鹰嘴豆素A(biochanin A)是植物生长过程中产生的次级代谢产物,图 1为其化学结构。鹰嘴豆素A主要存在于红三叶草和鹰嘴豆等豆科植物,属于异黄酮类植物雌激素,是大豆异黄酮的主要成分之一,占异黄酮总量的40%,其分子结构和大小类似于雌激素,尤其是雌二醇(17-β-雌二醇),可以发挥雌激素作用[1],与人类的健康密切相关。随着人们对饮食平衡和健康生活追求的不断提高,对鹰嘴豆素A生物活性的研究也更加深入。大量体内和体外研究结果表明,鹰嘴豆素A具有抗炎[2-3]、抗氧化[4-5]、抗菌[6-8]和抗癌[9-12]等生物学功能。除此之外,鹰嘴豆素A还能抗骨质疏松[13]、预防和治疗心血管疾病[14]、维持认知[15]以及缓解妇女绝经后的症状[13],是开发新型保健品和药品重要的潜在来源。大豆异黄酮已经被多个国家列入食物保健品名录,鹰嘴豆素A也因其广泛的生物学功能而闻名。近年来,人们开始关注鹰嘴豆素A对反刍动物瘤胃发酵和生产性能的影响,但是已有的文献报道基本都是体外发酵研究,体内研究较少,关于单胃动物的研究还未见报道。
|
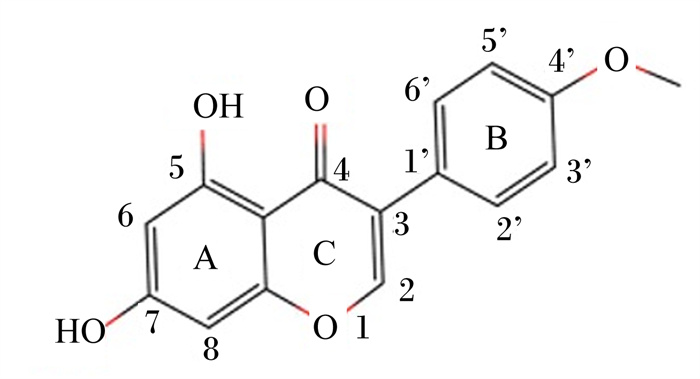
图 1 鹰嘴豆素A的化学结构 Fig. 1 Chemical structure of biochanin A |
鹰嘴豆素A是豆科植物中异黄酮类植物雌激素的主要成分之一,最早发现于红三叶草的茎和叶[16]。除了红三叶草,人们从花生[17]、大豆[6]和鹰嘴豆[18]中也都分离得到了鹰嘴豆素A。研究表明,红三叶草中鹰嘴豆素A的含量最高,其中红三叶草叶和芽的干物质中含量分别为10.30和1.62 mg/g[19-20];鹰嘴豆的干物质中鹰嘴豆素A的含量为1.58 mg/g[18],而花生的干物质中鹰嘴豆素A的含量只有0.76 mg/100 g[17]。除此之外,鹰嘴豆素A的天然来源还包括苜蓿[21]和传统中药黄芪[22]等豆科植物,但是鹰嘴豆素A的含量非常低。表 1总结了几种鹰嘴豆素A的常见来源和含量。
|
|
表 1 不同来源干物质中鹰嘴豆素A的含量 Table 1 Contents of biochanin A in dry matter of different sources |
炎症通常被认为是宿主对组织损伤的应答或机体对感染或任何其他刺激的保护性攻击,在慢性炎症的情况下,免疫细胞、基质细胞和癌细胞在微环境中共存,这些细胞协同产生炎症介质,调节炎症信号通路,如生长因子和细胞因子[14]。
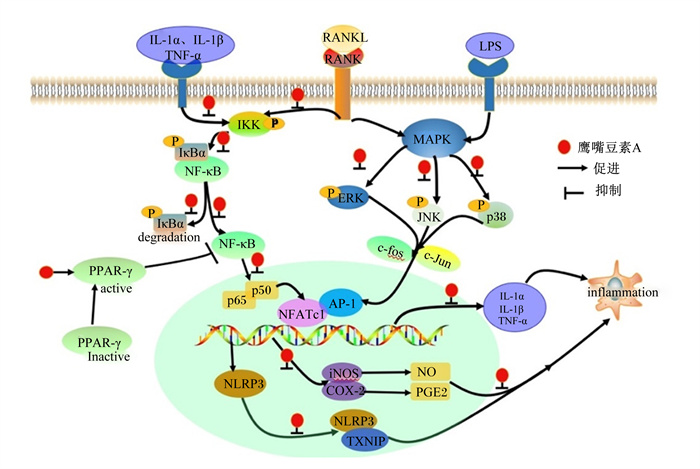
鹰嘴豆素A在核因子-κB受体激活剂配体(receptor-activated nuclear factor-κB ligand, RANKL)诱导的过度激活破骨细胞中,通过下调肿瘤坏死因子-α(TNF-α)、白细胞介素-1α(IL-1α)和白细胞介素-1β(IL-1β)的分泌水平抑制炎症反应;鹰嘴豆素A还通过抑制包括p38丝裂原活化蛋白激酶(p38 mitogen-activated protein kinase, p38)、细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)与c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)在内的丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)和包括核因子-κB抑制蛋白α(IκBα)与p65丝裂原活化蛋白激酶(p65 mitogen-activated protein kinase, p65)在内的核因子-κB(NF-κB)信号通路,下调活化T细胞1和c-Fos的核因子表达[23]。在酵母多糖诱导的小鼠关节炎中,鹰嘴豆素A的抗炎作用与17-β雌二醇相似,是通过抑制促炎因子TNF-α和γ-干扰素(IFN-γ)的产生,并增加抗炎细胞因子白细胞介素-4(IL-4)和白细胞介素-10(IL-10)的水平发挥抗炎作用;鹰嘴豆素A还通过剂量依赖的方式诱导中性粒细胞的凋亡[24]。鹰嘴豆素A在脂多糖(LPS)诱导的急性肝损伤中以剂量介导的方式增强血红素氧合酶-1(HO-1)和核因子E2相关因子2(Nrf2)的表达;另外,它还通过减少核苷酸结合寡聚化结构域样受体蛋白3(NOD-like receptor protein 3, NLRP3)和人硫氧还蛋白相互作用蛋白(thioredoxin-interacting protein, TXNIP)之间的相互作用来抑制炎症小体的活化[25]。在LPS诱导的BV2小神经胶质细胞中鹰嘴豆素A通过抑制NF-κB、p65、TNF-α、白细胞介素-6(IL-6)、IL-1β、一氧化氮(NO)和前列腺素E2(PGE2)与活性氧(ROS)的释放,诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)、环氧合酶-2(COX-2)、髓样分化因子(myeloid differentiation factor 88, MyD88)和Toll样受体-4(Toll-like receptor-4, TLR-4)蛋白的表达,以及蛋白激酶B(protein kinase B, Akt)与ERK1/2通路的激活发挥抗炎作用[3]。在另一项研究中鹰嘴豆素A在HUVEC细胞中通过上调过氧化物酶体增殖物激活受体-γ(peroxisome proliferators-activated receptor-γ, PPAR-γ)的表达而发挥抗炎作用,从而抑制NF-κB的活化和TNF-α、白细胞介素-8(IL-8)、血管细胞黏附因子-1(vascular cell adhesion molecule-1, VCAM-1)与细胞间黏附因子-1(intercellular cell adhesion molecule-1, ICAM-1)的表达[26]。在THP-1巨噬细胞衍生的泡沫细胞中鹰嘴豆素A还可以通过激活PPAR-γ/肝X受体α(LXRα)或PPAR-γ/HO-1途径增加三磷酸腺苷结合盒转运体A1(ATP-binding cassette transporter A1, ABCA1)和三磷酸腺苷结合盒转运体G1(ATP-binding cassette transporter G1, ABCG1)的表达,抑制促炎细胞因子(TNF-α、IL-1β和IL-6)的分泌[14]。鹰嘴豆素A能通过抑制炎症因子(IL-1β、TNF-α)的释放和ROS的生成保护牙周组织免受炎症破坏[2]。鹰嘴豆素A还能抑制小鼠JB6 P+细胞和人HaCaT角质细胞中紫外线诱导的COX-2的表达[27]。鹰嘴豆素A和根皮素通过抑制细胞内ROS的产生而减缓过敏性细胞因子的生成,表明它们在RBL-2H3细胞中可能具有较强的抗过敏特性[28]。鹰嘴豆素A抗炎活性的分子机制见图 2。
