过去几十年,抗生素由于其能有效抑制动物肠道病原微生物的生长而被广泛应用于畜禽生产,但长期使用抗生素导致了微生物耐药性增强及在动物产品中的残留的问题,使畜牧行业认识到必须开发抗生素替代品[1]。含有大量活性物质的天然植物提取物具备抗氧化、抗菌、抗病毒、抗炎、抑制胃肠道甲烷排放等功能[2-3],被认为是具有巨大开发和应用潜力的饲料添加剂,而受到越来越多的关注。认识并优化饲用天然植物提取物的制备工艺,对揭示植物活性功能组分、生物学作用及机制,建立功能组分结构、含量和饲喂效果的关系至关重要[4]。因此,本文对主要天然植物功能组分制备方面的研究进展作一综述,旨在为未来的研究、开发和应用提供借鉴。
1 饲用天然植物活性功能组分研发前景与行业现状2018年,我国将黄芩和甘草等117种药食同源的天然植物列入《饲料原料目录》。2020年,宣布退出除中药外促生长类药物饲料添加剂品种后,农业农村部改革和完善了新饲料添加剂产品审批制度,针对天然植物提取物在事前咨询、缩短研发周期、数据共享和适度放宽检测要求方面进行了制度优化,这激发了众多企业和科研单位研制开发新产品的积极性,天然植物活性功能组分应用研究与开发将迎来新阶段。
在医药健康和食品化工等领域对植物活性物质需求的带动下,我国专业的植物提取物企业已经超过1 000家[5]。国内诸多企业已经开始针对饲用提取物产品进行投入,并开发了特定产品,如黄芪提取物和丝兰提取物等[6]。但是,针对饲用产品开发的专业化企业数量还非常少,且规模小,不具备自主研发能力,产品制备方法不一、标准缺乏、价格较高。虽然一些产品已进行了饲喂试验,但在畜禽生产上并不具备效益优势,限制了饲用天然植物活性功能组分在动物上的商业化应用。这与中小企业在提取、分离、纯化和鉴定所需的新技术应用缺乏、制备工艺优化不足有关。
另外,科学研究方面,动物营养学家往往对功能组分制备这一环节认识不足。诚然,现代色谱和光谱技术的发展使生物活性化合物的分析比以前更容易,但产品效果仍然取决于制备方法和优化的参数等[7]。制备方法不同有可能导致同类产品在动物上的研究数据难以相互比较,影响了研究结果的深入解读和归纳总结。而随着制备仪器和工艺的不断发展,植物活性功能组分的提取效率、分离纯度和鉴定准确度正在不断提高,这也将有助于其生理活性的全面研究,促进饲用开发和产业升级。
1 主要制备工艺概述饲用天然植物活性功能组分包括酚类、生物碱、挥发油、苷类、多糖和有机酸等。要获得大量且较纯的植物活性功能组分是一个复杂的过程,涉及几个步骤,包括植物初步筛选、提取、分离纯化和结构解析等[8]。图 1展示了饲用天然植物活性功能组分制备研究的基本流程。
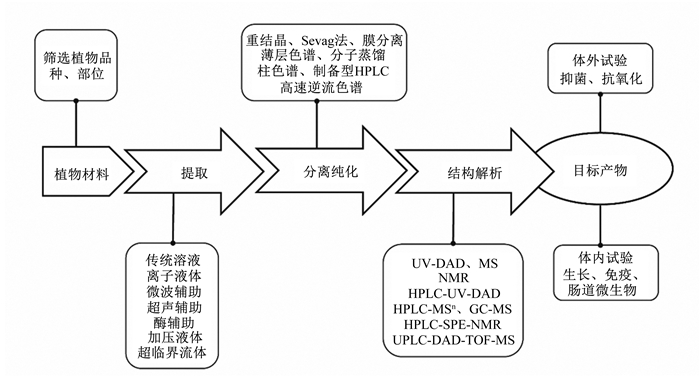
|
UV-DAD: 紫外-光电二极管阵列检测器ultra violet-diode-array detector;MS: 质谱mass spectrum;NMR:核磁共振nuclear magnetic resonance;HPLC-MSn:高效液相色谱-质谱联用high performance liquid chromatography-mass spectrum;GC-MS:气相色谱-质谱联用gas chromatography-mass spectrum;HPLC-SPE-NMR:高效液相-固体萃取-核磁共振联用high performance liquid chromatography-solid phase extraction-nuclear magnetic resonance;UPLC-DAD-TOF-MS:超高压液相色谱-电二极管阵列检测器-飞行时间质谱ultra-performance liquid chromatography-diode-array detector-time of flight-mass spectrum。 图 1 天然植物活性功能组分制备工艺流程 Fig. 1 Flow chart of natural plant active functional component preparation[9] |
第1步是选择植物品种和部位。一方面,可根据文献报道及传统的应用经验,评估某种植物试验数据的可靠性,选择合适的物种和部位;另一方面,基于化学分类学研究,也就是说,某些植物类别含有特定类型的化合物或次级代谢物,因此与它们的分类相关的植物也可能含有相同的化合物或代谢物。选择植物后,要收集和鉴定植物材料,即收集特定植物或其部分,如叶、茎、树皮、花、种子或根,以供进一步分析[10]。
第2步是活性功能组分的提取。传统溶剂萃取(CSE)主要包括液液萃取(LLE)、固相萃取(SPE)和固相微萃取(SPME)。CSE工艺虽然简便易上手,但制备效率低,耗费大量有机溶剂,造成环境污染。近年来,超声辅助萃取(UAE)、微波辅助萃取(MAE)、酶解辅助萃取(EAE)、加压液体萃取(PLE)、亚临界水萃取(SWE)、瞬时控制压降萃取(DIC)、超临界二氧化碳萃取(SCE)等新型绿色方法开始涌现[7]。
第3步是活性功能组分的分离纯化。植物材料的粗提过程中会有多种杂质同时被提取出来,因此,活性功能组分的分离纯化十分有必要。传统的分离纯化方法主要是重结晶法、纸色谱(PC)、柱色谱(CC)和薄层色谱(TLC)。CC和TLC因其方便、经济,适用多种固定相而仍被广泛使用。新型的分离方法有分子蒸馏(MD)、凝胶渗透色谱(GPC)、离子交换色谱(IEC)、大孔吸附树脂色谱(MARC)、制备型高效液相色谱法(Pre-HPLC)、高速逆流色谱法(HSCCC)、超临界流体色谱法(SFC)和分子印迹技术(MIT)等。表 1总结了主要的新型绿色提取、分离纯化工艺的原理、所需仪器设备和优缺点。天然植物功能组分多样且复杂,制备工艺也呈现出多样化特征[22-23]。
|
|
表 1 天然植物活性功能组分的主要新型制备工艺 Table 1 Main new preparation technology of natural plant active functional components |
第4步是活性功能组分的结构解析。通常采用的手段包括薄层色谱、柱色谱、快速色谱、高效液相色谱-傅里叶变换红外光谱、气相色谱-质谱、液相色谱-质谱、核磁共振光谱、HPLC-二极管阵列检测、毛细管电泳-二极管阵列检测、电喷雾等[24]。最后是设计体内、体外试验对活性功能组分进行生物学效果验证。
所有这些过程中,活性功能组分提取和分离纯化所用的时间占比最多,因此选择合适的制备方法不仅关系到目标产物得率和纯度,也关系到结构解析的准确性,更关系到体内、体外评价的可靠性。基于文献总结,图 2展示了饲用天然植物六大活性功能组分常用的制备方法,下文详细介绍这些活性组分的提取和分离纯化研究进展。
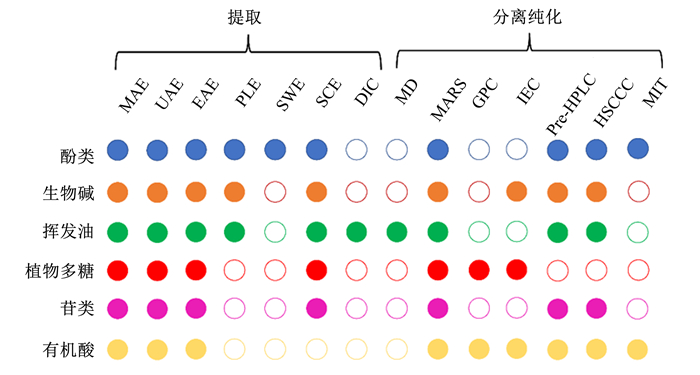
|
MAE:微波辅助萃取microwave-assisted extraction;UAE:超声辅助萃取ultrasound-assisted extraction;EAE:酶解辅助萃取enzyme-assisted extraction;PLE:加压液体萃取pressurized liquid extraction;SWE:亚临界水萃取subcritical water extraction;SCE:超临界二氧化碳萃取supercritical CO2 extraction;DIC:瞬时控制压降萃取instant controlled pressure-drop;MD:分子蒸馏molecular distillation;MARC:大孔吸附树脂色谱macroporous adsorption resin chromatography;GPC:凝胶渗透色谱gel permeation chromatography;IEC:离子交换色谱ion exchange chromatography;Pre-HPLC:制备型高效液相色谱preparative high performance liquid chromatography;HSCCC:高速逆流色谱high-speed counter-current chromatography;MIT:分子印迹技术molecular imprinting technique。实心圆圈表示常用方法solid circles denote common methods。 图 2 不同植物活性功能组分常用的制备工艺 Fig. 2 Common preparation technology of different natural plant active functional components |
酚类化合物是饲用天然植物功能组分中最大的一类,结构及分子质量丰富多变,在畜禽上被证明具有抗氧化、提高免疫力等功能,主要包括酚酸、黄酮、黄酮醇、二氢黄酮醇、异黄酮、香豆素、花青素和多酚等。表 2列举了国内外部分关于酚类化合物制备方法研究中的植物来源、方法、优化条件。众多提取方法中,MAE和UAE的方法较为成熟,应用最广,最具代表性[25]。Kumar等[26]报道,采用CSE、EAE和MAE提取大豆皮中的花青素,发现MAE优化后的产量为5 094.9 mg/L,而CSE和EAE分别为(1 246.89±68.45) mg/L和(4 064.77±163.23) mg/L,可见微波辅助具有显著优势。Goltz等[27]研究表明,与CSE相比,在优化条件下,应用UAE使甘菊花中酚类物质产量提高了6.1倍,抗氧化能力提高了3.4倍。因此,UAE可以提高酚类物质的回收率并减少溶剂用量,降低成本,可以作为CSE的最佳替代方法之一。此外,植物基质的酶解可以释放结合的酚类物质,果胶酶、纤维素酶、半纤维素酶、淀粉酶等被证明可以用于酚类物质的提取[7]。
|
|
表 2 酚类化合物的新型制备工艺 Table 2 New preparation technology of phenolic compounds |
常用的酚类纯化方法主要有MARC、超滤膜法和反相HPLC[28]。应用最多的是MARC,大孔树脂是一种常用的有机高分子吸附剂,已广泛应用于植物活性物质的分离纯化,特别是黄酮类化合物。树脂的选择应考虑其结构和极性,如表面积、孔径、极性较低的树脂对极性低或非极性化合物有较强的吸附能量[29]。Zhang等[30]采用EAE提取红松中的黄酮,然后用大孔树脂进行纯化,使黄酮的纯度从33.8%提升到61.7%,进而体外试验证明了纯化产物具有较强的抗氧化性。另外,金属络合也非常适用黄酮类化合物的分离纯化,利用黄酮和金属离子形成黄酮-金属离子络合物。经过滤,提取物中不能与金属反应的杂质被去除,然后,具有较强络合能力的物质与络合物反应,捕获黄酮-金属离子络合物中的金属离子,并释放出黄酮类成分,从而增加黄酮类化合物的含量。该方法简单、高效,容易实现工业化。如Wang等[31]采用MAE结合胶束萃取了甘草中的黄酮,然后采用金属络合+反溶剂重结晶法纯化,黄酮纯度从36.47%提高到90.32%,且具有较强的2, 2-联苯基-1-苦基肼基(DPPH)自由基清除能力。
