2. 内蒙古农业大学兽医学院, 呼和浩特 010018
2. College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot 010018, China
近年来,人们的生活水平随着我国经济的迅速发展在快速地提高,各类优质蛋白质在膳食比例中占比越来越大,高蛋白质、低胆固醇的牛肉越来越受到人们的青睐[1]。2019年我国牛肉折算胴体基础的总产量达685.0万t,已成为世界第三大牛肉生产国。但牛肉市场仍供不应求,2019年我国牛肉进口量为165.93万t,是2018年同期的1.6倍[2]。在这一形势下,国家出台各项政策不断加大肉牛标准化规模养殖推进力度,通过引导深入研究提高母牛繁殖力以及犊牛健康的营养调控技术,构建一体化的营养与高效繁育技术体系[3]。
繁殖是影响肉牛生产效率的主要决定因素。肉牛的繁殖效率受到多种因素的影响,比如饲粮营养、肉牛品种、饲养管理水平、机体健康状况和环境温湿度,以及这些因素的交互作用[4-6]。在实际生产中,母畜繁殖性能下降的主要原因是营养摄入不足和由此引起的休情期延长。Butler[7]的研究表明母牛产后能量负平衡和产后发情间隔呈密切正相关。Short等[8]研究发现,降低饲粮能量水平可导致育成牛初情期的推迟,还可导致成年母牛产后乏情,进而延长产后发情间隔,最终导致牛群繁殖效率下降。Houghton等[9]发现为母牛提供高能量饲粮对缩短产后间隔和提高受胎率是必要的。还有学者报道母牛产犊时的体况与其产后发情间隔密切相关[10]。
除了母畜体况与其繁殖性能密切相关以外,营养摄入量和机体能量储存可以显著影响母畜血液中能量底物和代谢激素的浓度,底物和代谢激素浓度的变化可向大脑、垂体或卵巢负反馈动物代谢状态的信号,进而影响下丘脑-垂体-卵巢轴,从而对母畜繁殖性能的发挥产生影响[11]。有学者研究发现通过改善繁殖母畜的饲粮营养水平,可有效地促进母畜繁殖激素的分泌,进而提高其发情率和受胎率[12]。Da Silva等[13]研究发现,通过给放牧饲养条件下的肉用母牛每天补充1.5 kg精料,显著提高了母牛血清中孕酮(P)的浓度,并有效地缩短了发情间隔。类似地,有学者指出营养补充可显著提高冷季放牧母牦牛血清中P的浓度[14]。因此,提高母牛的饲粮营养水平对改善其机体状况和代谢水平具有积极意义。
在实际肉牛生产中,饲喂高精料饲粮是满足肉牛高能量需求和提高效益的常用策略。与低精料饲粮相比,高精料饲粮中富含的非纤维性碳水化合物能被瘤胃微生物快速发酵产生短链脂肪酸(SCFA),特别是丙酸和丁酸,这有利于提高反刍动物的生长性能和饲料利用率[15-16]。但目前关于饲粮精粗比对肉牛的影响研究主要集中于各个育肥阶段的肉牛,关于空怀期母牛的报道较少[17-18]。因此,本试验以空怀母牛为试验动物,在不同饲粮精粗比(蛋能比和钙磷比一致)饲喂条件下,从增重、体况评分、发情率、血清繁殖激素和血浆生化指标几个方面开展系统的研究,以期为体况较瘦弱的空怀母牛的饲养提供生产参数,并为提高母牛繁殖性能和养殖效益提供理论基础。
1 材料与方法 1.1 试验设计与饲粮本试验在内蒙古自治区多伦县博赫牛场进行。采用完全随机设计,将90头体况相近、年龄相近且体况偏瘦,产后2个月以上未见明显发情的空怀安格斯初产母牛[(387.2±22.6) kg]平均分为3组,分别饲喂高精粗比饲粮(精粗比65 ∶ 35,HCD组)、中精粗比饲粮(精粗比50 ∶ 50,MCD组)及低精粗比饲粮(精粗比35 ∶ 65,LCD组)。以全株玉米青贮和干稻草作为主要的粗饲料来源,根据NRC(2000)空怀期肉用母牛饲养标准配制试验饲粮,试验饲粮组成及营养水平见表 1。试验牛采用散栏饲养,于每日08:00和18:00各饲喂1次。整个试验期间所有试验牛自由采食,自由饮水;试验饲粮采用全混合日粮(TMR)形式饲喂,每天记录投料量和剩料量,保证每天早晨剩料量在5%~10%。试验期为75 d,其中预试期15 d,正试期60 d。
|
|
表 1 试验饲粮组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of experimental diets (DM basis) |
试验期间,每隔7 d分别采集精饲料、粗饲料及TMR各500 g,分装于自封袋并保存于-20 ℃冰箱,用于干物质含量测定和饲料营养成分分析。
1.3 体重和体况的测定于正试期开始前(第1天)和结束(第60天)时分别连续2 d测定各试验牛的体重,并计算平均日增重(ADG)。测定体重后,由3名肉牛饲养管理人员根据NRC(2000)推荐的9分制BCS系统(1分代表极瘦,9分代表过胖),利用眼观和触摸的方式对试验牛进行体况评分。
1.4 血液采集与测定在正试期第0(开始前)、15、30、45、60天及发情时的早晨饲喂前,通过颈静脉处采集10 mL血液样品于无菌去热源的真空采血管中,立即在4 ℃下以3 000×g离心15 min,分离血清,并采用移液枪将血清转移至2 mL无菌离心管中,置于液氮中带回实验室保存于-20 ℃,用于血清繁殖激素的测定。
在正试期第20、40、60天晨饲前进行颈静脉空腹采血,通过颈静脉处采集10 mL血液样品于含有肝素钠作为抗凝剂的真空采血管中,采集的血液样品于3 000×g离心15 min,分离血浆并保存于-20 ℃,用于检测血浆生化指标。
血清繁殖激素的测定:采用酶联免疫吸附测定(ELISA)试剂盒(泉州市睿信生物科技有限公司)测定促黄体素(LH)、促卵泡素(FSH)、雌二醇(E2)、P的浓度,具体操作步骤按照试剂盒说明书进行。
