2. 中国科学院亚热带农业生态研究所, 中国科学院 亚热带农业过程重点实验室, 动物营养生理学与代谢过程湖南省重点实验室, 长沙 410125;
3. 河南金百合生物科技股份有限公司, 汤阴 456150
2. Hunan Provincial Key Laboratory of Animal Nutrition Physiology and Metabolic Process, Key Laboratory of Agricultural Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China;
3. Henan Golden Lily Biotechnology Co., Ltd., Tangyin 456150, China
多胺是存在于所有生物系统中的一种脂肪胺,有季氮基团的脂肪族烃小分子,在生理pH下带有净正电荷。多胺(腐胺、亚精胺和精胺)对正常细胞生长至关重要[1]。腐胺(1,4-二氨基丁烷)和尸胺(1,5-二氨基戊烷)是各自带有2个氨基的一级二胺,而亚精胺[N-(3氨基丙基)丁烷-1,4-二胺]和精胺[N,N′-双(3氨基丙基)丁烷-1,4-二胺]分别含有3和4个氨基[2]。多胺是相对稳定的化合物,能够抵抗酸性和碱性条件,并且可以与羟基溶剂如水和醇建立氢键[3-4]。动物体内的多胺分为内源性和外源性来源,其中内源性来源是通过生物合成和相互逆转以及消化分泌物和肠道自身的催化产物;外源性来源主要是膳食和人乳,其吸收主要发生在十二指肠和空肠前段[5-6]。由于多胺是阳离子,能与DNA、ATP、磷脂、特定种类的蛋白质相互作用[7],因此多胺对细胞代谢、生长和组织更新至关重要,是哺乳动物细胞普遍存在且必不可少的成分[1]。
1 多胺的来源及代谢多胺广泛存在于动物和植物来源的食物中,含量较高的食物类别是谷物、豆类与大豆衍生物和动物肝脏[3]。青椒、玉米等食物中腐胺含量均在800 nmol/g以上;在发酵大豆、清酒酒糟、黑豆、大豆中尸胺含量超过800 nmol/g;小麦胚芽、干蘑菇、黑豆、大豆、西米菇、莲子菇、纳豆、青椒等食物中亚精胺含量超过700 nmol/g;鸡肝、猪肝、牛肝、麦胚、咸鱼子、鸡心、鳗鱼肝等食物中精胺含量超过500 nmol/g[8]。食品的加工方式和储存条件也显著影响膳食中多胺的含量。Kozová等[9]研究发现,鸡胸部和大腿在-18 ℃下储存3个月后,精胺和亚精胺的含量减少,而6个月后则明显增加,且此过程中未检测到腐胺。将鸡胸肉在有氧包装的条件下储存,精胺和亚精胺的含量与屠宰后并无显著差异。对于精胺和亚精胺, 与煮沸和炖煮相比,新鲜鸡胸肉的烘烤、烧烤和油炸造成的损失更多,造成此差异原因可能是烧烤、油炸等过程温度更高,更容易破坏精胺和亚精胺[10]。此外,成熟也可能潜在地影响亚精胺的含量,在香蕉成熟期间亚精胺含量相对稳定,几乎不受蒸煮或煎炸的影响[11]。
多胺的代谢分为合成代谢和分解代谢,合成代谢分为传统途径和替代途径。合成过程中,S-腺苷甲硫氨酸脱羧酶(S-adenosylmethionine decarboxylase, AdoMetDC)作为亚精胺合酶(spermidine synthase,SPDSY)和精胺合酶(spermine synthase,SPMSY)的氨丙基供体,SPDSY和SPMSY分别用于亚精胺和精胺的合成[12]。在多胺合成的传统途径中,精氨酸酶1(argininase 1,ARG1)将精氨酸转化为鸟氨酸,鸟氨酸通过鸟氨酸脱羧酶(ornithine decarboxylase,ODC)的脱羧作用生成腐胺,后者在SPDSY的作用下转化为亚精胺, SPMSY再将亚精胺转化为精胺[13]。多胺合成的替代途径即精氨酸通过精氨酸脱羧酶(arginine decarboxylase,ADC)脱羧产生胍丁胺,胍丁胺再通过胍丁胺酶转化为腐胺[14]。精胺可以通过亚精胺/精胺乙酰转移酶1(spermidine/spermine acetyltransferase 1,SAT1)和多胺氧化酶(polyamine oxidase,PAOX)的作用进一步分解代谢产生亚精胺和腐胺[15]。在动物体内,多胺分解代谢始于亚精胺/亚精胺N1-乙酰基转移酶(spermidine/spermidine N1-acetyltransferase,SSAT),该酶通过将乙酰基从乙酰辅酶A转移到精胺/亚精胺的N1位置来催化N1-乙酰基衍生物的形成[16]。随后,过氧化物酶体和组成型PAOX将乙酰基衍生物转化为亚精胺或腐胺,同时生成3-乙酰氨基丙醛(3-acetylaminopropanal,3-AAP)和过氧化氢(H2O2)。3-AAP可以通过醛脱氢酶和N-乙酰基-β-丙氨酸脱乙酰酶的顺序反应进一步分解为β-丙氨酸[17](图 1)。
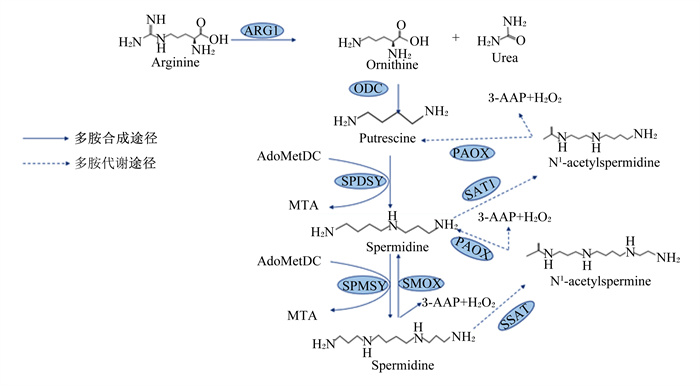
|
Arginine: 精氨酸;ARG1:精氨酸酶1 argininase 1;Ornithine: 鸟氨酸;Urea: 尿素;ODC: 鸟氨酸脱羧酶ornithine decarboxylase;putrescine:腐胺;AdoMetDC:S-腺苷甲硫氨酸脱羧酶S-adenosylmethionine decarboxylase;SAT1:亚精胺/精胺乙酰转移酶1 spermidine/spermine acetyltransferase 1;PAOX:多胺氧化酶polyamine oxidase;SSAT:亚精胺/亚精胺N1-乙酰基转移酶spermidine/spermidine N1-acetyltransferase;MTA:甲硫基腺苷methylthioadenosine;SPDSY:亚精胺合酶spermidine synthase;SPMSY:精胺合酶spermine synthase;SMOX:精胺氧化酶spermine oxidase;3-AAP:3-乙酰氨基丙醛3-acetylaminopropanal;3-AP:3-氨基丙醛3-aminopropanal;Spermidine:亚精胺;Spermine:精胺;N1-acetylspermine:N1-乙酰精胺。 图 1 多胺的合成与代谢 Fig. 1 Synthesis and metabolism of polyamines[13-17] |
肠道屏障能阻挡肠道内的内源性微生物、毒素向肠腔外组织和器官扩散,保护机体免受病原菌和毒素的侵害,而细胞增殖和迁移是维持肠上皮屏障功能的关键过程[18]。多胺在肠上皮细胞的完整性和相互作用中起着重要作用[19]。
