107(3): 689-697. |
| [18] |
POMPILI S, LATELLA G, GAUDIO E, et al. The charming world of the extracellular matrix: a dynamic and protective network of the intestinal wall[J]. Frontiers in Medicine, 2021, 8: 610189. DOI:10.3389/fmed.2021.610189 |
| [19] |
SLEBODA D A, STOVER K K, ROBERTS T J. Diversity of extracellular matrix morphology in vertebrate skeletal muscle[J]. Journal of Morphology, 2020, 281(2): 160-169. DOI:10.1002/jmor.21088 |
| [20] |
CAI Z Y, GONG Z, LI Z Q, et al. Vascular extracellular matrix remodeling and hypertension[J]. Antioxidants & Redox Signaling, 2021, 34(10): 765-783. |
| [21] |
MA Z H, MAO C F, JIA Y T, et al. Extracellular matrix dynamics in vascular remodeling[J]. American Journal of Physiology.Cell Physiology, 2020, 319(3): C481-C499. DOI:10.1152/ajpcell.00147.2020 |
| [22] |
SHIRAI K, HAGIWARA N, HORIGOME T, et al. Extracellularly extruded syntaxin-4 binds to laminin and syndecan-1 to regulate mammary epithelial morphogenesis[J]. Journal of Cellular Biochemistry, 2017, 118(4): 686-698. DOI:10.1002/jcb.25661 |
| [23] |
EBLE J A, NILAND S. The extracellular matrix in tumor progression and metastasis[J]. Clinical & Experimental Metastasis, 2019, 36(3): 171-198. |
| [24] |
MICKEL W, MVNSTER S, JAWERTH L M, et al. Robust pore size analysis of filamentous networks from three-dimensional confocal microscopy[J]. Biophysical Journal, 2008, 95(12): 6072-6080. DOI:10.1529/biophysj.108.135939 |
| [25] |
DIDERIKSEN K, SINDBY A K R, KROGSGAARD M, et al. Effect of acute exercise on patella tendon protein synthesis and gene expression[J]. SpringerPlus, 2013, 2(1): 109. DOI:10.1186/2193-1801-2-109 |
| [26] |
CHANG J, GARVA R, PICKARD A, et al. Circadian control of the secretory pathway maintains collagen homeostasis[J]. Nature Cell Biology, 2020, 22(1): 74-86. DOI:10.1038/s41556-019-0441-z |
| [27] |
BOYADJIEV S A, KIM S D, HATA A, et al. Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion[J]. Clinical Genetics, 2011, 80(2): 169-176. DOI:10.1111/j.1399-0004.2010.01550.x |
| [28] |
SARMAH S, BARRALLO-GIMENO A, MELVILLE D B, et al. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis[J]. PLoS One, 2010, 5(4): e10367. DOI:10.1371/journal.pone.0010367 |
| [29] |
MALHOTRA V, ERLMANN P. The pathway of collagen secretion[J]. Annual Review of Cell and Developmental Biology, 2015, 31: 109-124. DOI:10.1146/annurev-cellbio-100913-013002 |
| [30] |
李亚楠, 李京宝, 商澎, 等. 调控纤连蛋白表达的信号通路[J]. 中国细胞生物学学报, 2013, 35(1): 98-103. LI Y N, LI J B, SHANG P, et al. The signal pathways involved in regulating the expression of fibronectin[J]. Chinese Journal of Cell Biology, 2013, 35(1): 98-103 (in Chinese). |
| [31] |
VAN DOREN S R. Matrix metalloproteinase interactions with collagen and elastin[J]. Matrix Biology, 2015, 44/46: 224-231. DOI:10.1016/j.matbio.2015.01.005 |
| [32] |
BEDDY D, MULSOW J, WATSON R W G, et al. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn's disease[J]. The British Journal of Surgery, 2006, 93(10): 1290-1296. DOI:10.1002/bjs.5431 |
| [33] |