血浆生化指标的测定:葡萄糖(GLU)、尿素氮(UN)、甘油三酯(TG)、总蛋白(TP)、白蛋白(ALB)及总胆固醇(TCHO)含量均采用北京华英生物技术研究所提供的试剂盒进行测定,具体操作步骤按照试剂盒说明书进行。
1.5 发情鉴定在正试期开始后,每天3次对各试验牛进行发情观察,若母牛外阴部红润肿胀并伴有黏液流出,鉴定为疑似发情;此外,根据上述发情标准判定为疑似发情的母牛,每天早上和晚上放入健康的种公牛试情,若其主动接近种公牛或接受种公牛爬跨,则鉴定该母牛发情。记录各发情母牛的耳标号和发情时间,并统计各组母牛的发情率。
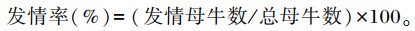
|
试验数据经过Excel 2010进行处理后,采用SAS 9.0软件对试验数据进行单因素方差分析(one-way ANOVA),试验结果显著性分析以P<0.05表示差异显著,P>0.05表示差异不显著。
2 结果与分析 2.1 饲粮精粗比对空怀母牛体重、体况和发情的影响由表 2可知,在正试期开始时,母牛的体重和体况评分各组之间均无显著差异(P>0.05)。在正试期第60天时,MCD组和HCD组母牛的体重和体况评分高于LCD组,但差异不显著(P>0.05);MCD组和HCD组母牛的ADG和体况变化均显著高于LCD组(P<0.05),但MCD组和HCD组之间差异不显著(P>0.05)。HCD和MCD组母牛发情率显著高于LCD组(P<0.05),且MCD组发情时间要显著早于LCD组(P<0.05)。
|
|
表 2 饲粮精粗比对空怀母牛体重、体况和发情的影响 Table 2 Effects of dietary concentrate to forage ratio on body weight, body condition and estrus of empty-breasted cows |
由表 3可知,在正试期第30天时,HCD组和MCD组血清LH浓度显著高于LCD组(P<0.05);MCD组血清E2浓度显著高于LCD组(P<0.05),但与HCD组无显著差异(P>0.05)。在正试期第45天时,HCD组和MCD组血清LH、FSH和E2浓度均显著高于LCD组,血清LH和FSH浓度均在发情时达到最高,且显著高于LCD组(P<0.05)。HCD组和MCD组血清P浓度在发情时有高于LCD组的趋势,但差异并不显著(P>0.05)。
|
|
表 3 饲粮精粗比对空怀母牛血清繁殖激素的影响 Table 3 Effects of dietary concentrate to forage ratio on serum reproductive hormones of empty-breasted cows |
由表 4可知,在正试期第20天时,HCD组和MCD组血浆葡萄糖含量均显著高于LCD组(P < 0.05);HCD组血浆甘油三酯含量有高于其他2组的趋势;血浆其他指标在3组之间差异不显著(P>0.05)。在正试期第40天时,HCD组血浆葡萄糖和甘油三酯含量均显著高于LCD组(P < 0.05),但均与MCD组无显著差异(P>0.05);HCD组和MCD组血清尿素氮含量显著低于LCD组(P < 0.05);血浆其他指标在各组之间无显著差异(P>0.05)。在正试期第60天时,HCD组和MCD组血清葡萄糖含量均显著高于LCD组(P < 0.05);MCD组血清尿素氮含量显著低于LCD组(P < 0.05),且有低于HCD组的趋势;其他指标在各组间无显著差异(P>0.05)。
|
|
表 4 饲粮精粗比对空怀母牛血浆生化指标的影响 Table 4 Effects of dietary concentrate to forage ratio on plasma biochemical indices of empty-breasted cows |
通过营养调控措施可有效改善母畜体况,增加其机体能量储备,这对其繁殖性能的发挥具有积极作用[19]。Beam等[20]研究发现饲粮能量水平对母牛产后发情和排卵均具有重要影响。景炜[21]通过比较研究饲粮能量水平对多浪羊繁殖性能的影响发现提高饲粮能量水平可显著提高多浪羊的发情率。此外,短期补充玉米也对绵羊的发情具有一定的积极作用[22]。还有研究发现母畜繁殖器官的发育和卵巢机能的活动均与饲粮营养水平密切相关[23-24]。本试验中,HCD组和MCD组母牛的发情率均显著高于LCD组,这是由于提高空怀母牛的饲粮营养水平显著提高了母牛的日增重并改善了其体况,母牛的体况与其发情率密切相关;上述结果表明提高饲粮营养水平可促使空怀母牛发情;此外,通过比较研究3组空怀母牛的发情时间发现,除了显著短于LCD组,MCD组母牛的发情时间还较HCD组早,但差异不显著,这说明饲粮营养水平过高或过低均可能对母牛的发情具有一定程度上的负面影响。刘宁[25]在比较研究饲粮营养水平对滩羊发情率的影响时也进行了类似的报道。
FSH和LH均是繁殖母畜机体内重要的内分泌调节因子,主要促进卵泡的发育和生长[26]。营养缺乏可减少FSH和LH的合成和分泌,对卵泡的最终成熟造成负面影响。FSH的主要生理作用是在卵泡腔形成后,在卵泡发育至成熟的过程中具有重要作用;与FSH不同的是,LH是促使母畜发情的重要激素之一,其主要生理作用是促进卵泡发育为黄体[27]。已有研究证实,母牛血清中LH分泌的减少与显著降低的营养摄入量密切相关[28]。有学者研究发现,通过提高繁殖母羊的饲粮营养水平可显著提高其血液中FSH和LH浓度[29];然而Xu等[30]等研究发现饲粮能量水平对母畜FSH的分泌无显著影响;也有研究表明LH的分泌受到饲粮能量水平的影响。本试验中,各组空怀母牛血清中FSH和LH浓度整体上随着营养调控时间的推进而逐步升高;饲粮精粗比对空怀母牛血清中FSH和LH浓度的影响较大,HCD组和MCD组血清LH浓度在正试期第45天和发情时均显著高于LCD组;在正试期第45天时,HCD组和MCD组血清FSH浓度均显著高于LCD组,且MCD组在发情时血清FSH浓度最高。这与Xie等[14]报道的提高空怀期牦牛营养摄入量可显著提高血清中FSH和LH浓度的结果相一致,也验证了饲粮营养水平与FSH和LH的合成和分泌之间的相关性。上述研究结果表明,提高饲粮营养水平有利于促进空怀母牛的卵泡正常性周期活动,进而改善母牛繁殖性能。