|
IL-1α:白细胞介素-1α interleukin-1α;IL-1β:白细胞介素-1β interleukin-1β;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;RANKL: 核因子-κB受体激活剂配体receptor-activated nuclear factor-κB ligand;RANK:核因子-κB受体激活剂receptor-activated nuclear factor-κB;LPS: 脂多糖lipopolysaccharide; IKK: 核因子抑制蛋白激酶inhibitor of nuclear factor kappa-B kinase; MAPK: 丝裂原活化蛋白激酶mitogen-activated protein kinase;NF-κB:核因子-κB nuclear factor-kappa B;IκBα:核因子-κB抑制蛋白α inhibitor of nuclear factor kappa B alpha;ERK: 细胞外信号调节激酶extracellular signal-regulated kinase; JNK: c-Jun氨基末端激酶c-Jun N-terminal kinase; p38: p38丝裂原活化蛋白激酶p38 mitogen-activated protein kinase; p50:p50丝裂原活化蛋白激酶p50 mitogen-activated protein kinase; p65:p65丝裂原活化蛋白激酶p65 mitogen-activated protein kinase; PPAR-γ: 过氧化物酶体增殖物激活受体-γ peroxisome proliferators-activated receptor-γ; AP-1:激活蛋白1 activator protein 1;NFATc:活化T细胞1的核因子nuclear factor of activated T cells 1;NLRP3:核苷酸结合寡聚化结构域样受体蛋白3 NOD-like receptor protein 3;iNOS: 诱导型一氧化氮合酶inducible nitric oxide synthase; COX-2:环氧合酶-2 cyclooxygenase-2;TXNIP: 硫氧还蛋白相互作用蛋白thioredoxin-interacting protein; NO: 一氧化氮nitric oxide; PGE2:前列腺素E2 prostaglandin E2;degradation: 降解;inflammation:炎症;active:有活性的;inactive:无活性的。 图 2 鹰嘴豆素A抗炎活性的分子机制 Fig. 2 Molecular mechanism of anti-inflammatory activity of biochanin A |
ROS生成和抗氧化防御系统之间的不平衡会引起组织器官的损伤,从而造成各种病症,如炎症、过敏和心血管疾病。氧化应激也是造成神经变性、衰老、癌症和糖尿病的关键因素[29-32]。因此,清除自由基和激活细胞保护防御系统有利于健康[33]。
鹰嘴豆素A具有较低的直接抗氧化活性,能通过激活Nrf2来保护HepG2细胞免受叔丁基过氧化氢(t-BHP)诱导的氧化损伤,从而刺激下游细胞保护酶[如HO-1和还原型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADPH)]的表达[4]。鹰嘴豆素A[20 mg/(kg BW·d)]通过上调大鼠肝脏中的谷胱甘肽(GSH)、超氧化物歧化酶(SOD)和过氧化氢酶(CAT)的表达来减轻砷诱导的大鼠肝毒性,表现出其抗氧化的潜力[5]。在糖尿病大鼠中,鹰嘴豆素A主要是通过降低丙二醛(MDA)的水平,增加内源性抗氧化酶(CAT、SOD)的活性来降低氧化应激,从而改变总抗氧化状态[34]。此外,鹰嘴豆素A还展现出了1, 1-联苯基-2-苦基肼基(1, 1-diphenyl-2-picrylhydrazyl, DPPH)自由基清除活性[半抑制浓度(IC50)=(129.11±2.60) μg/mL]、NO自由基清除活性[IC50=(60.76±5.30) μg/mL]、金属螯合活性[IC50=(112.62±6.12) μg/mL]和脂质过氧化抑制作用[IC50=(58.66±3.40) μg/mL][35]。
2.3 抑菌活性鹰嘴豆素A能抑制H5N1病毒的复制,阻断H5N1诱导的ERK1/2、Akt和NF-κB的活化以及病毒诱导的IL-6、IL-8、IL-10的产生[36]。已有报道表明,鹰嘴豆素A具有抗利什曼原虫前鞭毛体的抗利什曼原虫活性[半最大效应浓度(EC50)=18.96 μg/mL][37]和抗肺链球菌的抗菌活性(IC50=12 μmol/L)[38]。据报道,鹰嘴豆素A的协同抗菌活性可以增强喹诺酮类药物环丙沙星或氧氟沙星对解脲脲原体的抑菌活性,两者联合使用时对解脲脲原体具有最大的抑菌活性[7]。鹰嘴豆素A和环丙沙星联合作用还可以增强环丙沙星对耐甲氧西林金黄色葡萄球菌的抑菌活性,鹰嘴豆素A的浓度为40 μg/mL时,环丙沙星的最小抑菌浓度由64 μg/mL降至8 μg/mL,这主要归因于鹰嘴豆素A的外排泵抑制作用[8]。除此之外,鹰嘴豆素A还能通过下调包括dnaA、dnaC和dnaN在内的几个DNA合成相关基因的表达,对大豆斑疹病菌(Xanthomonas axonopodis pv. glycines)发挥抑菌活性(最小抑菌浓度<100 μg/mL)[6]。此外,鹰嘴豆素A对8个梭状芽孢杆菌也表现出了抑菌潜力[39]。
2.4 抗癌活性鹰嘴豆素A具有在各种癌细胞的G1、G2/M和G0/G1期阻断细胞周期的潜力,鹰嘴豆素A的抗癌活性与通过介导B淋巴细胞瘤-2(Bcl-2)家族蛋白(Bad、Bid、Bax等)、促炎性细胞因子[9-10, 12, 40]和MAPK信号网络(p38 MAPK、c-Jun、ERK)[9, 41]、NF-κB[10]、细胞色素c[9]的释放以及激活包括半胱氨酸蛋白酶(cysteinyl aspartate specific proteinase,Caspase)-3、Caspase-8、Caspase-9在内的Caspase家族成员[12]来诱导细胞凋亡有关。鹰嘴豆素A和索拉非尼联合处理可诱导HepG2细胞G0/G1期的终止,下调细胞周期蛋白D和Ki-67基因的表达及存活率[12]。此外,鹰嘴豆素A还能通过上调p53及其下游靶点p21的表达,同时降低细胞周期蛋白A和细胞周期蛋白依赖性激酶2(cyclin-dependent kinases 2, CDK2)的表达,调节结肠癌细胞SW-480在G2/M期、A549细胞在S期和神经胶质瘤细胞U87在G1期的细胞周期终止[11, 42]。据报道,鹰嘴豆素A通过抑制NF-κB的激活而抑制头颈癌的增殖,它还通过下调由p38、MAPK和Akt通路介导的基质金属蛋白酶MMP-2/-9(matrix metalloproteinase-2/-9, MMP-2/-9)的表达来抑制FaDu细胞的生长和迁移[10]。另外,用鹰嘴豆素A和替莫唑胺联合处理降低了神经胶质瘤(T98、U87)细胞中表皮生长因子受体(epidermal growth factor receptor, EGFR)、磷酸化细胞外信号调节激酶(phosphorylated extracellular signal-regulated kinase, p-ERK)、磷酸化蛋白激酶(phosphorylated protein kinase B, p-Akt)和c-Myc的水平[42]。