Pre-HPLC是经典的分离纯化手段,柱效高、分离重复性好,可进行紫外-质谱在线检测,因而被广泛使用。Pre-HPLC可进一步分为一维(1D-RPLC)和二维(正相×反相,2D-NPLC/RPLC),其中二维在分离复杂混合物上效率更高。研究较多还有HSCCC,它是一种无载体的全液体分配色谱系统。与硅胶、制备型反相HPLC等方法相比,具有样品回收率高、耗时少、环保等特点[32]。因此它特别适用于从植物中分离纯化高极性化合物(如多酚)。由于其优越的分离能力和回收率,HSCCC在天然植物产物的制备中的应用正在稳步增长[33]。例如,Shu等[32]首次采用HSCCC从芍药花中分离了8种酚类化合物,且纯度均高于97%。鉴于酚类化合物分布的广泛性及其改善动物健康的重要性,大规模的工业化生产是发展趋势,因此,探索低成本的Pre-HPLC和HSCCC将极大促进畜牧业对植物酚类物质的利用。
2.2 生物碱生物碱是存在于天然植物中的碱性含氮有机化合物,大多含有复杂的环状结构。饲用上有研究的生物碱包括苦参碱、石松碱、血根碱,来源包括苦参根、石松、野百合、博落回等。传统工艺提取生物碱普遍使用的有机溶剂有己烷、甲醇、乙醇等,通过这些溶剂萃取成功制备了生物碱,并证实了它们的许多特性,如制备后产物得抗胆碱酯酶、抗炎、抗菌、抗真菌和抗病毒活性等[42]。应用新方法SCE、PLE、MAE等提取生物碱[42-43],缩短了提取时间,减少了溶剂消耗,提高了产物质量。与有机溶剂相比,深共晶溶剂(DES)具有低毒和生物可降解等优点,被证明是适合生物碱的新型高效萃取介质[44]。Kang等[45]首次采用DES提取苦参根中的苦参碱,结果表明,DES-2(氯化胆碱∶丙二酸=1 ∶ 2,与50%水)和DES-8(氯化胆碱∶乙二醇=1 ∶ 2,与30%水)产物得率最高,达到21.04 mg/g。另外,多技术组合应用也是研究的热点,如Zhang等[43]组合MAE与双水相萃取(APTE)从苦参根中提取苦参碱;Tang等[46]组合MAE与浊点萃取,使用Triton X-100-NaCl-HCl制备体系提取野百合中生物碱,这些方法的优化和组合使得生物碱得率更高。
生物碱的分离应用较多的是CC,研究方向主要集中在填料选择,包括大孔树脂、硅胶等。研究表明,吸附生物碱应选择弱极性树脂,以AB-8、D-101和HPD100应用最多,需要优化上样量、吸附温度和样品液浓度等[47]。但由于大孔树脂质量参差不齐,导致纯化效果不稳定,往往回收率很低,特别是含量低的生物碱容易损失。硅胶作为另一种常用的吸附剂也被不断改进,质量比大孔树脂更稳定,Azadbakht等[48]和Mishig等[49]采用硅胶分别作为TLC和快速柱色谱的填料成功分离了秋水仙和蒙古莸的生物碱。除了常见的大孔树脂和硅胶外,羟丙基葡聚糖凝胶填料(Sephadex-20 LH)作为新型填料也进入研究人员视线,它是以分子筛作用力为主、兼具分配作用机制、分离效率良好的填料,可在化合物分离后期使用。例如,Xiu等[50]采用乙醇粗提马齿苋生物碱,然后运用正-反相硅胶柱色谱分离,再采用Sephadex-20 LH柱色谱进行分离得到了一种具有抗乙酰胆碱酯酶的新生物碱。表 3列举了采用新型工艺制备生物碱的研究。
|
|
表 3 生物碱的新型制备工艺 Table 3 New preparation technology of alkaloid |
挥发油也被称作精油,是具有挥发性芳香气味的物质,以萜类为主,包括单帖、倍半萜以及含氧衍生物。饲用上关注较多有牛至精油、大蒜精油、桉树精油、沙葱精油、艾叶精油、肉桂精油等。传统的制备方法是水蒸馏法,但产量较低[58]。UAE、MAE和EAE的应用有效提高了挥发油萃取效率。目前研究最多的是SCE[59],其不使用易爆或有毒溶剂,无毒性残留物、产物得率高、芳香族化合物保留率较好,适合分离植物精油[60]。Wang等[60]应用优化的SCE使莎草精油得率达到1.82%,是传统提取方法的3倍多。但是,尹浩等[61]用3种方法酶辅助溶剂萃取法(4 h)、酶辅助水蒸气蒸馏法(4 h)和SCE(30 min)提取油樟叶精油,结果显示,SCE工艺效率虽高,但产物得率和抑菌活性均次于MAE和水蒸气蒸馏法。这说明对于SCE的应用仍需要进一步验证优化。
挥发油组成较为复杂,直接采用色谱分离纯化很难获得单一成分。因此,挥发油一般需要经过MD初步分离后,再进行色谱分离[62]。MD是新型的液蒸馏技术,适用热敏性化合物的分离,可与SCE联合用于挥发油等组分的提取分离[15]。随后,可采用GS-MS对挥发油进一步分离鉴定。Marques等[20, 63]用HSCCC分别从胡椒叶和红叶果的水蒸馏产物中成功分离了4种和3种挥发油组分,纯度均高于90%,用时低于2 h。进而,Wang等[64]建立了离线DPPH-GC-MS-HSCCC流程,先用DPPH和GC-MS筛选抗氧化活性强的温郁金精油,后采用HSCCC进行分离,这是一套高效的精油筛选、鉴定和分离方法,具有分离分辨率高、检测灵敏度高、结构鉴定容易,目标化合物可以定向分离。总之,挥发油的提取、分离和纯化界限往往并不明显,SCE同时兼有提取和纯化的能力,但SCE的维持成本较高,目前还处于实验室小试阶段,对于饲用天然植物挥发油的制备仍是巨大挑战。表 4列举了采用新工艺的研究进展。
|
|
表 4 挥发油的新型制备工艺 Table 4 New preparation technology of volatile oil |
植物多糖是由多个单糖以糖苷键形式连接起来的大分子活性物质。目前,沙蒿多糖、茯苓多糖、马齿苋多糖、当归多糖、黄芪多糖、苜蓿多糖、枸杞多糖等已在畜禽上开展了饲喂效果评价,证明植物多糖具备抗炎、抗氧化、调节机体免疫功能,可作为新型饲料添加剂[70]。植物多糖的研究较多,因而提取工艺也更成熟,应用最广泛的方法是水提醇沉法,其利用多糖的羟基与水易形成氢键、而不易与醇类形成氢键的特点,加入乙醇即可使多糖沉淀,缺点是容易造成了有效成分的大量流失。另一种传统的制备方法是热水提取,但存在提取时间长、温度高等缺点。Ying等[71]比较了3种方法即CSE、MAE和UAE提取桑叶多糖的效果,发现UAE优化条件的得率较高,这表明了UAE在制备多糖上的优势。也有报道称,MAE制备可能导致部分多糖降解,因此使用MAE时,微波功率和时间需要进一步优化[72]。Dong等[73]则检验了MAE-UAE联合提取方法,产物枸杞多糖的抗氧化活性比传统方法更高,体现了方法组合的提取优势。但是,也有报道称,EAE同样可以用于植物多糖的提取,如Wang等[74]组合纤维素酶、果胶酶、木瓜蛋白酶,优化提取条件,使苜蓿多糖得率达到5.05%。但EAE很少单独使用,通常与其他提取方法组合使用,以提高多糖的得率。
植物多糖的分离纯化工艺相对简单。粗多糖的提取液往往与蛋白质、色素、无机小分子等杂质混在一起,需要将这些杂质去除,以免影响多糖的质量和纯度及后续结构表征的可靠性。在众多植物多糖的纯化方法中,常用的是Sevag法、MARC、GPC和IEC等[75]。Sevag法利用氯仿-正丁醇溶液可以将游离蛋白变性成为不溶性物质,从而过滤蛋白,是多糖制备除蛋白最有效的方法,应用最为普遍[17, 76]。除蛋白之后,一般需要再经CC进一步纯化。种分离纯化色谱柱组合使用可以取得更好的效果。Fan等[18]对藜麦种子多糖粗提物进行Sevag法除蛋白,然后使用离子交换柱DEAE-52 Cellulose洗脱,洗脱液装载到凝胶柱Sephadex G-50,进一步纯化得到7种多糖。基于文献总结,植物多糖分离纯化常用的离子交换色谱柱包括DEAE-Cellulose、DEAE-Cellulose 52、DEAE-Sepharose CL-6B和DEAE-Sepharose FF等,凝胶渗透色谱柱有Sephadex G、Sephacryl S和Sepharose CL[77]。植物多糖分离纯化方法简单经济,但如何选择最佳的色谱柱和纯化条件优化仍鲜有报道,针对种类繁多的饲用植物多糖,分离纯化工艺的进一步优化选择是今后重点研究的方向。表 5列举了采用新工艺提取植物多糖的研究。
|
|
表 5 植物多糖的新型制备工艺 Table 5 New preparation technology of plant polysaccharide |
苷类是糖或糖的衍生物与另一非糖物质通过糖的端基碳原子连接而成的一类化合物,又被称为配糖体。按化学结构类型,可以分为香豆素苷、蒽醌苷、皂苷、黄酮苷等。饲用上关注度最高的是皂苷,其可调控反刍动物瘤胃发酵,抑制甲烷排放。除了MAE和UAE外,新型绿色溶剂DES也普遍用于苷类的制备,如Milani等[84]采用DES(氯化四甲铵+乙二醇)提取甜叶菊中的甜菊醇苷,辅以超声萃取,试验结果表明,UAE+DES提取的甜菊糖苷是传统溶剂的3倍,该研究证明了UAE+DES在提取苷类化合物上的优势。目前,高速剪切均质萃取(HSHE)和离子液体(IL)萃取是苷类制备工艺的研究热点。高速剪切技术是一种新型的均质粉碎技术,已用于多种天然植物活性物质的制备。Xu等[85]使用59%乙醇作为溶剂,采用HSHE提取甜叶菊中制备甜菊糖苷,证明HSHE的制备效率比MAE和UAE更高,这也为苷类制备提出了一个新思路。IL具有不挥发或极低挥发性、热稳定性好、溶解性强等特点,是新型的绿色溶剂。Ji等[86]研究证明,与传统UAE相比,基于IL溶剂(1-丁基-3-甲基咪唑醋酸盐)的UAE(IL-UAE)对甘草微观结构的破坏更大,从而使得黄酮苷和三萜皂苷提取效率更高,且IL-UAE的提取时间短,液料比小。因此,IL作为绿色萃取溶剂在苷类萃取上具有广阔的应用前景。
苷类的分离纯化方面,国内外应用最多的MARC、Pre-HPLC和HSCCC等。在提取苷类成分时,亲水成分如糖和单宁常成为粗提物中的杂质。弱极性大孔树脂吸附杂质后,杂质容易被水洗脱,然后用不同乙醇洗下吸附的苷类,得到纯度较高的目标化合物,此方法在皂苷的分离中十分常用。根据许多文献中皂苷制备的大孔树脂吸附和解析特性,证明AB-8和D101型吸附量大、易解析且重复性能好,是分离苷类化合物较为理想的树脂类型[87]。但是,在分离粗提物中多聚体与苷类方面,CC还存在不足,而Pre-HPLC和HSCCC作为先进的分离技术可以有效地实现两者分离。李媛媛等[88]采用HSCCC,从红葡萄皮中分离出3种花色苷,纯度均高于95%。另外,MARC也可联合Pre-HPLC或HSCCC制备分离苷类。例如,Li等[89]制备荷叶青中的皂苷,先用D101树脂纯化,再用硅胶柱色谱洗脱,然后结合Pre-HPLC分离得到4种三萜皂苷。因此,优化以MARC为代表的柱色谱与Pre-HPLC联用,发挥各自特点,是苷类化合物高效提取的关键。表 6列举了苷类化合物的新型制备工艺。
|
|
表 6 苷类的新型制备工艺 Table 6 Novel preparation technology of glycosides |
有机酸是存在于天然植物中的具有羧基的一类有机化合物,如草酸、苹果酸、苯甲酸、水杨酸、绿原酸、柠檬酸、富马酸、阿魏酸、咖啡酸等。有机酸的酸性可以降低动物肠道pH,抑制有害菌,有利于肠道有益菌生长,从而促进养分消化吸收,也被视作替代抗生素的新型饲料添加剂[97]。相较于其他功能组分,有机酸的制备工艺受到的关注度较低。饲用上关注度较高的阿魏酸主要采用酶法提取,Lau等[98]采用阿魏酸酯酶(0.02 U/g)和木聚糖酶(3 475.3 U/g)提取玉米穗富含的阿魏酸,产物得率达到了1.69 g/kg,提取效率明显提高。封易成等[99]联合应用离子液体、酶解和超声辅助(IL-EAE-UAE)并优化条件提取山楂中柠檬酸,与水浸提法比较,2种方法提取到的柠檬酸含量接近,但前者提取时间是25.3 min,远低于后者的4 h。可见,UAE与EAE组合运用可加强对植物细胞壁的破坏,释放更多有机酸,两者在协同萃取有机酸上具有明显优势。