FRANZōE, DINALLO V, LAUDISI F, et al. Interleukin-34 stimulates gut fibroblasts to produce collagen synthesis[J]. Journal of Crohn's & Colitis, 2020, 14(10): 1436-1445. |
| [34] |
SCHEIBE K, KERSTEN C, SCHMIED A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation[J]. Gastroenterology, 2019, 156(4): 1082-1097. DOI:10.1053/j.gastro.2018.11.029 |
| [35] |
RAHAL K, SCHMIEDLIN-REN P, ADLER J, et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn's disease[J]. Inflammatory Bowel Diseases, 2012, 18(4): 613-623. DOI:10.1002/ibd.21843 |
| [36] |
唐雪梅. PRMT1介导的细胞外基质沉积及维生素D3的缓解机制研究[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2019. TANG X M. PRMT1-mediated extracellular matrix deposition and mechanism of alleviation by vitamin D3[D]. Master's Thesis. Yangling: Northwest A & F University, 2019. (in Chinese) |
| [37] |
SULLI G, ROMMEL A, WANG X J, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence[J]. Nature, 2018, 553(7688): 351-355. DOI:10.1038/nature25170 |
| [38] |
HIROTA T, LEE J W, ST JOHN P C, et al. Identification of small molecule activators of cryptochrome[J]. Science, 2012, 337(6098): 1094-1097. DOI:10.1126/science.1223710 |
| [39] |
LU H W, JIA C, WU D Y, et al. Fibroblast growth factor 21(FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway[J]. Cell Death & Disease, 2021, 12(10): 865. |
| [40] |
DA COSTA FERNANDES C Jr, ZAMBUZZI W F. Fibroblast-secreted trophic factors contribute with ECM remodeling stimulus and upmodulate osteocyte gene markers in osteoblasts[J]. Biochimie, 2020, 168: 92-99. DOI:10.1016/j.biochi.2019.10.013 |
| [41] |
LATELLA G, VETUSCHI A, SFERRA R, et al. Localization of ανβ6 integrin-TGF-β1/Smad3, mTOR and PPARγ in experimental colorectal fibrosis[J]. European Journal of Histochemistry, 2013, 57(4): e40. DOI:10.4081/ejh.2013.e40 |
| [42] |
MARONI L, NINFOLE E, PINTO C, et al. Gut-liver axis and inflammasome activation in cholangiocyte pathophysiology[J]. Cells, 2020, 9(3): 736. DOI:10.3390/cells9030736 |
| [43] |
张京, 戴兆来, 朱伟云. 肠道必需氨基酸代谢及其功能的研究进展[J]. 肠外与肠内营养, 2010, 17(1): 55-59. ZHANG J, DAI Z L, ZHU W Y. Recent progress in intestinal essential amino acids metabolism and its function[J]. Parenteral & Enteral Nutrition, 2010, 17(1): 55-59 (in Chinese). DOI:10.3969/j.issn.1007-810X.2010.01.019 |
| [44] |
朱伟云, 余凯凡, 慕春龙, 等. 猪的肠道微生物与宿主营养代谢[J]. 动物营养学报, 2014, 26(10): 3046-3051. ZHU W Y, YU K F, MU C L, et al. Gut microbiota and host nutrition metabolism in pigs[J]. Chinese Journal of Animal Nutrition, 2014, 26(10): 3046-3051 (in Chinese). DOI:10.3969/j.issn.1006-267x.2014.10.016 |
| [45] |
孙志洪, 李貌, 许庆庆, 等. 猪氨基酸代谢节俭机制新假说[J]. 动物营养学报, 2016, 28(11): 3369-3376. SUN Z H, LI M, XU Q Q, et al. A new hypothesis for the mechanism of metabolic saving of amino acids of pigs[J]. Chinese Journal of Animal Nutrition, 2016, 28(11): 3369-3376 (in Chinese). DOI:10.3969/j.issn.1006-267x.2016.11.001 |
| [46] |
夏耀耀, 宾朋, 朱国强, 等. 氨基酸代谢调控猪免疫细胞命运研究进展[J]. 中国科学(生命科学), 2020, 50(9): 895-913. XIA Y Y, BIN P, ZHU G Q, et al. Amino acid metabolism in fate decision of porcine immune cells: advances and beyond[J]. Scientia Sinica Vitae, 2020, 50(9): 895-913 (in Chinese). |