2.5 其他生物学功能鹰嘴豆素A对卵巢切除大鼠具有降血压作用[43]。鹰嘴豆素A的胃保护活性可归因于细胞代谢途径的提高,如提高NO和SOD的作用以及增加MDA的水平和热休克蛋白70(heat shock protein 70,Hsp70)的表达[33]。有报道称,鹰嘴豆素A对链脲酶素诱导的糖尿病大鼠具有潜在的降血压作用,其有益的降血压作用在雄性Wistar大鼠中也进行了研究[44-45]。在高脂饮食激发的高血脂症小鼠中,鹰嘴豆素A降低了总胆固醇水平,这为鹰嘴豆素A作为降血脂药物提供了新的见解[46]。鹰嘴豆素A也被证明是终止四氯二苯并二英(TCDD)激发的消耗综合征的有效药物,其作用机制包括上调脂肪生成因子[如PPAR-γ和脂联素(TCDD使其减少)]的表达以及抑制TCDD介导的葡萄糖转运蛋白4和胰岛素受体底物1形成的减少[47]。另一项研究揭示了包括鹰嘴豆素A在内的植物雌激素的仿生和亲脂特性,该研究结果表明鹰嘴豆素A和其他异黄酮具有较高的亲脂性、α-1-酸性糖蛋白(α-1-acid-glycoprotein, AGP)结合性和Caco-2细胞通透性[48]。鹰嘴豆素A作为雌性激素的替代品对改善绝经后妇女的皮肤和肾脏的变化可能是有益的[49]。在脑缺血/再灌注损伤模型中,鹰嘴豆素A通过抑制Nrf2介导的大鼠氧化应激和炎症信号通路发挥神经保护作用[3]。在鱼藤酮诱导的神经毒性小鼠中鹰嘴豆素A能通过防止多巴胺神经元退化,恢复抗氧化能力,抑制COX-2和炎症因子(CD1-1b、iNOS、IL-1β和TNF-α)的mRNA表达来发挥神经保护作用[50]。
3 鹰嘴豆素A的生物利用率生物利用率是指在系统循环中药物在作用部位的吸收率。一些研究报道了异黄酮在给药后在人体中的生物利用率大部分都小于1%[51]。与其他异黄酮相似,鹰嘴豆素A的口服生物利用率低。据报道,大鼠口服剂量为5 mg/kg BW时鹰嘴豆素A的生物利用率为2.6%,而口服剂量为50 mg/kg BW时为1.2%;大鼠血浆中鹰嘴豆素A的含量为1.5%,鹰嘴豆素A由于其高渗透性使其吸收迅速,而胆汁的清除和机体的新陈代谢可能是其生物利用率较低的主要原因[52]。在给大鼠同时饲喂5种黄酮类化合物时,鹰嘴豆素A的生物利用率为21.3%,说明黄酮类化合物的组合可能能够提高鹰嘴豆素A的生物利用率[53]。
4 鹰嘴豆素A的构效关系与二氢异黄酮相比,异黄酮由于其结构上的修饰而表现出生物学活性,C环上存在的2,3-双键对异黄酮的活性起着至关重要的作用,鹰嘴豆素A的2,3-双键和A环上的7-OH能增强活性,由于A环7-OH和B环上的4-OCH3的存在,极大地增强了鹰嘴豆素A的抗氧化活性[54]。对鹰嘴豆素A不同衍生物的研究表明,A环中的7号位、B环中的4号位和C环中的2, 3-双键在反应过程中非常重要,7号位的游离羟基增强了抗癌活性,而5号位的游离羟基由于阻止活性自由基的产生而降低了抗氧化活性[32]。例如,A环中7号位的脂化作用增强了对MCF-7细胞的抗增殖活性[55]。鹰嘴豆素A的抗菌活性可能与染料木素相似(抑制DNA拓扑异构酶),因为它们两者在5号位上的甲氧基和羟基在结构上是同源的[56]。
5 鹰嘴豆素A在反刍动物上的研究体外发酵结果表明,鹰嘴豆素A具有选择性抗菌活性和协同抗菌活性,可以改变瘤胃菌群结构,抑制高产氨菌、淀粉分解菌和乳酸菌的活性,减少氨基酸发酵脱氨和淀粉分解,导致氨和乳酸的生成受到抑制;鹰嘴豆素A还可以促进纤维素分解菌和乳酸利用菌的活性,增强纤维素的分解和乳酸的代谢[56-58]。此外,Liu等[59]研究显示,鹰嘴豆素A可以通过抑制蛋白质分解菌和尿素分解菌的活性抑制蛋白质和尿素的分解,提高瘤胃微生物蛋白的合成效率。饲养试验也得到了相同的结果,鹰嘴豆素A不仅可以抑制牛高产氨菌发酵干酒糟(dried-distillers’ grains,DDG)和瘤胃中氨基酸的降解,使更多的氨基酸在后消化道吸收,提高DDG中粗蛋白质的消化率和肉牛的增重性能,还可以增加细菌素等瘤胃微生物产生的抗菌化合物,代替莫能菌素改善瘤胃pH,防止瘤胃酸中毒[60-62]。
鹰嘴豆素A进入瘤胃以后首先在瘤胃微生物的作用下脱去B环4号位上的甲基生成染料木素,染料木素在瘤胃液中进一步开环产生没有雌激素活性的对乙基苯酚和有机酸(4-羟基苯基-2-丙酸)[63]。除此之外,染料木素还可以被瘤胃微生物直接转化成二氢染料木素[64]。研究表明,人肠道内的细枝真杆菌也可以进一步将染料木素降解为二氢染料木素,然后将裂解C环生成乙基苯酚和6-羟基去氧甲基安哥拉紫檀素(6-methoxyl-O-desmethylangolensin,6-OH-O-DMA),6-OH-O-DMA随后生成4-羟基苯基-2-丙酸[65]。鹰嘴豆素A在瘤胃中的代谢程度随着饲喂时间的延长逐渐提高,6~10 d后鹰嘴豆素A几乎被完全代谢成对乙基苯酚和有机酸,然后主要通过尿液被排出体外,山羊奶中也有对乙基苯酚被检出[66-67]。
6 小结与展望目前,大量的研究表明鹰嘴豆素A具有多种生物学功能,在抗炎、抗氧化和抗癌等方面都具有良好的作用,但是其作用机制仍有待于深入解析,而且鹰嘴豆素A在反刍动物生产中的研究也鲜有报道。鹰嘴豆素A的植物提取率低,难以获取高纯度鹰嘴豆素A,而且生物利用率也低。因此,应该加强鹰嘴豆素A的提取工艺方面的研究,提高鹰嘴豆素A的纯度,以减少研究过程中其他异黄酮的影响;同时,还应继续对其生物活性进行更深入的研究,绘制出清晰完整的作用机制图,并且加强其在反刍动物生产中的研究,找出鹰嘴豆素A在瘤胃内完整的代谢途径,为新型药物和保健品以及饲料添加剂的开发提供理论依据。
| [1] |
DADÁKOVÁ K, TRNKOVÁ A, KAŠPAROVSKÁ J, et al. In vitro metabolism of red clover isoflavones in rumen fluid[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(6): 1647-1654. DOI:10.1111/jpn.13402 |