有机酸分离纯化方面,以MARC、Pre-HPLC、HSCCC和pH区带精制逆流色谱(pH-zone-refining CCC)等方法为主。胡居吾等[100]联用大孔树脂306与GPC,进而使用Pre-HPLC对蔓三七叶中的绿原酸和异绿原酸进行分离制备,两者纯度均达到96%以上,具有良好的自由基清除作用。HSCCC的制备工艺也在不断得到优化,如溶剂体系的改良。张伟等[101]将离子液体[C6min] [PF6]作为HSCCC两相溶剂体系的改良剂,分离制备金银花提取物中的绿原酸,纯度为97.5%。pH-zone-refining CCC是近年来在传统逆流色谱上发展起来特殊逆流色谱分配技术,根据化合物的解离常数和疏水性质差异而实现分离,上样量是传统HSCCC的十几倍,且目标产物纯度更高,非常适合生物碱和有机酸的制备[102]。Tang等[102]为制备分离麦麸中阿魏酸的2种立体异构体,采用了两步分离措施,先使用HSCCC(己烷-乙酸乙酯-甲醇-水=2 ∶ 5 ∶ 2 ∶ 4),再运用pH-zone-refining CCC (己烷-乙酸乙酯-乙腈-水= 2 ∶ 5 ∶ 2 ∶ 2)得到反式-阿魏酸和顺式-阿魏酸的纯度分别为99%和98%,说明了pH-zone-refining CCC分离制备有机酸上的高纯度优势。表 7列举了采用新工艺制备有机酸的研究进展。
|
|
表 7 有机酸的新型制备工艺 Table 7 New preparation technology of organic acid |
卢德勋[108]于2004年首次提出了饲料营养活性物质组学理论,强调多种营养活性物质组合和整体功能,产品应用应融入动物营养工程技术体系,这为活性功能组分产品研发提供了理论指导。当前,虽然涌现了以微波、超声、酶、超临界流体等为代表的新型辅助萃取手段,以及以柱色谱、Pre-HPLC和HSCCC等新型分离纯化方法,但方法学研究也存在诸多问题。1)制备工艺的研究大多只关注某植物中的一种(类)活性功能组分,多种植物活性功能组分在动物体内代谢可能产生协同效应提高生物活性和生物利用度,因此对于单一组分追求太高纯度,则可能造成制备成本提高。2)制备工艺优化的条件不一,即使同是MAE和UAE,但选择的优化参数不同,导致大量研究无法进行比较和归纳;研究结果更注重产物的得率,而对制备过程中植物活性功能组分的分子结构损害程度缺乏关注,而这直接关系到制备后物质的生物活性。3)研究文献多基于实验室规模,大量试验数据是在小试条件下优化的,优化的工艺参数与工厂化差距大,甚至不适合工业化生产。
对此,适合畜禽生产的天然植物活性功能组分制备工艺体系亟待建立。1)应进一步优化工艺,吸纳各种组分制备的特点,建立并优化同时提取多种组分的工艺,分离纯化后要结合结构解析和体外体内试验综合评价多组分提取物的相互作用,确定适宜的纯度,最大化地利用天然植物的价值。2)建立制备工艺优化的标准体系,明确MAE、UAE、EAE、IL、SCE等独立或联合应用应优化的条件,针对不同植物及不同目标化合物,建立一套优化参数值的参考范围。制备工艺研究不仅要关注产物的得率,也要注重植物材料的来源和标准化,采用光谱等检测手段,评估产物的降解或结构受损情况。3)以市场化为导向,联合畜牧企业开展调研,可选定某一类植物或某一类活性功能组分作为突破口,建立适合工业化生产的质量控制参数,广泛开展中试试验,降低制备成本。此外,组分制备应充分认识到产品融入饲用技术体系的必要性,考虑活性功能组分与饲粮营养成分的相互作用、动物不同生理阶段、不同饲养决策目标和环境因素等,以使活性物质在动物体内达到精准营养效果。
4 小结天然植物是一个十分复杂的体系,从繁多的植物中提取和分离各种活性功能组分,是促进天然植物活性功能组分饲用化必须要解决的课题。本文总结了植物各活性功能组分的主要制备方法,重点介绍了具有饲用潜力的六大活性组分制备研究进展。未来,应重点推进绿色制备工艺优化,加强方法的联用,强调制备效率,提高目标化合物得率和纯度。针对畜牧业特点,建立从植物筛选到组分制备,再到体内、体外试验,从实验室研究到工厂化生产的研发流程,以不断开发低成本、环境友好且利于动物生长和健康的植物活性功能组分产品。
| [1] |
BATIHA G E S, ALKAZMI L M, WASEF L G, et al. Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities[J]. Biomolecules, 2020, 10(2): 202. DOI:10.3390/biom10020202 |
| [2] |
OH J, HARPER M, GIALLONGO F, et al. Effects of rumen-protected Capsicum oleoresin on immune responses in dairy cows intravenously challenged with lipopolysaccharide[J]. Journal of Dairy Science, 2017, 100(3): 1902-1913. DOI:10.3168/jds.2016-11666 |
| [3] |
PANIAGUA M, CRESPO J, BACH A, et al. Effects of flavonoids extracted from Citrus aurantium on performance, eating and animal behavior, rumen health, and carcass quality in Holstein bulls fed high-concentrate diets[J]. Animal Feed Science and Technology, 2018, 246: 114-126. DOI:10.1016/j.anifeedsci.2018.08.010 |
| [4] |
熊安然, 熊本海, 蒋林树. 基于指纹图谱技术的饲料营养活性物质结构-谱-量-效关系的研究进展[J]. 动物营养学报, 2021, 33(8): 4263-4270. XIONG A R, XIONG B H, JIANG L S. Research progress on structure-spectrum-quantity-effect relationship of feed nutritional active substances based on fingerprint technology[J]. Chinese Journal of Animal Nutrition, 2021, 33(8): 4263-4270 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.08.007 |
| [5] |
范小燕. 植物提取物全球市场对比研究与我国发展对策[D]. 硕士学位论文. 上海: 上海交通大学, 2018. FAN X Y. Comparative study of global market on botanic extracts and the development strategy for China[D]. Master's Thesis. Shanghai: Shanghai Jiaotong University, 2018. (in Chinese) |
| [6] |
曾建国. 植物提取物及其饲料添加剂注册开发建议[J]. 饲料工业, 2020, 41(10): 1-8. ZENG J G. Plant extracts and recommendations for the registration and development of plant extract feed additives[J]. Feed Industry, 2020, 41(10): 1-8 (in Chinese). |
| [7] |
KUMAR M, DAHUJA A, TIWARI S, et al. Recent trends in extraction of plant bioactives using green technologies: a review[J]. Food Chemistry, 2021, 353: 129431. DOI:10.1016/j.foodchem.2021.129431 |
| [8] |
PATRA J K, DAS G, LEE S, et al. Selected commercial plants: a review of extraction and isolation of bioactive compounds and their pharmacological market value[J]. Trends in Food Science and Technology, 2018, 82: 89-109. DOI:10.1016/j.tifs.2018.10.001 |
| [9] |
BRUSOTTI G, CESARI I, DENTAMARO A, et al. Isolation and characterization of bioactive compounds from plant resources: the role of analysis in the ethnopharmacological approach[J]. Journal of Pharmaceutical and Biomedical Analysis, 2014, 87: 218-228. DOI:10.1016/j.jpba.2013.03.007 |
| [10] |
ROOPAN S M, DEVI RAJESWARI V, KALPANA V N, et al. Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview[J]. Applied Microbiology and Biotechnology, 2016, 100(3): 1153-1162. DOI:10.1007/s00253-015-7190-0 |
| [11] |
AJILA C M, BRAR S K, VERMA M, et al. Extraction and analysis of polyphenols: recent trends[J]. Critical Reviews in Biotechnology, 2011, 31(3): 227-249. DOI:10.3109/07388551.2010.513677 |
| [12] |
AZMIR J, ZAIDUL I S M, RAHMAN M M, et al. Techniques for extraction of bioactive compounds from plant materials: a review[J]. Journal of Food Engineering, 2013, 117(4): 426-436. DOI:10.1016/j.jfoodeng.2013.01.014 |
| [13] |
JOANA GIL-CHÁVEZ G, VILLA J A, FERNANDO AYALA-ZAVALA J, et al. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview[J]. Comprehensive Reviews in Food Science and Food Safety, 2013, 12(1): 5-23. DOI:10.1111/1541-4337.12005 |