E2是动物卵巢内卵泡颗粒细胞分泌的类固醇激素,在不同品种和动物的各个繁殖阶段具有不同的生理作用。E2可促进初情期前母畜繁殖器官的发育,还可参与卵泡成熟和排卵相关激素的释放过程,对妊娠的建立也具有积极作用,因此被广泛应用于牛、羊及猪等各种家畜诱导发情和同期发情处理的生产实践当中[31-32]。本试验中,3组空怀母牛血清中E2的浓度均在发情时达到最高。本试验中,MCD组血清E2浓度在正试期第30和45天时均显著高于LCD组,MCD组和HCD组在发情时血清P浓度有高于LCD组的趋势,但差异不显著。这与Xie等[14]的研究结果相似,他们比较研究了补充精料对放牧饲养条件下冷季空怀牦牛繁殖性能的影响,结果表明补饲组牦牛血清E2和P浓度均显著高于放牧组。此外,本研究结果还表明,MCD组血清E2浓度在整个试验期间的各个时间点也相对高于其他2组,这可能解释了MCD组的发情时间较其他2组早的原因。本试验中,各组试验牛血清P浓度在各个时间点均无显著差异,这与郑家三等[33]报道的饲粮营养水平对奶牛血清P浓度无显著影响的研究结果一致。但有学者指出补充营养可显著提高冷季放牧母牛血清P浓度[14]。除了动物品种差异可对上述存在差异的结果进行解释,有学者指出饲粮营养水平的提高对繁殖激素分泌的影响程度远没有营养限制所造成的影响大[34]。
3.2 饲粮精粗比对空怀母牛血浆生化指标的影响动物的血液生化指标不仅可以较直观地反映机体的生理状况和代谢水平,还可以用来监测机体的健康状态[35]。当动物处于正常生理状况时,其各项血液生化指标维持在正常范围内波动。血糖含量可反映反刍动物机体内糖的吸收、转运和代谢等状况,是衡量其能量代谢水平的重要指标[36]。反刍动物机体内90%以上的血糖是依靠糖异生合成,丙酸是糖异生的重要原料,当反刍动物采食粗饲料比例较高的饲粮时,瘤胃内丙酸产量不足进而导致血糖含量较低[37]。袁庆启等[38]比较研究了饲粮精粗比对中国荷斯坦奶牛血糖含量的影响,结果表明饲粮中精料水平的增加可导致奶牛血糖含量显著升高;夏传齐[39]的研究结果表明,当荷斯坦奶公牛饲粮中精料水平由35%提高至65%时,血糖含量随之显著升高。本试验研究结果与上述研究结论一致,这表明瘤胃内丙酸的产生和吸收随着饲粮中精料水平的增加而增强,进而促进肝脏的糖异生作用和机体糖代谢。
瘤胃内栖息的大量微生物可将饲粮中的含氮物质分解为氨,并将其中一部分氨合成菌体蛋白为机体所利用,另一部分被瘤胃吸收后参与机体尿素氮循环,因此血液中尿素氮含量通常被用于衡量机体蛋白质代谢水平和氨基酸平衡状况的重要指标[40]。姜南等[41]研究发现,饲喂60%精料水平饲粮的牦牛血清尿素氮含量显著高于饲喂40%精料水平饲粮的牦牛。相反地,有学者发现湖羊血清中尿素氮含量随着饲粮精料水平的提高而极显著降低[42]。还有学者指出饲粮精料水平对淘汰母牛血清尿素氮含量和蛋白质代谢无显著影响[43]。上述研究结论出现较大差异,这表明饲粮精粗比对反刍动物血清尿素氮含量的影响,除了精料水平,很可能还受到粗饲料来源这一因素的影响。有学者指出,当以三叶草等富含瘤胃降解蛋白质的植物作为奶牛主要粗饲料来源时,可促进瘤胃内氨气(NH3)的产生,进而提高血液中尿素氮的含量[44]。本试验中,MCD组空怀母牛血浆尿素氮含量在正试期第40和60天时均显著低于LCD组,这表明MCD组母牛具有更好的氮沉积状况和蛋白质代谢水平;此外,MCD组血清尿素氮含量在正试期第60天时有低于HCD组的趋势,这可能是由于HCD组母牛采食高精料饲粮后,释放的大量瘤胃氨无法被瘤胃微生物迅速利用所致[45]。
当动物摄入高能量饲粮后,过量的能量可通过血液进入肝脏并以脂质的形式储存;而脂质在血液中的主要存在形式是甘油三酯和胆固醇[46]。因此,这两者是反映动物机体能量代谢和脂肪代谢的重要代谢参数。本试验结果表明,空怀母牛血清甘油三酯含量除了在正试期第40天时有显著差异,在其他时间点均各组之间无显著差异。夏传齐[39]、杨宇为等[43]的研究结果均表明,血液中甘油三酯含量随着饲粮精料水平的提高而显著增加,提高饲粮精料水平可促进肉牛机体脂肪代谢并进一步加速脂肪合成;相反地,有学者报道,通过提高饲粮精料水平可显著降低奶牛血清中甘油三酯的含量[47]。上述研究结果不一致的原因可能是由于各项研究中试验肉牛的营养摄入和体脂率存在差别,也可能是由于母牛生理阶段的差异所导致的,已有大量学者指出处于能量负平衡状态下的围产期母牛体脂降解加快,可引起血液中甘油三酯含量的迅速升高[48-49]。
4 结论本试验条件下,通过对体况较差的空怀母牛进行营养调控,得出以下结论:提高饲粮精料水平可改善体况偏瘦空怀母牛的能量和蛋白质代谢水平,进一步改善母牛体况,且可有效地促进母牛机体内繁殖相关激素的分泌并提高空怀母牛的发情率。
| [1] |