| [2] |
ZHANG S D, NIU Y L, YANG Z, et al. Biochanin A alleviates gingival inflammation and alveolar bone loss in rats with experimental periodontitis[J]. Experimental and Therapeutic Medicine, 2020, 20(6): 251. |
| [3] |
BERKÖZ M, KROŚNIAK M, ÖZKAN-YILMAZ F, et al. Prophylactic effect of biochanin A in lipopolysaccharide-stimulated BV2 microglial cells[J]. Immunopharmacology and Immunotoxicology, 2020, 42(4): 330-339. DOI:10.1080/08923973.2020.1769128 |
| [4] |
LIANG F Q, CAO W W, HUANG Y T, et al. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells[J]. BioFactors, 2019, 45(4): 563-574. |
| [5] |
JALALUDEEN A M, HA W T, LEE R, et al. Biochanin A ameliorates arsenic-induced hepato- and hematotoxicity in rats[J]. Molecules, 2016, 21(1): 69. DOI:10.3390/molecules21010069 |
| [6] |
HU K X, SHI X C, XU D, et al. Antibacterial mechanism of biochanin A and its efficacy for the control of Xanthomonas axonopodis pv. glycines in soybean[J]. Pest Management Science, 2021, 77(4): 1668-1673. DOI:10.1002/ps.6186 |
| [7] |
JIN H, QI C, ZOU Y P, et al. Biochanin A partially restores the activity of ofloxacin and ciprofloxacin against topoisomerase IV mutation-associated fluoroquinolone-resistant Ureaplasma species[J]. Journal of Medical Microbiology, 2017, 66(11): 1545-1553. DOI:10.1099/jmm.0.000598 |
| [8] |
ZOU D, XIE K P, WANG H T, et al. [Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA)][J]. Acta Microbiologica Sinica, 2014, 54(10): 1204-1211. |
| [9] |
HSU Y N, SHYU H W, HU T W, et al. Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis[J]. Food and Chemical Toxicology, 2018, 112: 194-204. DOI:10.1016/j.fct.2017.12.062 |
| [10] |
CHO I A, YOU S J, KANG K R, et al. Biochanin-A induces apoptosis and suppresses migration in FaDu human pharynx squamous carcinoma cells[J]. Oncology Reports, 2017, 38(5): 2985-2992. DOI:10.3892/or.2017.5953 |
| [11] |
LI Y, YU H Y, HAN F F, et al. Biochanin A induces S phase arrest and apoptosis in lung cancer cells[J]. BioMed Research International, 2018, 2018: 3545376. |
| [12] |
YOUSSEF M M, TOLBA M F, BADAWY N N, et al. Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells[J]. Scientific Reports, 2016, 6: 30717. DOI:10.1038/srep30717 |
| [13] |
LEE K H, CHOI E M. Biochanin A stimulates osteoblastic differentiation and inhibits hydrogen peroxide-induced production of inflammatory mediators in MC3T3-E1 cells[J]. Biological & Pharmaceutical Bulletin, 2005, 28(10): 1948-1953. |
| [14] |
YU X H, CHEN J J, DENG W Y, et al. Biochanin A mitigates atherosclerosis by inhibiting lipid accumulation and inflammatory response[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 8965047. |
| [15] |
KO K P. Isoflavones: chemistry, analysis, functions and effects on health and cancer[J]. Asian Pacific Journal of Cancer Prevention, 2014, 15(17): 7001-7010. DOI:10.7314/APJCP.2014.15.17.7001 |