| [47] |
HUANG T J, TERRELL J A, CHUNG J H, et al. Electrospun microfibers modulate intracellular amino acids in liver cells via integrin β1[J]. Bioengineering, 2021, 8(7): 88. DOI:10.3390/bioengineering8070088 |
| [48] |
YE Z C, SONTHEIMER H. Modulation of glial glutamate transport through cell interactions with the extracellular matrix[J]. International Journal of Developmental Neuroscience, 2002, 20(3/5): 209-217. |
| [49] |
BERTERO T, OLDHAM W M, GRASSET E M, et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy[J]. Cell Metabolism, 2019, 29(1): 124-140. DOI:10.1016/j.cmet.2018.09.012 |
| [50] |
LENZINI S, BARGI R, CHUNG G, et al. Matrix mechanics and water permeation regulate extracellular vesicle transport[J]. Nature Nanotechnology, 2020, 15(3): 217-223. DOI:10.1038/s41565-020-0636-2 |
| [51] |
VALLABHANENI K C, PENFORNIS P, DHULE S, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites[J]. Oncotarget, 2015, 6(7): 4953-4967. DOI:10.18632/oncotarget.3211 |
| [52] |
ZHENG D W, HONG S, ZHANG Q L, et al. Controllable gelation of artificial extracellular matrix for altering mass transport and improving cancer therapies[J]. Nature Communications, 2020, 11(1): 4907. DOI:10.1038/s41467-020-18493-7 |
| [53] |
SULLIVAN W J, MULLEN P J, SCHMID E W, et al. Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization[J]. Cell, 2018, 175(1): 117-132. DOI:10.1016/j.cell.2018.08.017 |
| [54] |
MASON J A, COCKFIELD J A, PAPE D J, et al. SGK1 signaling promotes glucose metabolism and survival in extracellular matrix detached cells[J]. Cell Reports, 2021, 34(11): 108821. DOI:10.1016/j.celrep.2021.108821 |
| [55] |
GRASSIAN A R, COLOFF J L, BRUGGE J S. Extracellular matrix regulation of metabolism and implications for tumorigenesis[J]. Cold Spring Harbor Symposia on Quantitative Biology, 2011, 76: 313-324. DOI:10.1101/sqb.2011.76.010967 |
| [56] |
KHAN T, MUISE E S, IYENGAR P, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen Ⅵ[J]. Molecular and Cellular Biology, 2009, 29(6): 1575-1591. DOI:10.1128/MCB.01300-08 |
| [57] |
ABEL E D, PERONI O, KIM J K, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver[J]. Nature, 2001, 409(6821): 729-733. DOI:10.1038/35055575 |
| [58] |
AIKIO M, ELAMAA H, VICENTE D, et al. Specific collagen XVⅢ isoforms promote adipose tissue accrual via mechanisms determining adipocyte number and affect fat deposition[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(30): E3043-E3052. |
| [59] |
LI Q X, WANG Q Y, ZHANG Q Y, et al. Collagen prolyl 4-hydroxylase 2 predicts worse prognosis and promotes glycolysis in cervical cancer[J]. American Journal of Translational Research, 2019, 11(11): 6938-6951. |
| [60] |
MORRIS B A, BURKEL B, PONIK S M, et al. Collagen matrix density drives the metabolic shift in breast cancer cells[J]. EBioMedicine, 2016, 13: 146-156. DOI:10.1016/j.ebiom.2016.10.012 |
| [61] |
FERNIE A R, ZHANG Y J, SAMPATHKUMAR A. Cytoskeleton architecture regulates glycolysis coupling cellular metabolism to mechanical cues[J]. Trends in Biochemical Sciences, 2020, 45(8): 637-638. DOI:10.1016/j.tibs.2020.04.003 |