和立文. 全株玉米青贮品质评价及其对肉牛育肥性能和牛肉品质的影响[D]. 博士学位论文. 北京: 中国农业大学, 2017: 6-15. HE L W. Quality evaluation of corn silage and its effect on the growth performance and beef quality of finishing cattle[D]. Ph. D. Thesis. Beijing: China Agricultural University, 2017: 6-15. (in Chinese) |
| [2] |
曹兵海, 李俊雅, 王之盛, 等. 2019年度肉牛牦牛产业技术发展报告[J]. 中国畜牧杂志, 2020, 56(3): 173-178. CAO B H, LI J Y, WANG Z S, et al. 2019 beef cattle and yak industry technology development report[J]. Chinese Journal of Animal Nutrition, 2020, 56(3): 173-178 (in Chinese). |
| [3] |
刘森挥. 我国肉牛养殖业全要素生产率变动及提升路径研究[D]. 博士学位论文. 长春: 吉林农业大学, 2019: 3-7. LIU S H. Study on the change and improvement path of total factor productivity of beef cattle breeding industry in China[D]. Ph. D. Thesis. Changchun: Jilin Agricultural University, 2019: 3-7. (in Chinese) |
| [4] |
封元. 日粮营养水平对繁殖母牛生产性能与繁殖性能及犊牛生长发育的影响[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2020: 15-21. FENG Y. Effects of diets with different nutritional leves on the weight gain and reproduction of breeding cow and calbes growth and development[D]. Master's Thesis. Yangling: Northwest A & F University, 2020: 15-21. (in Chinese) |
| [5] |
MADUREIRA E D, MATURANA FILHO M, LEMES K M, et al. Análise crítica de fatores que interferem na fertilidade de vacas zebuínas[C]//Proceedings of 9th Symposium of Beef Cattle Production, Viçosa: Universidade Federal de Viçosa, 2014: 367-400.
|
| [6] |
WETTEMANN R P, LENTS C A, CICCIOLI N H, et al. Nutritional- and suckling-mediated anovulation in beef cows[J]. Journal of Animal Science, 2003, 81(14_suppl_2): E48-E59. |
| [7] |
BUTLER W R. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows[J]. BSAP Occasional Publication, 2001, 26(1): 133-145. DOI:10.1017/S0263967X00033644 |
| [8] |
SHORT R E, ADAMS D C. Nutritional and hormonal interrelationships in beef cattle reproduction[J]. Canadian Journal of Animal Science, 1988, 68(1): 29-39. DOI:10.4141/cjas88-003 |
| [9] |
HOUGHTON P L, LEMENAGER R P, HORSTMAN L A, et al. Effects of body composition, pre- and postpartum energy level and early weaning on reproductive performance of beef cows and preweaning calf gain[J]. Journal of Animal Science, 1990, 68(5): 1438-1446. DOI:10.2527/1990.6851438x |
| [10] |
MURPHY M G, ENRIGHT W J, CROWE M A, et al. Effect of dietary intake on pattern of growth of dominant follicles during the oestrous cycle in beef heifers[J]. Journal of Reproduction and Fertility, 1991, 92(2): 333-338. DOI:10.1530/jrf.0.0920333 |
| [11] |