| [14] |
ROMBAUT N, TIXIER A S, BILY A, et al. Green extraction processes of natural products as tools for biorefinery[J]. Biofuels Bioproducts and Biorefining, 2014, 8(4): 530-544. DOI:10.1002/bbb.1486 |
| [15] |
ZHONG J L, MUHAMMAD N, CHEN S Q, et al. Pilot-scale supercritical CO2 extraction coupled molecular distillation and hydrodistillation for the separation of essential oils from Artemisia argyi Lévl. et Vant[J]. Separation Science and Technology, 2021, 56(18): 3127-3135. DOI:10.1080/01496395.2021.1875239 |
| [16] |
PANG J M, DONG W J, LI Y H, et al. Purification of Houttuynia cordata Thunb. essential oil using macroporous resin followed by microemulsion encapsulation to improve its safety and antiviral activity[J]. Molecules, 2017, 22(2): 293. DOI:10.3390/molecules22020293 |
| [17] |
陆娟, 谢东雪, 贺柳洋, 等. 洋甘菊多糖的分离纯化、性质结构及抗氧化活性分析[J]. 食品与发酵工业, 2021, 47(3): 72-78. LU J, XIE D X, HE L Y, et al. Purification, structure analysis and antioxidant activity of polysaccharides from Matricaria chamomilla L.[J]. Food and Fermentation Industries, 2021, 47(3): 72-78 (in Chinese). |
| [18] |
FAN S H, LI J N, BAI B Q. Purification, structural elucidation and in vivo immunity-enhancing activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds[J]. Bioscience, Biotechnology, and Biochemistry, 2019, 83(12): 2334-2344. DOI:10.1080/09168451.2019.1650635 |
| [19] |
TURNER M W, ROSSI M, CAMPFIELD V, et al. Steroidal alkaloid variation in Veratrum californicum as determined by modern methods of analytical analysis[J]. Fitoterapia, 2019, 137: 104281. DOI:10.1016/j.fitote.2019.104281 |
| [20] |
MARQUES A M, DE AQUINO V H C, CORREIA V G, et al. Isolation of two major sesquiterpenes from the leaf essential oil of Eugenia uniflora by preparative-scale high-speed countercurrent chromatography[J]. Separation Science Plus, 2018, 1(12): 785-792. |
| [21] |
NOLVACHAI Y, KULSING C, BOYSEN R I, et al. Miniaturized molecularly imprinted polymer extraction method for the gas chromatographic analysis of flavonoids[J]. Journal of Separation Science, 2014, 37(8): 1018-1025. DOI:10.1002/jssc.201301009 |
| [22] |
潘予琮, 蒋林树, 熊本海. 指纹图谱技术及其在饲料营养活性物质评价中的应用研究进展[J]. 动物营养学报, 2020, 32(9): 4044-4052. PAN Y C, JIANG L S, XIONG B H. Research progress of fingerprinting technique and its application in evaluation of nutricines in feeds[J]. Chinese Journal of Animal Nutrition, 2020, 32(9): 4044-4052 (in Chinese). |
| [23] |
YAHYA N A, ATTAN N, WAHAB R A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds[J]. Food and Bioproducts Processing, 2018, 112: 69-85. DOI:10.1016/j.fbp.2018.09.002 |
| [24] |
SCHEFER A B, BRAUMANN U, TSENG L H, et al. Application of high-performance liquid chromatography-nuclear magnetic resonance coupling to the identification of limonoids from mahogany tree (Switenia macrophylla, Meliaceae) by stopped-flow 1D and 2D NMR spectroscopy[J]. Journal of Chromatography A, 2006, 1128(1/2): 152-163. |
| [25] |
PERERA C O, ALZAHRANI M A J. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: a review[J]. LWT, 2021, 142: 111114. DOI:10.1016/j.lwt.2021.111114 |
| [26] |
KUMAR M, DAHUJA A, SACHDEV A, et al. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat[J]. International Journal of Biological Macromolecules, 2019, 135: 1070-1081. DOI:10.1016/j.ijbiomac.2019.06.034 |
| [27] |
GOLTZ C, ÁVILA S, BARBIERI J B, et al. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts[J]. Industrial Crops and Products, 2018, 115: 227-234. DOI:10.1016/j.indcrop.2018.02.013 |
| [28] |
FAN Y P, FU Y H, FU Q, et al. Purification of flavonoids from licorice using an off-line preparative two-dimensional normal-phase liquid chromatography/reversed-phase liquid chromatography method[J]. Journal of Separation Science, 2016, 39(14): 2710-2719. DOI:10.1002/jssc.201501393 |
| [29] |
LIU Y F, LIU J X, CHEN X F, et al. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins[J]. Food Chemistry, 2010, 123(4): 1027-1034. DOI:10.1016/j.foodchem.2010.05.055 |
| [30] |
ZHANG M Y, MA W C, WANG C, et al. Optimization of enzyme-assisted extraction and purification of flavonoids from Pinus koraiensis nut-coated film and antioxidant activity evaluation[J]. Molecules, 2021, 26(7): 1950. DOI:10.3390/molecules26071950 |
| [31] |
WANG Z J, ZHAO X H, ZHAO D M, et al. Ultrasonic microwave-assisted micellar extraction and purification of flavonoids from licorice by metal complex and antisolvent recrystallization[J]. LWT, 2021, 147: 111501. DOI:10.1016/j.lwt.2021.111501 |
| [32] |
SHU X K, DUAN W J, LIU F, et al. Preparative separation of polyphenols from the flowers of Paeonia lactiflora Pall. by high-speed counter-current chromatography[J]. Journal of Chromatography B, 2014, 947/948: 62-67. DOI:10.1016/j.jchromb.2013.12.004 |
| [33] |
WANG X, LIU J H, GENG Y L, et al. Preparative separation of alkaloids from Nelumbo nucifera Gaertn by pH-zone-refining counter-current chromatography[J]. Journal of Separation Science, 2010, 33(4/5): 539-544. |