多胺通过典型瞬时受体电位1(canonical transient receptor potential 1,TRPC1)介导Ca2+内流调节肠道上皮细胞的迁移[20]。钙离子感受器蛋白介导的钙池操纵钙内流(store-operated Ca2+ entry,SOCE)是一种重要的Ca2+信号产生机制[21]。钙释放激活钙通道蛋白(calcium release-activated calcium channel protein, Orai)是Ca2+释放激活通道的关键蛋白,基质相互作用分子1(stromal interaction molecule 1,STIM1)是Ca2+的传感器。因此,STIM1在Ca2+贮存耗尽后激活TRPC1和Orai介导的Ca2+内流中发挥重要作用[22]。多胺通过刺激蛋白质合成调节STIM1,促进STIM1转移到质膜[2]。在质膜中,STIM1激活TRPC1、Orai1,增加SOCE,促进Ca2+内流[20]。此外,多胺以电压依赖的方式阻断通道孔,引起K+通道内向整流增强[23-24]。多胺刺激电压门控钾离子通道(Kv通道)活性并导致膜的超极化,增强钙释放激活钙通道(Ca2+ release activated Ca2+,CRAC)通道活性,导致Ca2+的流入增加[25]。由于Ca2+进入是胞质游离钙离子([Ca2+]cyt)的主要来源,因此增强Ca2+内流会增加[Ca2+]cyt浓度。[Ca2+]cyt浓度的升高部分通过改变肠上皮细胞RhoA蛋白合成稳定性并增加RhoA活性,RhoA活性增加导致Rho激酶(ROK)/Rho相关激酶(ROCK)激活。ROK/ROCK的最终激活增加了肌球蛋白轻链(myosin light chain,MLC)磷酸化,促进肌动球蛋白应激纤维的形成,并刺激肠上皮细胞的迁移[26]。小鼠出生后口服精胺和亚精胺可促进肠道免疫细胞的成熟,使肠道上皮和黏膜通透性的形态学发生变化[27](图 2)。总之,多胺通过刺激STIM1和Kv通道活性介导Ca2+内流进一步促进肠道上皮细胞的迁移,调节肠道屏障的完整性。
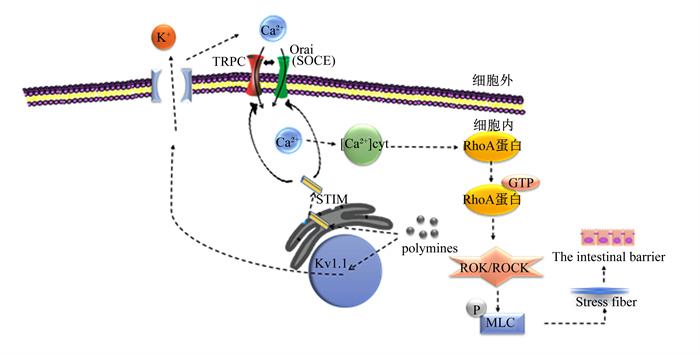
|
TRPC: 典型瞬时受体电位canonical transient receptor potential;SOCE:钙池操纵钙内流store-operated Ca2+ entry;Orai:钙释放激活钙通道蛋白calcium release-activated calcium channel protein;STIM: 基质相互作用分子stromal interaction molecule;MLC: 肌球蛋白轻链myosin light chain;ROK:Rho激酶Rho kinase;ROCK:Rho相关激酶Rho-associated kinase;Kv:电压门控钾离子通道voltage-gated potassium channel;polyamines: 多胺;The intestinal barrier: 肠道屏障;Stress fiber: 应激纤维;[Ca2+]cyt:胞质游离钙离子浓度cytoplasmic Ca2+。 图 2 多胺调节肠道上皮细胞迁移机制 Fig. 2 Mechanisms of regulation of intestinal epithelial cell migration by polyamines[28] |
多胺被称为内源性免疫调节剂,膳食多胺的摄入会提高机体精胺浓度[29]。精胺通过SSAT和PAOX转化为亚精胺。精胺和亚精胺的增加可以保护细胞和基因免受有害刺激[16]。
精胺增加能激活负反馈系统,抑制ODC和AdoMetDC的活性[16]。而AdoMetDC可以催化S-腺苷甲硫氨酸(S-adenosylmethionine,SAM)转化为脱羧SAM(dcSAM)。当AdoMetDC的活性被抑制时,机体内SAM浓度增加,而dcSAM浓度降低[30]。研究表明,SAM在许多甲基转移反应中充当甲基供体,包括DNA甲基化[16],SAM浓度的升高增强了甲基化催化酶DNA甲基转移酶(DNA methyltransferase,DNMT)的活性[31]。dcSAM浓度的降低能重新激活DNMT3a和DNMT3b[24]。在SAM存在的情况下,DNA的甲基化由DNMT1、DNMT3a和DNMT3b调节[30],因此,dcSAM浓度的降低能增强DNA甲基化。DNA过甲基化促使淋巴细胞功能相关分子-1(lymphocyte function-associated antigen-1,LFA-1)启动子区域(ITGAL)高甲基化,进而增强ITGAL(CD11a)启动子侧翼序列活性,从而抑制CD11a信使RNA表达[32],DNA过甲基化通过对编码CD11a基因的作用来抑制LFA-1[33]。LFA-1通过与内皮细胞上的细胞间黏附分子(intercellular adhesion molecules,ICAMs)结合并产生协同刺激信号抑制淋巴细胞的增殖活化、迁移和凋亡等,引发银屑病、冠心病、风湿性关节炎等一系列疾病,同时,在临床器官移植后可引发急性排斥反应等并发症[34]。DNA甲基化的异常是导致肿瘤发展、转移和恶化的重要因素[35]。因此,精胺浓度的增加能增强DNA甲基化,进一步抑制LFA-1的功能,抑制LFA-1/ICAM-1结合,从而抑制免疫功能和炎症,这为自身免疫性疾病的临床治疗、器官移植和移植排斥反应的控制提供了新方法[31](图 3)。衰老小鼠摄入含有高水平精胺和亚精胺(分别为374和1 540 nmol/g)的饮食增加了血液中多胺的浓度,并降低了年龄相关的DNA甲基化、肾小球萎缩和死亡率[29];同时,高剂量的亚精胺增加自噬活性,清除细胞中受损的蛋白质和细胞器,从而抑制由DNA甲基化引起的衰老过程[32, 36]。
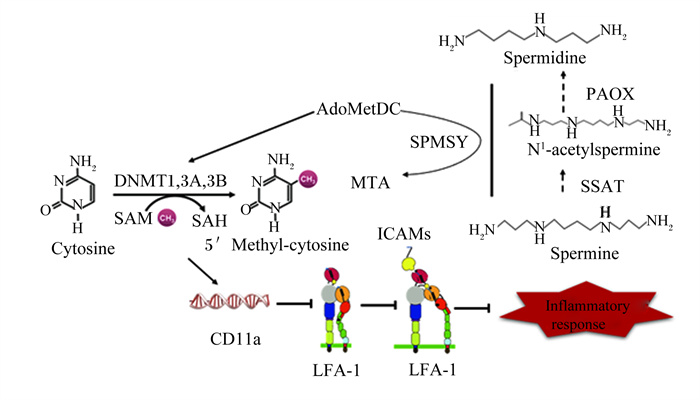
|