| [62] |
PARK J S, BURCKHARDT C J, LAZCANO R, et al. Mechanical regulation of glycolysis via cytoskeleton architecture[J]. Nature, 2020, 578(7796): 621-626. DOI:10.1038/s41586-020-1998-1 |
| [63] |
ROMANI P, VALCARCEL-JIMENEZ L, FREZZA C, et al. Crosstalk between mechanotransduction and metabolism[J]. Nature Reviews.Molecular Cell Biology, 2021, 22(1): 22-38. |
| [64] |
LIU Q P, LUO Q, DENG B, et al. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling[J]. Cancers, 2020, 12(2): 490. DOI:10.3390/cancers12020490 |
| [65] |
ROMANI P, BRIAN I, SANTINON G, et al. Extracellular matrix mechanical cues regulate lipid metabolism through lipin-1 and SREBP[J]. Nature Cell Biology, 2019, 21(3): 338-347. DOI:10.1038/s41556-018-0270-5 |
| [66] |
AL HASAN M, MARTIN P E, SHU X H, et al. Type Ⅲ collagen is required for adipogenesis and actin stress fibre formation in 3T3-L1 preadipocytes[J]. Biomolecules, 2021, 11(2): 156. DOI:10.3390/biom11020156 |
| [67] |
LIU X L, LONG X Y, GAO Y F, et al. Type I collagen inhibits adipogenic differentiation via YAP activation in vitro[J]. Journal of Cellular Physiology, 2020, 235(2): 1821-1837. DOI:10.1002/jcp.29100 |
| [68] |
LIU G N, LI M H, XU Y T, et al. ColXV promotes adipocyte differentiation via inhibiting DNA methylation and cAMP/PKA pathway in mice[J]. Oncotarget, 2017, 8(36): 60135-60148. DOI:10.18632/oncotarget.18550 |
| [69] |
GESTA S, GUNTUR K, MAJUMDAR I D, et al. Reduced expression of collagen Ⅵ alpha 3(COL6A3) confers resistance to inflammation-induced MCP1 expression in adipocytes[J]. Obesity, 2016, 24(8): 1695-1703. DOI:10.1002/oby.21565 |
| [70] |
OH J, KIM C S, KIM M, et al. Type Ⅵ collagen and its cleavage product, endotrophin, cooperatively regulate the adipogenic and lipolytic capacity of adipocytes[J]. Metabolism, 2021, 114: 154430. DOI:10.1016/j.metabol.2020.154430 |
| [71] |
JI E, JUNG M Y, PARK J H, et al. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid[J]. International Journal of Obesity, 2014, 38(8): 1035-1043. DOI:10.1038/ijo.2013.202 |
| [72] |
VAICIK M K, THYBOLL KORTESMAA J, MOVÉRARE-SKRTIC S, et al. Laminin α4 deficient mice exhibit decreased capacity for adipose tissue expansion and weight gain[J]. PLoS One, 2014, 9(10): e109854. DOI:10.1371/journal.pone.0109854 |
| [73] |
ZHANG Z Y, MAI Y, YANG H, et al. CTSB promotes porcine preadipocytes differentiation by degrading fibronectin and attenuating the Wnt/β-catenin signaling pathway[J]. Molecular and Cellular Biochemistry, 2014, 395(1/2): 53-64. |
| [74] |
ZÖLLER N, SCHREINER S, PETRY L, et al. Collagen Ⅰ promotes adipocytogenesis in adipose-derived stem cells in vitro[J]. Cells, 2019, 8(4): 302. DOI:10.3390/cells8040302 |
| [75] |
BUECHLER C, KRAUTBAUER S, EISINGER K. Adipose tissue fibrosis[J]. World Journal of Diabetes, 2015, 6(4): 548-553. DOI:10.4239/wjd.v6.i4.548 |
| [76] |
SUN K, TORDJMAN J, CLÉMENT K, et al. Fibrosis and adipose tissue dysfunction[J]. Cell Metabolism, 2013, 18(4): 470-477. DOI:10.1016/j.cmet.2013.06.016 |