| [34] |
NAYAK B, DAHMOUNE F, MOUSSI K, et al. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels[J]. Food Chemistry, 2015, 187: 507-516. DOI:10.1016/j.foodchem.2015.04.081 |
| [35] |
KOYU H, KAZAN A, OZTURK T K, et al. Optimizing subcritical water extraction of Morus nigra L. fruits for maximization of tyrosinase inhibitory activity[J]. The Journal of Supercritical Fluids, 2017, 127: 15-22. DOI:10.1016/j.supflu.2017.03.007 |
| [36] |
KRAKOWSKA A, RAFIŃSKA K, WALCZAK J, et al. Enzyme-assisted optimized supercritical fluid extraction to improve Medicago sativa polyphenolics isolation[J]. Industrial Crops and Products, 2018, 124: 931-940. DOI:10.1016/j.indcrop.2018.08.004 |
| [37] |
王虹玲, 吴优, 于子箫, 等. 酿酒葡萄皮渣中白藜芦醇的提取及抗氧化、抗肿瘤活性研究[J]. 中国酿造, 2018, 37(7): 112-116. WANG H L, WU Y, YU Z X, et al. Extraction, antioxidant and antitumor activity of resveratrol from wine grape pomace[J]. China Brewing, 2018, 37(7): 112-116 (in Chinese). |
| [38] |
MUNEKATA P E S, ALCÁNTARA C, ŽUGČIĆ T, et al. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary[J]. Food Research International, 2020, 134: 109242. DOI:10.1016/j.foodres.2020.109242 |
| [39] |
崔艳平, 聂玮, 迟晓君, 等. 蒲公英多酚提取工艺优化及抗氧化活性研究[J]. 安徽农业科学, 2021, 49(8): 175-180. CUI Y P, NIE W, CHI X J, et al. Study on optimization of extraction technology and antioxidant activity of dandelion polyphenols[J]. Journal of Anhui Agricultural Sciences, 2021, 49(8): 175-180 (in Chinese). DOI:10.3969/j.issn.0517-6611.2021.08.046 |
| [40] |
张静, 高园, 万力, 等. 大孔吸附树脂分离纯化葡萄枝条中多酚类物质[J]. 西北农业学报, 2013, 22(3): 173-177. ZHANG J, GAO Y, WAN L, et al. Macroporous resin adsorption for purification of grape polyphenols from grapevine cane[J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2013, 22(3): 173-177 (in Chinese). |
| [41] |
李媛, 谢岩黎. 制备型色谱柱对黑豆皮花青素分离纯度的影响[J]. 河南工业大学学报(自然科学版), 2017, 38(6): 21-25. LI Y, XIE Y L. Purification of anthocyanins from black bean coats by preparative chromatographic columns[J]. Journal of Henan University of Technology (Natural Science Edition), 2017, 38(6): 21-25 (in Chinese). DOI:10.3969/j.issn.1673-2383.2017.06.004 |
| [42] |
DYMEK A, WIDELSKI J, WOJTANOWSKI K K, et al. Optimization of pressurized liquid extraction of lycopodiaceae alkaloids obtained from two Lycopodium species[J]. Molecules, 2021, 26(6): 1626. DOI:10.3390/molecules26061626 |
| [43] |
ZHANG W, ZHU D, FAN H J, et al. Simultaneous extraction and purification of alkaloids from Sophora flavescens Ait. by microwave-assisted aqueous two-phase extraction with ethanol/ammonia sulfate system[J]. Separation and Purification Technology, 2015, 141: 113-123. DOI:10.1016/j.seppur.2014.11.014 |
| [44] |
JIANG Z M, WANG L J, GAO Z, et al. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents[J]. Microchemical Journal, 2019, 145: 345-353. DOI:10.1016/j.microc.2018.10.057 |
| [45] |
KANG X, DENG L L, QUAN T, et al. Selective extraction of quinolizidine alkaloids from Sophora flavescens Aiton root using tailor-made deep eutectic solvents and magnetic molecularly imprinted polymers[J]. Separation and Purification Technology, 2021, 261: 118282. DOI:10.1016/j.seppur.2020.118282 |
| [46] |
TANG X Y, ZHU D, HUAI W B, et al. Simultaneous extraction and separation of flavonoids and alkaloids from Crotalaria sessiliflora L. by microwave-assisted cloud-point extraction[J]. Separation and Purification Technology, 2017, 175: 266-273. DOI:10.1016/j.seppur.2016.11.038 |
| [47] |
刘岩, 陈伟豪, 亢迪, 等. 大孔树脂分离富集生物碱类成分研究进展[J]. 中草药, 2020, 51(6): 1650-1659. LIU Y, CHEN W H, KANG D, et al. Research progress on macroporous resin application in enriching and separating alkaloids[J]. Chinese Traditional and Herbal Drugs, 2020, 51(6): 1650-1659 (in Chinese). |
| [48] |
AZADBAKHT M, DAVOODI A, HOSSEINIMEHR S J, et al. Tropolone alkaloids from Colchicum kurdicum (Bornm.) Stef. (Colchicaceae) as the potent novel antileishmanial compounds; purification, structure elucidation, antileishmanial activities and molecular docking studies[J]. Experimental Parasitology, 2020, 213: 107902. DOI:10.1016/j.exppara.2020.107902 |
| [49] |
MISHIG D, GRUNER M, LÜBKEN T, et al. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge[J]. Scientific Reports, 2021, 11(1): 13740. DOI:10.1038/s41598-021-93010-4 |
| [50] |
XIU F, LI X, ZHANG W, et al. A new alkaloid from Portulaca oleracea L. and its antiacetylcholinesterase activity[J]. Natural Product Research, 2019, 33(18): 2583-2590. DOI:10.1080/14786419.2018.1460833 |
| [51] |
叶甜, 谢翰祥, 严喜鸾. 桑叶中生物碱提取工艺的优化和功能活性研究[J]. 中国食物与营养, 2019, 25(9): 42-45. YE T, XIE H X, YAN X L. Optimization of extraction process and functional activity of alkaloids from mulberry leaves[J]. Food and Nutrition in China, 2019, 25(9): 42-45 (in Chinese). DOI:10.3969/j.issn.1006-9577.2019.09.010 |
| [52] |