Spermidine: 亚精胺;Spermine: 精胺;SPMSY: 精胺合酶spermine synthase;N1-acetylspermine: N1-乙酰精胺;PAOX: 多胺氧化酶polyamine oxidase;SSAT: 亚精胺/亚精胺N1-乙酰基转移酶spermidine/spermidine N1-acetyltransferase;AdoMetDC: S-腺苷甲硫氨酸脱羧酶S-adenosylmethionine decarboxylase; MTA: 甲硫基腺苷methylthioadenosine; Cytosine: 胞嘧啶; 5'Methyl-cytosine: 5'甲基胞嘧啶; SAM: S-腺苷甲硫氨酸S-adenosylmethionine; SAH: S-腺苷同型半胱氨酸S-adenosine homocysteine; DNMT: DNA甲基转移酶DNA methyltransferase; LFA-1:淋巴细胞功能相关分子-1 lymphocyte function-associated antigen-1;ICAMs: 细胞间黏附分子intercellular adhesion molecules;inflammatory response: 炎症反应。 图 3 多胺抑制炎症反应机制 Fig. 3 Mechanisms of inhibition of inflammatory response by polyamine[37-38] |
羟自由基是已知的最强的氧化剂,几乎可以和所有的细胞成分反应,是导致机体氧化应激损伤的主要自由基类物质。羟自由基产生的最典型的途径是H2O2与呼吸链的过渡金属(如Fe)相互作用,通过芬顿型反应产生[39]。多胺与Cu2+和Zn2+等金属离子形成络合物,防止氢过氧化物的形成,并延迟二次氧化化合物的生成,从而发挥抗氧化作用[3, 40]。此外。多胺在生理酸碱度下带正电荷,可以与带负电荷的亲核大分子如DNA、磷脂和带电蛋白质紧密结合,而这些大分子通常是亲电和氧化攻击的目标[41]。多胺通过阻止它们与自由基结合发挥其抗氧化特性,防止其结构和功能的损伤[13]。
研究表明,抗氧化反应元件(antioxidant reaction element,ARE)是基础和诱导型2期基因表达的核心调节元件,可保护细胞免受环境胁迫[42]。Kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein 1,Keap1)通过与其N端结构域结合并将其保留在胞质溶胶中作为核因子E2相关因子2(nuclear factor E2 related factor 2, Nrf2)的阻遏物[43]。氧化应激刺激状态下,Keap1-Nrf2的结合稳定性降低,Nrf2被释放出来,转移到细胞核,并与ARE结合,激活下游抗氧化酶基因的转录,翻译出一系列相关蛋白,发挥其生理功能[44]。在SSAT存在的情况下,细胞内多胺的增加上调转录因子多胺调节因子-1(polyamine-modulated factor-1,PMF-1)的表达,PMF-1首先与Nrf2结合来激活转录,Nrf2启动子与肌腱膜性纤维肉瘤(musculoaponeurotic fibrosarcoma,Maf)蛋白结合形成异二聚体,并与ARE结合,通过相关激活程序,使醌氧化还原酶1[NAD(P)H: quinone oxidoreductase 1, NQO1]、谷胱甘肽硫基转移酶(glutathione-S-transferse, GST)、谷氨酰半胱氨酸连接酶(catalytic subunit glutamatecysteine ligase, GCLC)和谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)等下游基因转录[45],增强抗氧化酶mRNA的转录水平,提高抗氧化酶的活性[46](图 4)。
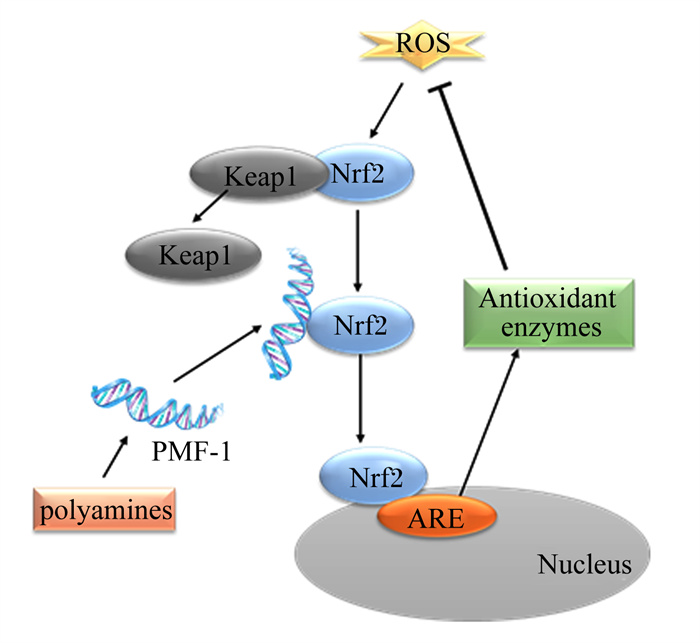
|
ROS: 活性氧自由基reactive oxygen radicals;Nrf2:核因子E2相关因子2 nuclear factor E2-related factor 2;Keap1:Kelch样环氧氯丙烷相关蛋白1 Kelch-like ECH-associated protein 1;ARE: 抗氧化反应元件antioxidant reaction element;Antioxidant enzymes: 抗氧化酶;Nucleus: 细胞核;PMF-1:多胺调节因子-1 polyamine-modulated factor-1;polyamines: 多胺。 图 4 多胺抑制氧化应激机制 Fig. 4 Mechanisms of inhibition of oxidative stress by polyamines[44] |
心肌细胞功能障碍激活了许多信号传导途径,导致心脏重塑,心脏重塑与心力衰竭密切相关[47]。外源性精胺能抑制Fas和FasL mRNA转录和蛋白质表达。而Fas/FasL是介导死亡受体通路的关键蛋白[48],Fas受体与FasL结合后,再与Fas相关死亡结构域(Fas-associated dead domains,FADD)蛋白和半胱氨酸天冬氨酸蛋白酶-8(cysteine aspartic acid specific protease-8,Caspase-8)结合形成死亡诱导信号复合体(death-inducing signaling complex,DISC),同时它激活Caspase-8,并通过激活下游相关信号通路诱导细胞凋亡,因此,精胺通过抑制Fas/FasL的转录和表达减轻心肌细胞凋亡[49]。此外,精胺还通过抑制蛋白激酶R样内质网激酶(protein kinase R-like ER kinase, PERK)-真核翻译起始因子2α(eukaryotic translation initiation factor 2α,eIF2α)途径激活来抑制内质网应激(endoplasmic reticulum stress,ERS),增强心肌细胞的活力同时降低其死亡率,从而抑制心肌细胞凋亡[50-51]。Madeo等[52]在对衰老小鼠的研究中,亚精胺已被证明能降低由年龄引起的动脉僵硬和内皮细胞的氧化损伤。此外,连续6周给小鼠饲喂精胺和亚精胺逆转了与年龄相关的心肌纤维化、心肌形态改变,并抑制了心脏的细胞凋亡[53]。综上所述,精胺能抑制ERS以及Fas、FasL mRNA转录和蛋白质表达来抑制心肌细胞凋亡,延缓心力衰竭并降低心血管疾病的发病率(图 5)。
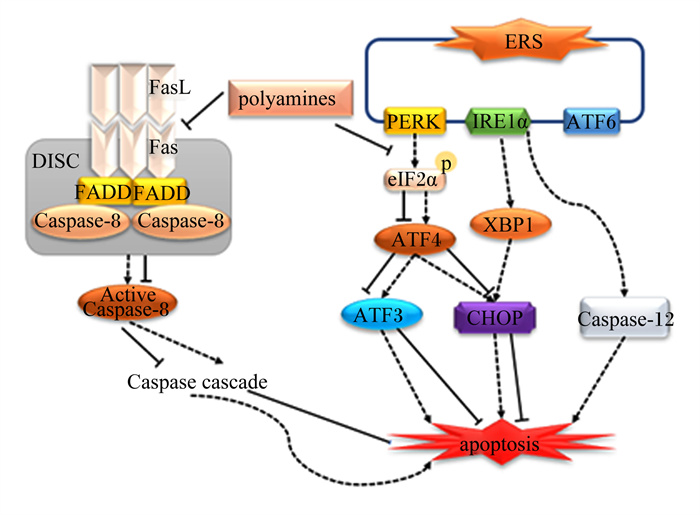
|