MEZA-HERRERA C A, HALLFORD D M, ORTIZ J A, et al. Body condition and protein supplementation positively affect periovulatory ovarian activity by non LH-mediated pathways in goats[J]. Animal Reproduction Science, 2008, 106(3/4): 412-420. |
| [12] |
武思同, 敖日格乐, 王纯洁, 等. 慢性热应激期营养调控对放牧妊娠牛生殖激素含量、免疫功能及抗氧化能力的作用[J]. 动物营养学报, 2021, 33(3): 1545-1554. WU S T, AO R G L, WANG C J, et al. Effects of nutritional regulation on reproductive hormone contents, immune function and antioxidant ability of grazing pregnant cattle during chronic heat stress period[J]. Chinese Journal of Animal Nutrition, 2021, 33(3): 1545-1554 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.03.036 |
| [13] |
DA SILVA A G, PAULINO M F, DETMANN E, et al. Energetic-protein supplementation in the last 60 days of gestation improves performance of beef cows grazing tropical pastures[J]. Journal of Animal Science and Biotechnology, 2017, 8: 78. DOI:10.1186/s40104-017-0209-x |
| [14] |
XIE J P, KALWAR Q, YAN P, et al. Effect of concentrate supplementation on the expression profile of miRNA in the ovaries of yak during non-breeding season[J]. Animals, 2020, 10(9): 1640. DOI:10.3390/ani10091640 |
| [15] |
KIM Y H, NAGATA R, OHKUBO A, et al. Changes in ruminal and reticular pH and bacterial communities in Holstein cattle fed a high-grain diet[J]. BMC Veterinary Research, 2018, 14(1): 310. DOI:10.1186/s12917-018-1637-3 |
| [16] |
PLAIZIER J C, DANESH MESGARAN M, DERAKHSHANI H, et al. Review: enhancing gastrointestinal health in dairy cows[J]. Animal, 2018, 12(Supplement 2): s399-s418. |
| [17] |
杨游. 高精料饲粮引起肉牛机体代谢组的变化及其营养调控效果研究[D]. 博士学位论文. 重庆: 西南大学, 2018. YANG Y. A study on the metabolomics profiling alterations associated with the high-concentrate diet in beef cattle and the effects of nutritional modulations[D]. Ph. D. Thesis. Chongqing: Southwest University, 2018. (in Chinese) |
| [18] |
田可. 日粮添加菊粉对育肥肉牛生长性能、瘤胃发酵和菌群、炎症反应的影响[D]. 硕士学位论文. 重庆: 西南大学, 2020. TIAN K. Effects of dietary supplementation of inulin on growth performance, rumen fermentation and bacterial microbiota, inflammatory response in finishing beef steers[D]. Master's Thesis. Chongqing: Southwest University, 2020. (in Chinese) |
| [19] |
MARTIN G B, RODGER J, BLACHE D. Nutritional and environmental effects on reproduction in small ruminants[J]. Reproduction, Fertility, and Development, 2004, 16(4): 491-501. DOI:10.1071/RD04035 |
| [20] |