郑梅霞, 苏海兰, 朱育菁. 博落回生物碱提取工艺优化[J]. 食品安全质量检测学报, 2020, 11(10): 3087-3091. ZHENG M X, SU H L, ZHU Y J. Optimization of extraction of alkaloids from Macleaya cordata[J]. Journal of Food Safety & Quality, 2020, 11(10): 3087-3091 (in Chinese). |
| [53] |
ZOU S H, WANG C H, LI J, et al. Microwave assisted solid phase microextraction for extraction and selective enrichment of four alkaloids in lotus leaf[J]. Sustainable Chemistry and Pharmacy, 2020, 18: 100345. DOI:10.1016/j.scp.2020.100345 |
| [54] |
李雪丽, 李静雅, 刘晓芳, 等. SP825树脂分离纯化益母草总黄酮和总生物碱的研究[J]. 中央民族大学学报(自然科学版), 2012, 21(1): 28-31. LI X L, LI J Y, LIU X F, et al. Separation and purification of total flavonoid and total alkaloid from leonurus japonicus with SP825 macroporous resin[J]. Journal of Minzu University of China (Natural Sciences Edition), 2012, 21(1): 28-31 (in Chinese). DOI:10.3969/j.issn.1005-8036.2012.01.007 |
| [55] |
刘德明, 陈淼芬, 王莹, 等. 高速逆流色谱法分离黄柏中4种生物碱的方法研究[J]. 湖南农业科学, 2018(12): 82-87. LIU D M, CHEN M F, WANG Y, et al. Separation of four alkaloids from phellodendron by high speed countercurrent chromatography[J]. Hunan Agricultural Sciences, 2018(12): 82-87 (in Chinese). |
| [56] |
LUO C M, YI F L, XIA Y L, et al. Comprehensive quality evaluation of the lateral root of Aconitum carmichaelii Debx. (Fuzi): simultaneous determination of nine alkaloids and chemical fingerprinting coupled with chemometric analysis[J]. Journal of Separation Science, 2019, 42(5): 980-990. |
| [57] |
LIU Y Y, WANG W J, CHE F F, et al. Isolation and purification of alkaloids from the fruits of Macleaya cordata by ionic-liquid-modified high-speed counter-current chromatography[J]. Journal of Separation Science, 2020, 43(12): 2459-2466. DOI:10.1002/jssc.201901242 |
| [58] |
LAWAL O A, OYEDEJI A O. Chemical composition of the essential oils of Cyperus rotundus L. from South Africa[J]. Molecules, 2009, 14(8): 2909-2917. DOI:10.3390/molecules14082909 |
| [59] |
ARUMUGHAM T, K R, HASAN S W, et al. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications-a review[J]. Chemosphere, 2021, 271: 129525. DOI:10.1016/j.chemosphere.2020.129525 |
| [60] |
WANG H W, LIU Y Q, WEI S L, et al. Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn.[J]. Food Chemistry, 2012, 132(1): 582-587. DOI:10.1016/j.foodchem.2011.10.075 |
| [61] |
尹浩, 王晨笑, 岳进, 等. 油樟叶精油的提取、化学成分、抗氧化以及抗菌活性研究[J]. 保鲜与加工, 2020, 20(4): 183-191. YIN H, WANG C X, YUE J, et al. Extraction of cinnamomum longipaniculatum essential oil and its chemical composition, antioxidation and antibacterial properties[J]. Storage and Process, 2020, 20(4): 183-191 (in Chinese). DOI:10.3969/j.issn.1009-6221.2020.04.029 |
| [62] |
李慧, 娄利峰, 李秀歌. 北五味子挥发油分离提纯及成分分析[J]. 食品科学, 2014, 35(14): 73-75. LI H, LOU L F, LI X G. Separation, extraction and component analysis of essential oil from Schisandra chinensis (Turcz.) Baill. fruits[J]. Food Science, 2014, 35(14): 73-75 (in Chinese). DOI:10.7506/spkx1002-6630-201414014 |
| [63] |
MARQUES A M, PEIXOTO A C C, PROVANCE D W, J r, et al. Separation of volatile metabolites from the leaf-derived essential oil of Piper mollicomum Kunth (Piperaceae) by high-speed countercurrent chromatography[J]. Molecules, 2018, 23(12): 3064. DOI:10.3390/molecules23123064 |
| [64] |
WANG X, ZUO G L, WANG C Y, et al. An off-line DPPH-GC-MS coupling countercurrent chromatography method for screening, identification, and separation of antioxidant compounds in essential oil[J]. Antioxidants, 2020, 9(8): 702. DOI:10.3390/antiox9080702 |
| [65] |
MARTINEZ-CORREA H A, BITENCOURT R G, KAYANO A C A V, et al. Integrated extraction process to obtain bioactive extracts of Artemisia annua L. leaves using supercritical CO2, ethanol and water[J]. Industrial Crops and Products, 2017, 95: 535-542. DOI:10.1016/j.indcrop.2016.11.007 |
| [66] |
王娣, 程柏, 丁莉, 等. 百里香精油的提取工艺及化学成分分析[J]. 中国调味品, 2019, 44(7): 76-80, 94. WANG D, CHENG B, DING L, et al. Extraction technology and chemical composition analysis of thyme essential oil[J]. China Condiment, 2019, 44(7): 76-80, 94 (in Chinese). DOI:10.3969/j.issn.1000-9973.2019.07.016 |
| [67] |
齐鑫裕, 孙涛, 张颖, 等. 枸杞精油的提取及工艺优化[J]. 山东化工, 2019, 48(20): 3-5. QI X Y, SUN T, ZHANG Y, et al. Extraction and process optimization of Lycium barbarum essential oil[J]. Shandong Chemical Industry, 2019, 48(20): 3-5 (in Chinese). DOI:10.3969/j.issn.1008-021X.2019.20.003 |
| [68] |
YU F, WAN N, ZHENG Q, et al. Effects of ultrasound and microwave pretreatments on hydrodistillation extraction of essential oils from Kumquat peel[J]. Food Science & Nutrition, 2021, 9(5): 2372-2380. |
| [69] |
LIU Z Z, LI H L, CUI G Q, et al. Efficient extraction of essential oil from Cinnamomum burmannii leaves using enzymolysis pretreatment and followed by microwave-assisted method[J]. LWT, 2021, 147: 111497. DOI:10.1016/j.lwt.2021.111497 |
| [70] |
吴香云, 刘亚娜, 周喆麒, 等. 多糖类化合物的抗菌作用及其机制研究进展[J]. 畜牧兽医学报, 2020, 51(6): 1167-1176. WU X Y, LIU Y N, ZHOU Z Q, et al. Research progress on antibacterial effect and mechanism of polysaccharides[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(6): 1167-1176 (in Chinese). |