ERS: 内质网应激endoplasmic reticulum stress;Caspase-8:半胱氨酸天冬氨酸蛋白酶-8 cysteine aspartic acid specific protease-8;CHOP: C/EBP同源蛋白C/EBP homologous protein; Caspase-12:半胱氨酸天冬氨酸蛋白酶-12 cysteine aspartic acid specific protease-12;DISC: 死亡诱导信号复合体death-inducing signaling complex;FADD: Fas相关死亡结构域Fas-associated dead domains;ATF4:转录激活因子4 recombinant activating transcription factor 4;ATF6:转录激活因子6 recombinant activating transcription factor 6;ATF3:转录激活因子3 recombinant activating transcription factor 3;PERK: 蛋白激酶R样内质网激酶protein kinase R-like ER kinase; XBP1:X-盒结合蛋白1 X-box binding protein 1;IRE1α: 肌醇必需酶1α inositol-requiring enzyme 1α;eIF2α: 真核翻译起始因子2α eukaryotic translation initiation factor 2α。 图 5 多胺抑制心肌细胞凋亡机制 Fig. 5 Mechanisms of polyamines inhibiting myocardial cell apoptosis[48-51] |
保障动物肠道健康是提高畜禽生产经济效益的关键所在。肠道是机体最大的免疫器官和消化器官,肠道菌群可以促进消化酶的分泌,形成保护屏障调节机体免疫。Van Wettere等[54]研究发现,在断奶时(产后第23天)与对照仔猪相比,口服2 mL 463 nmol/mL的精胺溶液可以增加绒毛高度,降低隐窝深度和绒毛高度与隐窝深度比值;此外,在产后14~18 d仔猪每天口服2 mL 463或2 013 nmol/mL的精胺溶液显著提高仔猪日增重,推测仔猪日增重增加的可能原因是补充精胺改善了仔猪肠道结构,使营养物质与肠道的接触面积增加,促进了营养物质的吸收,进而使仔猪日增重增加。有研究发现,补充0.4 mmol/kg BW的精胺显著增加空肠中消化酶活性及其基因的表达水平,提高氨基酸转运体的mRNA表达水平以及甘油磷胆碱、脂质和牛磺酸浓度,提示精胺可以提高仔猪的消化能力,调节仔猪空肠氨基酸和脂质代谢[55]。仔猪早期断奶是提高生产效益的关键技术之一,研究发现,仔猪断奶可诱导氧化应激反应,造成胸腺、脾脏等内脏器官的损伤,进而影响仔猪的生长性能[56]。研究表明,饲喂0.4 mmol/kg BW的精胺可以显著提高仔猪的抗氧化能力[57]。精胺主要是通过调控Nrf2、Keap1、哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)mRNA表达水平,提高GST、GSH-Px等抗氧化酶的活性来增强机体抗氧化能力[58]。值得注意的是,在补充精胺的第6天,仔猪获得最佳抗氧化效果[58]。因此,补充精胺可以通过提高机体抗氧化能力使得胸腺、脾脏等器官免受断奶造成的氧化损伤。
Chowdhury等[59]研究了添加不同水平腐胺对蛋鸡蛋品质的改善作用,饲喂含0.05%腐胺的饲粮会导致蛋壳重量和蛋壳重量占蛋重量的百分比增加,并减少蛋壳变形;添加较高水平(0.10%和0.15%)的腐胺降低了钙沉积和钙平衡,这可能是导致蛋壳重量和蛋壳厚度降低,蛋壳变形改善的原因。有研究发现,腐胺对肉鸡生长性能的影响与年龄存在关系。在14日龄时,0.04%的腐胺对粗蛋白质的消化率产生不利影响,但在21日龄时没有观察到这种影响;在饲粮中添加0.01%、0.02%、0.03%和0.05%的腐胺能显著促进21日龄雏鸡的体重增长,主要是由于腐胺增加了肠绒毛高度和隐窝深度,从而增加了营养物质的吸收利用率[60]。需要注意的是,高水平的腐胺会使家禽出现腐胺中毒现象,根据现有研究,腐胺添加水平可控制在0.05%以内。
4 小结多胺具有调节心肌细胞的凋亡、调节肠道上皮细胞迁移、抑制氧化应激、抑制炎症等生理作用,在禁抗严峻形势下,多胺可开发为饲料添加剂。但目前多胺的研究大多集中在临床医学上,在畜禽生产上的研究较少,特别是在家禽和反刍动物上。在以往的研究中,多胺的补充仅局限于短期内添加,且研究对象大多为仔猪。因此,多胺在畜禽生产上的应用还需进一步的研究,探究多胺在畜禽生产中的最适添加量、最佳给药期等,此外,多胺与其他一些营养素和生物活性物质的配伍作用还有待进一步的探究。
| [1] |
PEGG A E. Functions of polyamines in mammals[J]. Journal of Biological Chemistry, 2016, 291(29): 14904-14912. DOI:10.1074/jbc.R116.731661 |
| [2] |
IGARASHI K, KASHIWAGI K. Modulation of protein synthesis by polyamines[J]. IUBMB Life, 2015, 67(3): 160-169. DOI:10.1002/iub.1363 |
| [3] |
MUÑOZ-ESPARZA N C, LATORRE-MORATALLA M L, COMAS-BASTÉ O, et al. Polyamines in food[J]. Frontiers in Nutrition, 2019, 6: 108. DOI:10.3389/fnut.2019.00108 |
| [4] |
AGOSTINELLI E, MARQUES M P M, CALHEIROS R, et al. Polyamines: fundamental characters in chemistry and biology[J]. Amino Acids, 2010, 38(2): 393-403. DOI:10.1007/s00726-009-0396-7 |
| [5] |
MADEO F, BAUER M A, CARMONA-GUTIERREZ D, et al. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans?[J]. Autophagy, 2019, 15(1): 165-168. DOI:10.1080/15548627.2018.1530929 |
| [6] |
RUIZ-CANO D, PÉREZ-LLAMAS F, ZAMORA S. Implicaciones de las poliaminas en la salud infantil[J]. Archivos Argentinos de Pediatría, 2012, 110(3): 244-250. DOI:10.5546/aap.2012.244 |