| [16] |
KŘÍŽOVÁ L, DADÁKOVÁ K, KAŠPAROVSKÁ J, et al. Isoflavones[J]. Molecules, 2019, 24(6): 1076. DOI:10.3390/molecules24061076 |
| [17] |
CHUKWUMAH Y C, WALKER L T, VERGHESE M, et al. Effect of frequency and duration of ultrasonication on the extraction efficiency of selected isoflavones and trans-resveratrol from peanuts (Arachis hypogaea)[J]. Ultrasonics Sonochemistry, 2009, 16(2): 293-299. DOI:10.1016/j.ultsonch.2008.07.007 |
| [18] |
GAO Y, YAO Y, ZHU Y Y, et al. Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions[J]. Journal of Agricultural and Food Chemistry, 2015, 63(10): 2701-2707. DOI:10.1021/jf5057524 |
| [19] |
ZGÓRKA G. Pressurized liquid extraction versus other extraction techniques in micropreparative isolation of pharmacologically active isoflavones from Trifolium L. species[J]. Talanta, 2009, 79(1): 46-53. DOI:10.1016/j.talanta.2009.03.011 |
| [20] |
KIM M R, KIM H J, YU S H, et al. Combination of red clover and hops extract improved menopause symptoms in an ovariectomized rat model[J]. Evidence-Based Complementary and Alternative Medicine, 2020, 2020: 7941391. |
| [21] |
KAGAN I A, GOFF B M, FLYTHE M D. Soluble phenolic compounds in different cultivars of red clover and alfalfa, and their implication for protection against proteolysis and ammonia production in ruminants[J]. Natural Product Communications, 2015, 10(7): 1263-1267. |
| [22] |
BUTKUT Ė B, DAGILYT Ė A, BENETIS R, et al. Mineral and phytochemical profiles and antioxidant activity of herbal material from two temperate Astragalus species[J]. BioMed Research International, 2018, 2018: 6318630. |
| [23] |
LIAO S J, FENG W Y, LIU Y, et al. Inhibitory effects of biochanin A on titanium particle-induced osteoclast activation and inflammatory bone resorption via NF-κB and MAPK pathways[J]. Journal of Cellular Physiology, 2021, 236(2): 1432-1444. DOI:10.1002/jcp.29948 |
| [24] |
FELIX F B, ARA U ' JO J M D, DE SOUZA E V, et al. Biochanin A attenuates zymosan-induced arthritis in mice similarly to 17-β estradiol: an alternative to hormone replacement therapy?[J]. Inflammation Research, 2020, 69(12): 1245-1256. DOI:10.1007/s00011-020-01403-4 |
| [25] |
LIU X K, WANG T, LIU X S B E, et al. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation[J]. International Immunopharmacology, 2016, 38: 324-331. DOI:10.1016/j.intimp.2016.06.009 |
| [26] |
MING X D, DING M F, ZHAI B, et al. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells[J]. Life Sciences, 2015, 136: 36-41. DOI:10.1016/j.lfs.2015.06.015 |
| [27] |
LIM T G, KIM J E, JUNG S K, et al. MLK3 is a direct target of biochanin A, which plays a role in solar UV-induced COX-2 expression in human keratinocytes[J]. Biochemical Pharmacology, 2013, 86(7): 896-903. DOI:10.1016/j.bcp.2013.08.002 |
| [28] |
CHUNG M J, SOHNG J K, CHOI D J, et al. Inhibitory effect of phloretin and biochanin A on IgE-mediated allergic responses in rat basophilic leukemia RBL-2H3 cells[J]. Life Sciences, 2013, 93(9/11): 401-408. |