| [71] |
YING Z, HAN X X, LI J R. Ultrasound-assisted extraction of polysaccharides from mulberry leaves[J]. Food Chemistry, 2011, 127(3): 1273-1279. DOI:10.1016/j.foodchem.2011.01.083 |
| [72] |
CHEN Y, YAO F K, MING K, et al. Polysaccharides from traditional Chinese medicines: extraction, purification, modification, and biological activity[J]. Molecules, 2016, 21(12): 1705. DOI:10.3390/molecules21121705 |
| [73] |
DONG J, WANG Z, WANG Y. Rapid extraction of polysaccharides from fruits of Lycium barbarum L.[J]. Journal of Food Biochemistry, 2011, 35(4): 1047-1057. DOI:10.1111/j.1745-4514.2010.00433.x |
| [74] |
WANG S P, DONG X F, TONG J M. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity[J]. International Journal of Biological Macromolecules, 2013, 62: 387-396. DOI:10.1016/j.ijbiomac.2013.09.029 |
| [75] |
SIMAYI Z, ROZI P, YANG X J, et al. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: systematic review[J]. International Journal of Biological Macromolecules, 2021, 183: 387-398. DOI:10.1016/j.ijbiomac.2021.04.099 |
| [76] |
周先泰, 陈蓉, 齐娜. 竹叶多糖提取分离及体外抗氧化自由基的研究[J]. 广州化工, 2021, 49(6): 80-83, 111. ZHOU X T, CHEN R, QI N. Extraction and separation of polysaccharides from bamboo and study on antioxidant free radicals in vitro[J]. Guangzhou Chemical Industry, 2021, 49(6): 80-83, 111 (in Chinese). DOI:10.3969/j.issn.1001-9677.2021.06.026 |
| [77] |
LUAN F, JI Y F, PENG L X, et al. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: a review[J]. Carbohydrate Polymers, 2021, 261: 117863. DOI:10.1016/j.carbpol.2021.117863 |
| [78] |
WANG J L, ZHANG J, WANG X F, et al. A comparison study on microwave-assisted extraction of Artemisia sphaerocephala polysaccharides with conventional method: molecule structure and antioxidant activities evaluation[J]. International Journal of Biological Macromolecules, 2009, 45(5): 483-492. DOI:10.1016/j.ijbiomac.2009.09.004 |
| [79] |
ZHAO Y, SHI Y Y, YANG H X, et al. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity[J]. International Journal of Biological Macromolecules, 2016, 88: 44-50. DOI:10.1016/j.ijbiomac.2016.01.113 |
| [80] |
李青宇, 孟哲, 王磊. 响应面法优化超临界CO2提取甘草多糖及抗氧化活性研究[J]. 食品工业, 2017, 38(9): 1-5. LI Q Y, MENG Z, WANG L. Optimization of supercritical carbon dioxide extraction of polysaccharide from glycyrrhiza using response surface methodology and its antioxidant activities[J]. The Food Industry, 2017, 38(9): 1-5 (in Chinese). DOI:10.3969/j.issn.1006-6195.2017.09.001 |
| [81] |
SONG Y R, HAN A R, LIM T G, et al. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects[J]. International Journal of Biological Macromolecules, 2019, 128: 546-555. DOI:10.1016/j.ijbiomac.2019.01.131 |
| [82] |
SHU X, ZHANG Y F, JIA J X, et al. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda[J]. International Journal of Biological Macromolecules, 2019, 128: 858-869. DOI:10.1016/j.ijbiomac.2019.01.214 |
| [83] |
魏晨业, 包晓玮, 王娟, 等. 沙棘多糖分离纯化及抗氧化活性[J]. 食品科学, 2021, 42(4): 227-232. WEI C Y, BAO X W, WANG J, et al. Isolation, purification and antioxidant activity of polysaccharides from the fruit of hippophae rhamnoides[J]. Food Science, 2021, 42(4): 227-232 (in Chinese). |
| [84] |
MILANI G, VIAN M, CAVALLUZZI M M, et al. Ultrasound and deep eutectic solvents: an efficient combination to tune the mechanism of steviol glycosides extraction[J]. Ultrasonics Sonochemistry, 2020, 69: 105255. DOI:10.1016/j.ultsonch.2020.105255 |
| [85] |
XU S H, WANG G Y, GUO R L, et al. Extraction of steviol glycosides from Stevia rebaudiana (Bertoni) leaves by high-speed shear homogenization extraction[J]. Journal of Food Processing and Preservation, 2019, 43(12): e14250. |
| [86] |
JI S, WANG Y J, SU Z Y, et al. Ionic liquids-ultrasound based efficient extraction of flavonoid glycosides and triterpenoid saponins from licorice[J]. RSC Advances, 2018, 8(25): 13989-13996. DOI:10.1039/C8RA01056K |
| [87] |
刘洋, 刘海霞, 马先红. 天然植物中苷类化合物提取纯化方法的研究进展[J]. 吉林化工学院学报, 2013, 30(9): 13-17. LIU Y, LIU H X, MA X H. Development of extraction and separation methods of the glycosidic compounds from natural plants[J]. Journal of Jilin Institute of Chemical Technology, 2013, 30(9): 13-17 (in Chinese). DOI:10.3969/j.issn.1007-2853.2013.09.004 |
| [88] |
李媛媛, 李灵犀, 崔艳, 等. 高速逆流色谱法分离红葡萄皮中的花色苷[J]. 中国酿造, 2017, 36(2): 157-161. LI Y Y, LI L X, CUI Y, et al. Isolation of anthocyanins from red grape skins by high-speed countercurrent chromatography[J]. China Brewing, 2017, 36(2): 157-161 (in Chinese). |
| [89] |
LI F, WU S T, QU M H, et al. Studies on isolation and structural identification of saponins from the herb Hylomecon japonica and their bioactivities[J]. Carbohydrate Research, 2021, 507: 108391. DOI:10.1016/j.carres.2021.108391 |
| [90] |
YILMAZ F M, GÖRGVÜÇ A, UYGUN Ö, et al. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques[J]. Separation Science and Technology, 2021, 56(5): 936-948. DOI:10.1080/01496395.2020.1743311 |