| [7] |
IGARASHI K, KASHIWAGI K. Modulation of cellular function by polyamines[J]. The International Journal of Biochemistry & Cell Biology, 2010, 42(1): 39-51. |
| [8] |
NISHIMURA K, SHIINA R, KASHIWAGI K, et al. Decrease in polyamines with aging and their ingestion from food and drink[J]. Journal of Biochemistry, 2006, 139(1): 81-90. DOI:10.1093/jb/mvj003 |
| [9] |
KOZOVÁ M, KALA Č P, PELIKÁNOVÁ T. Contents of biologically active polyamines in chicken meat, liver, heart and skin after slaughter and their changes during meat storage and cooking[J]. Food Chemistry, 2009, 116(2): 419-425. DOI:10.1016/j.foodchem.2009.02.057 |
| [10] |
KRAUSOVÁ P, KALA ŘP, K ČÍ Ž EK M, et al. Changes in the content of biologically active polyamines during pork loin storage and culinary treatments[J]. European Food Research and Technology, 2008, 226(5): 1007-1012. DOI:10.1007/s00217-007-0625-9 |
| [11] |
BORGES C V, BELIN M A F, AMORIM E P, et al. Bioactive amines changes during the ripening and thermal processes of bananas and plantains[J]. Food Chemistry, 2019, 298: 125020. DOI:10.1016/j.foodchem.2019.125020 |
| [12] |
IKEGUCHI Y, BEWLEY M C, PEGG A E. Aminopropyltransferases: function, structure and genetics[J]. Journal of Biochemistry, 2006, 139(1): 1-9. DOI:10.1093/jb/mvj019 |
| [13] |
LENIS Y Y, ELMETWALLY M A, MALDONADO-ESTRADA J G, et al. Physiological importance of polyamines[J]. Zygote, 2017, 25(3): 244-255. DOI:10.1017/S0967199417000120 |
| [14] |
WANG X Q, YING W, DUNLAP K A, et al. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses[J]. Biology of Reproduction, 2014, 90(4): 84. |
| [15] |
MOUNCE B C, OLSEN M E, VIGNUZZI M, et al. Polyamines and their role in virus infection[J]. Microbiology and Molecular Biology Reviews, 2017, 81(4): e00029-17. |
| [16] |
SODA K. Polyamine metabolism and gene methylation in conjunction with one-carbon metabolism[J]. International Journal of Molecular Sciences, 2018, 19(10): 3106. DOI:10.3390/ijms19103106 |
| [17] |
CASERO R A, J r, MURRAY STEWART T, PEGG A E. Polyamine metabolism and cancer: treatments, challenges and opportunities[J]. Nature Reviews Cancer, 2018, 18(11): 681-695. DOI:10.1038/s41568-018-0050-3 |
| [18] |
SHARKEY K A, BECK P L, MCKAY D M. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium[J]. Nature Reviews Gastroenterology & Hepatology, 2018, 15(12): 765-784. |
| [19] |
GUO X, RAO J N, LIU L, et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines[J]. American Journal of Physiology: Cell Physiology, 2003, 285(5): C1174-C1187. DOI:10.1152/ajpcell.00015.2003 |
| [20] |
RAO J N, RATHOR N, ZHUANG R, et al. Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2[J]. American Journal of Physiology: Cell Physiology, 2012, 303(3): C308-C317. DOI:10.1152/ajpcell.00120.2012 |
| [21] |