| [29] |
FINKEL T, HOLBROOK N J. Oxidants, oxidative stress and the biology of ageing[J]. Nature, 2000, 408(6809): 239-247. DOI:10.1038/35041687 |
| [30] |
GORRINI C, HARRIS I S, MAK T W. Modulation of oxidative stress as an anticancer strategy[J]. Nature Reviews Drug Discovery, 2013, 12(12): 931-947. DOI:10.1038/nrd4002 |
| [31] |
ABRAMOV A Y, POTAPOVA E V, DREMIN V V, et al. Interaction of oxidative stress and misfolded proteins in the mechanism of neurodegeneration[J]. Life, 2020, 10(7): 101. DOI:10.3390/life10070101 |
| [32] |
LI P S, SHI X J, WEI Y, et al. Synthesis and biological activity of isoflavone derivatives from chickpea as potent anti-diabetic agents[J]. Molecules, 2015, 20(9): 17016-17040. DOI:10.3390/molecules200917016 |
| [33] |
HAJREZAIE M, SALEHEN N, KARIMIAN H, et al. Biochanin A gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats[J]. PLoS One, 2015, 10(3): e0121529. DOI:10.1371/journal.pone.0121529 |
| [34] |
DE CAMARGO A C, FAVERO B T, MORZELLE M C, et al. Is chickpea a potential substitute for soybean?Phenolic bioactives and potential health benefits[J]. International Journal of Molecular Sciences, 2019, 20(11): 2644. DOI:10.3390/ijms20112644 |
| [35] |
KUMARI S, ELANCHERAN R, KOTOKY J, et al. Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam.by UPLC-ESI-MS/MS[J]. Food Chemistry, 2016, 203: 521-529. DOI:10.1016/j.foodchem.2016.02.101 |
| [36] |
SITHISARN P, MICHAELIS M, SCHUBERT-ZSILAVECZ M, et al. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells[J]. Antiviral Research, 2013, 97(1): 41-48. DOI:10.1016/j.antiviral.2012.10.004 |
| [37] |
SARTORELLI P, CARVALHO C S, REIMÃO J Q, et al. Antiparasitic activity of biochanin A, an isolated isoflavone from fruits of Cassia fistula (Leguminosae)[J]. Parasitology Research, 2009, 104(2): 311-314. DOI:10.1007/s00436-008-1193-z |
| [38] |
HANSKI L, GENINA N, UVELL H, et al. Inhibitory activity of the isoflavone biochanin A on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation[J]. PLoS One, 2014, 9(12): e115115. DOI:10.1371/journal.pone.0115115 |
| [39] |
SKLENICKOVA O, FLESAR J, KOKOSKA L, et al. Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria[J]. Molecules, 2010, 15(3): 1270-1279. DOI:10.3390/molecules15031270 |
| [40] |
WANG Y, LI J J, CHEN Y M. Biochanin A extirpates the epithelial-mesenchymal transition in a human lung cancer[J]. Experimental and Therapeutic Medicine, 2018, 15(3): 2830-2836. |
| [41] |
JAIN A, LAI J C K, BHUSHAN A. Biochanin A inhibits endothelial cell functions and proangiogenic pathways: implications in glioma therapy[J]. Anti-Cancer Drugs, 2015, 26(3): 323-330. DOI:10.1097/CAD.0000000000000189 |
| [42] |
DESAI V, JAIN A, SHAGHAGHI H, et al. Combination of biochanin A and temozolomide impairs tumor growth by modulating cell metabolism in glioblastoma multiforme[J]. Anticancer Research, 2019, 39(1): 57-66. DOI:10.21873/anticanres.13079 |