BEAM S W, BUTLER W R. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows[J]. Journal of Reproduction and Fertility. Supplement, 1999, 54: 411-424. |
| [21] |
景炜. 日粮不同能量和蛋白水平对多浪羊母羊繁殖性能、血清生化指标及生殖激素的影响[D]. 硕士学位论文. 乌鲁木齐: 新疆农业大学, 2010. JING W. Effect of different energy and protein diet on reproduction, biochemical serum and hormone serum indexes performance of Daolang ewes[D]. Master's Thesis. Urumqi: Xinjiang Agricultural University, 2010. (in Chinese) |
| [22] |
郭云霞. 黄体期短期优饲对绵羊卵泡发育影响及其调控机理[D]. 博士学位论文. 保定: 河北农业大学, 2018. GUO Y X. Effect of short-term nutritional supplementation on folliculogenesis and its mechanism of regulation in sheep during the luteal phase[D]. Ph. D. Thesis. Baoding: Hebei Agricultural University, 2018. (in Chinese) |
| [23] |
FITZ-RODRÍGUEZ G, DE SANTIAGO-MIRAMONTES M A, SCARAMUZZI R J, et al. Nutritional supplementation improves ovulation and pregnancy rates in female goats managed under natural grazing conditions and exposed to the male effect[J]. Animal Reproduction Science, 2009, 116(1/2): 85-94. |
| [24] |
景小平. 冷季补饲对藏绵羊母羊生长性能、胃肠道及繁殖器官发育的影响[D]. 硕士学位论文. 雅安: 四川农业大学, 2016. JING X P. Effects of supplementation on the growth performance and the development of gastrointestinal tract and reproductive organ of Tibetan sheep ewes in cold season[D]. Master's Thesis. Ya'an: Sichuan Agricultural University, 2016. (in Chinese) |
| [25] |
刘宁. 不同营养水平日粮对育成后期滩母羊生长发育及繁殖激素的影响[D]. 硕士学位论文. 银川: 宁夏大学, 2016. LIU N. Effect of ration at different nutrition levels on growth-development and reproductive hormone of late-breed Tan sheep[D]. Master's Thesis. Yinchuan: Ningxia University, 2016. (in Chinese) |
| [26] |
姜怀志, 李向军, 戢爽, 等. 辽宁绒山羊母羊FSH全年分泌规律的研究[J]. 中国畜牧杂志, 2008, 44(15): 15-17. JIANG H Z, LI X J, JI S, et al. Study on the year round secreting regularity of FSH in peripheral plasma of female Liaoning cashmere goats[J]. Chinese Journal of Animal Science, 2008, 44(15): 15-17 (in Chinese). |
| [27] |
ULUG U, TURAN E, TOSUN S B, et al. Comparison of preovulatory follicular concentrations of epidermal growth factor, insulin-like growth factor-Ⅰ, and inhibins A and B in women undergoing assisted conception treatment with gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists[J]. Fertility and Sterility, 2007, 87(4): 995-998. DOI:10.1016/j.fertnstert.2006.08.102 |
| [28] |
VIÑOLES GIL C. Effect of nutrition on follicle development and ovulation rate in the ewe[D]. Ph. D. Thesis. Uppsala: Swedish University of Agricultural Sciences, 2003.