LIOU J, KIM M L, HEO W D, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx[J]. Current Biology, 2005, 15(13): 1235-1241. DOI:10.1016/j.cub.2005.05.055 |
| [22] |
JOHNSTONE L S, GRAHAM S J L, DZIADEK M A. STIM proteins: integrators of signalling pathways in development, differentiation and disease[J]. Journal of Cellular & Molecular Medicine, 2010, 14(7): 1890-1903. |
| [23] |
NICHOLS C G, LEE S J. Polyamines and potassium channels: a 25-year romance[J]. Journal of Biological Chemistry, 2018, 293(48): 18779-18788. DOI:10.1074/jbc.TM118.003344 |
| [24] |
OLIVER D, BAUKROWITZ T, FAKLER B. Polyamines as gating molecules of inward-rectifier K+ channels[J]. European Journal of Biochemistry, 2000, 267(19): 5824-5829. DOI:10.1046/j.1432-1327.2000.01669.x |
| [25] |
OHYA S, KITO H. Ca2+-activated K+ channel KCa3.1 as a therapeutic target for immune disorders[J]. Biological and Pharmaceutical Bulletin, 2018, 41(8): 1158-1163. DOI:10.1248/bpb.b18-00078 |
| [26] |
CHUNG H K, RATHOR N, WANG S R, et al. RhoA enhances store-operated Ca2+ entry and intestinal epithelial restitution by interacting with TRPC1 after wounding[J]. American Journal of Physiology: Gastrointestinal and Liver Physiology, 2015, 309(9): G759-G767. DOI:10.1152/ajpgi.00185.2015 |
| [27] |
PÉREZ-CANO F J, GONZÁLEZ-CASTRO A, CASTELLOTE C, et al. Influence of breast milk polyamines on suckling rat immune system maturation[J]. Developmental and Comparative Immunology, 2010, 34(2): 210-218. DOI:10.1016/j.dci.2009.10.001 |
| [28] |
WANG J Y. Cellular signaling in rapid intestinal epithelial restitution: implication of polyamines and K+ channels[J]. Acta Physiologica Sinica, 2003, 55(4): 365-372. |
| [29] |
SODA K, DOBASHI Y, KANO Y, et al. Polyamine-rich food decreases age-associated pathology and mortality in aged mice[J]. Experimental Gerontology, 2009, 44(11): 727-732. DOI:10.1016/j.exger.2009.08.013 |
| [30] |
FUKUI T, SODA K, TAKAO K, et al. Extracellular spermine activates DNA methyltransferase 3A and 3B[J]. International Journal of Molecular Sciences, 2019, 20(5): 1254. DOI:10.3390/ijms20051254 |
| [31] |
KANO Y, SODA K, KONISHI F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area[J]. PLoS One, 2013, 8(2): e56056. DOI:10.1371/journal.pone.0056056 |
| [32] |
SODA K. Spermine and gene methylation: a mechanism of lifespan extension induced by polyamine-rich diet[J]. Amino Acids, 2020, 52(2): 213-224. DOI:10.1007/s00726-019-02733-2 |
| [33] |
LU Q J, KAPLAN M, RAY D, et al. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus[J]. Arthritis and Rheumatism, 2002, 46(5): 1282-1291. DOI:10.1002/art.10234 |
| [34] |
VERMA N K, KELLEHER D. Not just an adhesion molecule: LFA-1 contact tunes the T lymphocyte program[J]. Journal of Immunology, 2017, 199(4): 1213-1221. DOI:10.4049/jimmunol.1700495 |
| [35] |