| [43] |
SACHDEVA C, MISHRA N, SHARMA S. Development and characterization of enteric-coated microparticles of biochanin A for their beneficial pharmacological potential in estrogen deficient-hypertension[J]. Drug Delivery, 2016, 23(6): 2044-2057. DOI:10.3109/10717544.2015.1114046 |
| [44] |
HARINI R, EZHUMALAI M, PUGALENDI K V. Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats[J]. European Journal of Pharmacology, 2012, 676(1/3): 89-94. |
| [45] |
AZIZI R, GOODARZI M T, SALEMI Z. Effect of biochanin A on serum visfatin level of streptozocin-induced diabetic rats[J]. Iranian Red Crescent Medical Journal, 2014, 16(9): e15424. |
| [46] |
XUE Z H, ZHANG Q, YU W C, et al. Potential lipid-lowering mechanisms of biochanin A[J]. Journal of Agricultural and Food Chemistry, 2017, 65(19): 3842-3850. DOI:10.1021/acs.jafc.7b00967 |
| [47] |
CHOI E M, SUH K S, PARK S Y, et al. Biochanin A prevents 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced adipocyte dysfunction in cultured 3T3-L1 cells[J]. Journal of Environmental Science and Health, Part A, Toxic/Hazardous Substances & Environmental Engineering, 2019, 54(9): 865-873. |
| [48] |
LASIĆ K, BOKULIĆ A, MILIĆ A, et al. Lipophilicity and bio-mimetic properties determination of phytoestrogens using ultra-high-performance liquid chromatography[J]. Biomedical Chromatography, 2019, 33(8): e4551. |
| [49] |
GALAL A A A, MOHAMED A A R, KHATER S I, et al. Beneficial role of biochanin A on cutaneous and renal tissues of ovariectomized rats treated with anastrozole[J]. Life Sciences, 2018, 201: 9-16. DOI:10.1016/j.lfs.2018.03.037 |
| [50] |
EL-SHERBEENY N A, SOLIMAN N, YOUSSEF A M, et al. The protective effect of biochanin A against rotenone-induced neurotoxicity in mice involves enhancing of PI3K/Akt/mTOR signaling and beclin-1 production[J]. Ecotoxicology and Environmental Safety, 2020, 205: 111344. DOI:10.1016/j.ecoenv.2020.111344 |
| [51] |
PASSAMONTI S, TERDOSLAVICH M, FRANCA R, et al. Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms[J]. Current Drug Metabolism, 2009, 10(4): 369-394. DOI:10.2174/138920009788498950 |
| [52] |
MOON Y J, SAGAWA K, FREDERICK K, et al. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats[J]. AAPS Journal, 2006, 8(3): E433-E442. DOI:10.1208/aapsj080351 |
| [53] |
MALLIS L M, SARKAHIAN A B, HARRIS H A, et al. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry[J]. Journal of Chromatography.B, Analytical Technologies in the Biomedical and Life Sciences, 2003, 796(1): 71-86. DOI:10.1016/j.jchromb.2003.08.003 |
| [54] |
CASTELLANO G, TORRENS F. Quantitative structure-antioxidant activity models of isoflavonoids: a theoretical study[J]. International Journal of Molecular Sciences, 2015, 16(6): 12891-12906. |
| [55] |