|
| [29] |
RHIND S M, LESLIE I D, GUNN R G, et al. Plasma FSH, LH, prolactin and progesterone profiles of Cheviot ewes with different levels of intake before and after mating, and associated effects on reproductive performance[J]. Animal Reproduction Science, 1985, 8(4): 301-313. DOI:10.1016/0378-4320(85)90046-6 |
| [30] |
XU Z Z, MCDONALD M F, MCCUTCHEON S N. The effects of nutritionally-induced liveweight differences on follicular development, ovulation rate, oestrous activity and plasma follicle-stimulating hormone levels in the ewe[J]. Animal Reproduction Science, 1989, 19(1/2): 67-78. |
| [31] |
KARSCH F J, GOODMAN R L, LEGAN S J. Feedback basis of seasonal breeding: test of an hypothesis[J]. Journal of Reproduction and Fertility, 1980, 58(2): 521-535. DOI:10.1530/jrf.0.0580521 |
| [32] |
应诗家, 王昌龙, 贾若欣, 等. 湖羊不同发育阶段卵泡内代谢产物和激素含量的比较研究[J]. 畜牧兽医学报, 2012, 43(2): 180-185. YING S J, WANG C L, JIA R X, et al. A comparative study of metabolites and hormone concentrations in different-sized follicles in Hu sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2012, 43(2): 180-185 (in Chinese). |
| [33] |
郑家三, 夏成, 朱玉哲, 等. 日粮能量水平对奶牛繁殖性能、血浆代谢产物和生殖激素的影响[J]. 中国奶牛, 2011(10): 28-31. ZHENG J S, XIA C, ZHU Y Z, et al. The effect of dietary energy levels on reproductive performances, plasma metabolites and reproductive hormones of lactating cows[J]. China Dairy Cattle, 2011(10): 28-31 (in Chinese). DOI:10.3969/j.issn.1004-4264.2011.10.008 |
| [34] |
O'CALLAGHAN D, YAAKUB H, HYTTEL P, et al. Effect of nutrition and superovulation on oocyte morphology, follicular fluid composition and systemic hormone concentrations in ewes[J]. Journal of Reproduction and Fertility, 2000, 118(2): 303-313. DOI:10.1530/jrf.0.1180303 |
| [35] |
STANLEY C C, WILLIAMS C C, JENNY B F, et al. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves[J]. Journal of Dairy Science, 2002, 85(9): 2335-2343. DOI:10.3168/jds.S0022-0302(02)74313-0 |
| [36] |
GIGER-REVERDIN S, RIGALMA K, DESNOYERS M, et al. Effect of concentrate level on feeding behavior and rumen and blood parameters in dairy goats: relationships between behavioral and physiological parameters and effect of between-animal variability[J]. Journal of Dairy Science, 2014, 97(7): 4367-4378. DOI:10.3168/jds.2013-7383 |
| [37] |
HWANG S Y, LEE M J, CHIOU P W S. Monitoring nutritional status of dairy cows in taiwan using milk protein and milk urea nitrogen[J]. Asian-Australasian Journal of Animal Sciences, 2000, 13(12): 1667-1673. DOI:10.5713/ajas.2000.1667 |
| [38] |
袁庆启, 姜发彬, 王亚琼, 等. 不同精粗比日粮对奶牛瘤胃发酵与肝脏VFA代谢及产奶性能的影响[J]. 家畜生态学报, 2016, 37(7): 36-43. YUAN Q Q, JIANG F B, WANG Y Q, et al. Effect of different concentrate to forage ratio of diet on rumen fermentation, VFA metabolism of liver and performance in dairy cows[J]. Acta Ecologae Animalis Domastici, 2016, 37(7): 36-43 (in Chinese). DOI:10.3969/j.issn.1673-1182.2016.07.007 |
| [39] |