AVRAHAMI D, LI C H, ZHANG J, et al. Aging-dependent demethylation of regulatory elements correlates with chromatin state and improved β cell function[J]. Cell Metabolism, 2015, 22(4): 619-632. DOI:10.1016/j.cmet.2015.07.025 |
| [36] |
KIECHL S, PECHLANER R, WILLEIT P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study[J]. The American Journal of Clinical Nutrition, 2018, 108(2): 371-380. DOI:10.1093/ajcn/nqy102 |
| [37] |
PFLUGFELDER S C, STERN M, ZHANG S, et al. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease[J]. Journal of Ocular Pharmacology and Therapeutics, 2017, 33(1): 5-12. DOI:10.1089/jop.2016.0105 |
| [38] |
VA NDER WIJST M G P, VENKITESWARAN M, CHEN H, et al. Local chromatin microenvironment determines DNMT activity: from DNA methyltransferase to DNA demethylase or DNA dehydroxymethylase[J]. Epigenetics, 2015, 10(8): 671-676. DOI:10.1080/15592294.2015.1062204 |
| [39] |
SAVA I G, BATTAGLIA V, ROSSI C A, et al. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria[J]. Free Radical Biology & Medicine, 2006, 41(8): 1272-1281. |
| [40] |
TORO-FUNES N, BOSCH-FUSTÉ J, VECIANA-NOGUÉS M T, et al. In vitro antioxidant activity of dietary polyamines[J]. Food Research International, 2013, 51(1): 141-147. DOI:10.1016/j.foodres.2012.11.036 |
| [41] |
RIDER J E, HACKER A, MACKINTOSH C A, et al. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide[J]. Amino Acids, 2007, 33(2): 231-240. DOI:10.1007/s00726-007-0513-4 |
| [42] |
BISWAS M, CHAN J Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond[J]. Toxicology and Applied Pharmacology, 2010, 244(1): 16-20. DOI:10.1016/j.taap.2009.07.034 |
| [43] |
ZHOU F Y, ZOU X H, ZHANG J, et al. Jian-Pi-Yi-Shen formula ameliorates oxidative stress, inflammation, and apoptosis by activating the Nrf2 signaling in 5/6 nephrectomized rats[J]. Frontiers in Pharmacology, 2021, 12: 630210. DOI:10.3389/fphar.2021.630210 |
| [44] |
王秀君, 李欣, 唐修文. Nrf2通路在肿瘤化学预防中的研究进展[J]. 化学进展, 2013, 25(9): 1544-1552. WANG X J, LI X, TANG X W. The role of Nrf2 in carcinogenesis[J]. Progress in Chemistry, 2013, 25(9): 1544-1552 (in Chinese). |
| [45] |
HEISS E H, SCHACHNER D, ZIMMERMANN K, et al. Glucose availability is a decisive factor for Nrf2-mediated gene expression[J]. Redox Biology, 2013, 1(1): 359-365. DOI:10.1016/j.redox.2013.06.001 |
| [46] |
KWAK M K, KENSLER T W, CASERO R A, J r. Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2:effect of the polyamine metabolite acrolein[J]. Biochemical and Biophysical Research Communications, 2003, 305(3): 662-670. DOI:10.1016/S0006-291X(03)00834-9 |
| [47] |
BRAUNWALD E, BRISTOW M R. Congestive heart failure: fifty years of progress[J]. Circulation, 2000, 102(20 Suppl 4): V14-IV23. |
| [48] |
LIU X M, YANG Z M, LIU X K. Fas/FasL induces myocardial cell apoptosis in myocardial ischemia-reperfusion rat model[J]. European Review for Medical and Pharmacological Sciences, 2017, 21(12): 2913-2918. |