FOKIALAKIS N, ALEXI X, ALIGIANNIS N, et al. Ester and carbamate ester derivatives of biochanin A: synthesis and in vitro evaluation of estrogenic and antiproliferative activities[J]. Bioorganic and Medicinal Chemistry, 2012, 20(9): 2962-2970. DOI:10.1016/j.bmc.2012.03.012 |
| [56] |
HARLOW B E, FLYTHE M D, AIKEN G E. Biochanin A improves fibre fermentation by cellulolytic bacteria[J]. Journal of Applied Microbiology, 2018, 124(1): 58-66. DOI:10.1111/jam.13632 |
| [57] |
HARLOW B E, FLYTHE M D, AIKEN G E. Effect of biochanin A on corn grain (Zea mays) fermentation by bovine rumen amylolytic bacteria[J]. Journal of Applied Microbiology, 2017, 122(4): 870-880. DOI:10.1111/jam.13397 |
| [58] |
FLYTHE M D, HARRISON B, KAGAN I A, et al. Antimicrobial activity of red clover (Trifolium pratense L.) extract on caprine hyper ammonia-producing bacteria[J]. Agriculture, Food and Analytical Bacteriology, 2013, 3(3): 176-185. |
| [59] |
LIU S J, ZHANG Z Y, HAILEMARIAM S, et al. Biochanin A inhibits ruminal nitrogen-metabolizing bacteria and alleviates the decomposition of amino acids and urea in vitro[J]. Animals, 2020, 10(3): 368. DOI:10.3390/ani10030368 |
| [60] |
HARLOW B E, FLYTHE M D, KAGAN I A, et al. Biochanin A (an isoflavone produced by red clover) promotes weight gain of steers grazed in mixed grass pastures and fed dried-distillers' grains[J]. Crop Science, 2017, 57(1): 506-514. DOI:10.2135/cropsci2016.07.0590 |
| [61] |
HARLOW B E, FLYTHE M D, KAGAN I A, et al. Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures[J]. PLoS One, 2020, 15(3): e0229200. DOI:10.1371/journal.pone.0229200 |
| [62] |
HARLOW B E, FLYTHE M D, KLOTZ J L, et al. Effect of biochanin A on the rumen microbial community of Holstein steers consuming a high fiber diet and subjected to a subacute acidosis challenge[J]. PLoS One, 2021, 16(7): e0253754. DOI:10.1371/journal.pone.0253754 |
| [63] |
DAEMS F, DECRUYENAERE V, AGNEESSENS R, et al. Changes in the isoflavone concentration in red clover (Trifolium pratense L.) during ensiling and storage in laboratory-scale silos[J]. Animal Feed Science and Technology, 2016, 217: 36-44. DOI:10.1016/j.anifeedsci.2016.04.008 |
| [64] |
ZHAO H, WANG X L, ZHANG H L, et al. Production of dihydrodaidzein and dihydrogenistein by a novel oxygen-tolerant bovine rumen bacterium in the presence of atmospheric oxygen[J]. Applied Microbiology and Biotechnology, 2011, 92(4): 803-813. DOI:10.1007/s00253-011-3278-3 |
| [65] |
SCHOEFER L, MOHAN R, BRAUNE A, et al. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus[J]. FEMS Microbiology Letters, 2002, 208(2): 197-202. DOI:10.1111/j.1574-6968.2002.tb11081.x |
| [66] |
NJÅSTAD K M. Metabolism of phytoestrogens in dairy cows-effect of botanical composition of silages[D]. Master's Thesis. Oslo: Norwegian University of Life Sciences, 2011.
|
| [67] |
SAKAKIBARA H, VIALA D, OLLIER A, et al. Isoflavones in several clover species and in milk from goats fed clovers[J]. BioFactors, 2004, 22(1/4): 237-239. |