夏传齐. 营养水平对荷斯坦奶公牛生长性能、血液生化指标、瘤胃发酵、屠宰性能及肉品质的影响[D]. 博士学位论文. 北京: 中国农业大学, 2018: 87-89. XIA C Q. Effects of dietary nutrition levels on growth performance, plasma parameters, rumen fermentation, carcass characteristics and meat quality of Holstein bulls[D]. Ph. D. Thesis. Beijing: China Agricultural University, 2018: 87-89. (in Chinese) |
| [40] |
HALL J B, STAIGMILLER R B, BELLOWS R A, et al. Body composition and metabolic profiles associated with puberty in beef heifers[J]. Journal of Animal Science, 1995, 73(11): 3409-3420. DOI:10.2527/1995.73113409x |
| [41] |
姜南, 朱彦宾, 孙光明, 等. 不同精粗比饲粮对牦牛养分表观消化率、血浆生化及抗氧化指标的影响[J]. 中国草食动物科学, 2021, 41(3): 43-48. JIANG N, ZHU Y B, SUN G M, et al. Effects of roughage to concentrate ratio on nutrient apparent digestibility, plasma biochemical parameters and antioxidant capacity of yak[J]. China Herbivore Science, 2021, 41(3): 43-48 (in Chinese). DOI:10.3969/j.issn.2095-3887.2021.03.008 |
| [42] |
占今舜, 杨群, 钟小军, 等. 不同精粗比饲粮对湖羊肉品质、血液指标和肠道发育的影响[J]. 草业科学, 2019, 36(12): 3166-3174. ZHAN J S, YANG Q, ZHONG X J, et al. Effects of total mixed rations with different concentration-roughage ratios on meat quality, serum indices, and intestinal tract development in Hu sheep[J]. Pratacultural Science, 2019, 36(12): 3166-3174 (in Chinese). DOI:10.11829/j.issn.1001-0629.2019-0176 |
| [43] |
杨宇为, 马吉锋, 王建东, 等. 不同精粗比对淘汰母牛生产性能及血液生化指标的影响[J]. 饲料工业, 2021, 42(3): 37-42. YANG Y W, MA J F, WANG J D, et al. Effects of different diets on growth performance and blood biochemical parameters in cull cows[J]. Feed Industry, 2021, 42(3): 37-42 (in Chinese). |
| [44] |
BEN MEIR Y A, NIKBACHAT M, PORTNIK Y, et al. Effect of forage-to-concentrate ratio on production efficiency of low-efficient high-yielding lactating cows[J]. Animal, 2021, 15(1): 100012. DOI:10.1016/j.animal.2020.100012 |
| [45] |
SERMENT A, SCHMIDELY P, GIGER-REVERDIN S, et al. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats[J]. Journal of Dairy Science, 2011, 94(8): 3960-3972. DOI:10.3168/jds.2010-4041 |
| [46] |
ANDERSON J W, JONES A E, RIDDELL-MASON S. Ten different dietary fibers have significantly different effects on serum and liver lipids of cholesterol-fed rats[J]. The Journal of Nutrition, 1994, 124(1): 78-83. DOI:10.1093/jn/124.1.78 |
| [47] |
杨靖, 崔巧荣, 张力莉, 等. 日粮精粗比对奶牛瘤胃挥发酸模式及血液糖脂代谢相关指标的影响[J]. 中国饲料, 2019(5): 33-35. YANG J, CUI Q R, ZHANG L L, et al. Effects of dietary concentrate to forage ratio on rumen volatile acid pattern and serum indices related with glucose and lipid metabolism in dairy cows[J]. China Feed, 2019(5): 33-35 (in Chinese). |
| [48] |
NELSON D L, COX M M. Lehninger principles of biochemistry[M]. 6th ed. New York: W.H. Freeman, 2013.
|
| [49] |
RAHMANI M, DEHGHAN-BANADAKY M, KAMALYAN R, et al. Effects of feeding rumen-protected choline and vitamin E on serum protein fractions, total thiol molecules and total antioxidant capacity in early lactating dairy cows[J]. Global Journal of Animal Scientific Research, 2014, 2(4): 337-344. |