| [49] |
韩丽萍, 李鸿珠, 姜春明, 等. 精胺抑制模拟缺血-再灌注心肌细胞Fas/FasL的表达[J]. 中国病理生理杂志, 2010, 26(4): 630-634. HAN L P, LI H Z, JIANG C M, et al. Spermine inhibits expression of Fas/FasL in simulated ischemia-reperfusion-injured cardiomyocytes of neonatal rat[J]. Chinese Journal of Pathophysiology, 2010, 26(4): 630-634 (in Chinese). DOI:10.3969/j.issn.1000-4718.2010.04.002 |
| [50] |
WEI C, WANG Y H, LI M X, et al. Spermine inhibits endoplasmic reticulum stress-induced apoptosis: a new strategy to prevent cardiomyocyte apoptosis[J]. Cellular Physiology and Biochemistry, 2016, 38(2): 531-544. DOI:10.1159/000438648 |
| [51] |
赵苗, 韩雅茹, 贺翼飞, 等. PERK/eIF2α信号通路在心肌保护中的研究进展[J]. 世界最新医学信息文摘, 2018, 18(102): 169-171. ZHAO M, HAN Y R, HE Y F, et al. PERK/eIF2α signaling pathway and its role in myocardial protection[J]. World Latest Medicine Information, 2018, 18(102): 169-171 (in Chinese). |
| [52] |
MADEO F, EISENBERG T, PIETROCOLA F, et al. Spermidine in health and disease[J]. Science, 2018, 359(6374): eaan2788. DOI:10.1126/science.aan2788 |
| [53] |
ZHANG H, WANG J, LI L, et al. Spermine and spermidine reversed age-related cardiac deterioration in rats[J]. Oncotarget, 2017, 8(39): 64793-64808. DOI:10.18632/oncotarget.18334 |
| [54] |
VAN WETTERE W H E J, WILLSON N L, PAIN S J, et al. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics[J]. Animal, 2016, 10(10): 1655-1659. DOI:10.1017/S1751731116000446 |
| [55] |
LIU G M, MO W W, CAO W, et al. Digestive abilities, amino acid transporter expression, and metabolism in the intestines of piglets fed with spermine[J]. Journal of Food Biochemistry, 2020, 44(5): e13167. |
| [56] |
LUO Z, ZHU W, GUO Q, et al. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets[J]. Oxidative Medicine and Cellular Longevity, 2016, 2016: 4768541. |
| [57] |
CAO W, XU X, JIA G, et al. Roles of spermine in modulating the antioxidant status and Nrf2 signalling molecules expression in the thymus and spleen of suckling piglets-new insight[J]. Journal of Animal Physiology and Animal Nutrition, 2018, 102(1): e183-e192. DOI:10.1111/jpn.12726 |
| [58] |
FANG T, ZHENG J, CAO W, et al. Effects of spermine on the antioxidant status and gene expression of antioxidant-related signaling molecules in the liver and longissimus dorsi of piglets[J]. Animal, 2018, 12(6): 1208-1216. DOI:10.1017/S1751731117002737 |
| [59] |
CHOWDHURY S R, SMITH T K. Effects of dietary 1, 4-diaminobutane (putrescine) on eggshell quality and laying performance of hens laying thin-shelled eggs[J]. Poultry Science, 2001, 80(12): 1702-1709. DOI:10.1093/ps/80.12.1702 |
| [60] |
HASHEMI S M. Growth performance and intestinal morphology of broilers fed low protein and low methionine diets supplemented with putrescine[J]. Ph.D.Thesis.Serdang: Universiti Putra Malaysia, 2013, 46-50. |