| [91] |
WANG Y, PENG B, ZHAO J, et al. Efficient extraction and determination of prenylflavonol glycosides in Epimedium pubescens Maxim. using deep eutectic solvents[J]. Phytochemical Analysis, 2020, 31(3): 375-383. DOI:10.1002/pca.2904 |
| [92] |
卢珊. 响应面设计的紫甘蓝花色苷色素提取技术研究[J]. 中国调味品, 2021, 46(2): 159-162. LU S. Study on the extraction technology of anthocyanin pigments from purple cabbage by response surface design[J]. China Condiment, 2021, 46(2): 159-162 (in Chinese). DOI:10.3969/j.issn.1000-9973.2021.02.035 |
| [93] |
LIU F, MA N, XIA F B, et al. Preparative separation of minor saponins from Panax notoginseng leaves using biotransformation, macroporous resins, and preparative high-performance liquid chromatography[J]. Journal of Ginseng Research, 2019, 43(1): 105-115. DOI:10.1016/j.jgr.2017.09.003 |
| [94] |
许永, 蔡为荣, 闻志莹, 等. 荷花花色苷的分离、纯化及鉴定[J]. 安徽工程大学学报, 2020, 35(3): 31-38. XU Y, CAI W R, WEN Z Y, et al. Seperation, purification and identification of anthocyanins from lotus[J]. Journal of Anhui Polytechnic University, 2020, 35(3): 31-38 (in Chinese). DOI:10.3969/j.issn.2095-0977.2020.03.006 |
| [95] |
XU T T, WU X M. Preparative separation of mangiferin glycosides by high speed counter current chromatography and comparison of their antioxidant and antitumor activities[J]. RSC Advances, 2020, 10: 25780-25785. DOI:10.1039/D0RA04307A |
| [96] |
PEIXIA L, NANXIANG G, YAN J, et al. Purification of paclobutrazol-free steroidal saponins and flavonoids from the fibrous roots of Ophiopogon japonicas[J]. Current Topics in Nutraceutical Research, 2020, 19(4): 484-492. DOI:10.37290/ctnr2641-452X.19:484-492 |
| [97] |
何荣香, 吴媛媛, 韩延明, 等. 复合有机酸对断奶仔猪生长性能、血清生化指标、营养物质表观消化率的影响[J]. 动物营养学报, 2020, 32(7): 3118-3126. HE X R, WU Y Y, HAN Y M, et al. Effects of compound organic acids on growth performance, serum biochemical indicators and nutrient apparent digestibility of weaning piglets[J]. Chinese Journal of Animal Nutrition, 2020, 32(7): 3118-3126 (in Chinese). |
| [98] |
LAU T, HARBOURNE N, ORUÑA-CONCHA M J. Optimization of enzyme-assisted extraction of ferulic acid from sweet corn cob by response surface methodology[J]. Journal of the Science of Food and Agriculture, 2020, 100(4): 1479-1485. DOI:10.1002/jsfa.10155 |
| [99] |
封易成, 牟德华. 离子液体酶法微波辅助提取山楂有机酸工艺优化及成分分析[J]. 食品研究与开发, 2019, 40(10): 87-95. FENG Y C, MOU D H. Optimization of cellulase and ionic liquid-based microwave-assisted extraction technique for organic acids from hawthorn[J]. Food Research and Development, 2019, 40(10): 87-95 (in Chinese). |
| [100] |
胡居吾, 吴磊, 涂招秀, 等. 蔓三七叶中分离绿原酸和异绿原酸及其抗氧化活性研究[J]. 天然产物研究与开发, 2019, 31(1): 38-43. HU J W, WU L, TU Z X, et al. Extraction and antioxidant activity of chlorogenic acids and isochlorogenic acids from Gynura procumbens (Lour.) Merr[J]. Natural Product Research and Development, 2019, 31(1): 38-43 (in Chinese). |
| [101] |
张伟, 董红敬, 刘伟, 等. 应用离子液体逆流色谱系统分离制备金银花中的绿原酸[J]. 食品工业, 2021, 42(2): 107-110. ZHANG W, DONG H J, LIU W, et al. Purification of chlorogenic acid from Lonicera japonica Thunb. by high-speed counter-current chromatography using[C6min][PF6] as the modifier of two-phase solvent system[J]. The Food Industry, 2021, 42(2): 107-110 (in Chinese). |
| [102] |
TANG Y Y, HAO J, FAN C, et al. Preparative separation of high-purity trans- and cis-ferulic acid from wheat bran by pH-zone-refining counter-current chromatography[J]. Journal of Chromatography A, 2021, 1636: 461772. |
| [103] |
赵文红, 冯丽然, 关二旗, 等. 均匀设计法优化麦麸阿魏酸的酶法提取工艺[J]. 食品工业, 2020, 41(5): 38-43. ZHAO W H, FENG L R, GUAN E Q, et al. Optimization of extraction process of wheat bran ferulic acid by uniform design method[J]. The Food Industry, 2020, 41(5): 38-43 (in Chinese). |
| [104] |
WU Z, LI Z J, XUE Z Z, et al. Optimization of extraction technology for determination of caffeic and chlorogenic acid in dandelion[J]. Banat's Journal of Biotechnology, 2020, 11(21): 26-37. |
| [105] |
WAN Z X, ZHU J J, TIAN R, et al. Quality evaluation for Dipacus asperoides from Enshi areas and optimization extraction of saponins and organic acids and its application[J]. Arabian Journal of Chemistry, 2021, 14(4): 103107. |
| [106] |
刘恩荔, 李青山. 大孔吸附树脂分离纯化金银花中总有机酸的研究[J]. 中草药, 2006, 37(12): 1792-1796. LIU E L, LI Q S. Separation and purification of total organic acids from immature flower of Lonicera japonica by macroporous resin[J]. Chinese Traditional and Herbal Drugs, 2006, 37(12): 1792-1796 (in Chinese). |
| [107] |
LIU M Z, LI X J, LIU Q, et al. Comprehensive profiling of α-glucosidase inhibitors from the leaves of Rubus suavissimus using an off-line hyphenation of HSCCC, ultrafiltration HPLC-UV-MS and prep-HPLC[J]. Journal of Food Composition and Analysis, 2020, 85: 103336. |
| [108] |
卢德勋. 系统动物营养学导论[M]. 北京: 中国农业出版社, 2004. LU D X. An introduction to systems-nutrition of animals[M]. Beijing: China Agriculture Press, 2004 (in Chinese). |




