2. 北京市农林科学院畜牧兽医研究所,北京 100097;
3. 北京市农林科学院-美国俄克拉荷马州立大学动物科学联合实验室,北京 100097
2. Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, China;
3. Joint Laboratory of Animal Science between Institute of Animal Husbandry and Veterinary Medicine of Beijing Academy of Agriculture and Forestry Sciences and Division of Agricultural Science and Natural Resource of Oklahoma State University, Beijing 100097, China
猪的肠道不仅是重要的消化吸收器官,也是重要的免疫器官,在维持肠道微生态的平衡、保障机体健康和正常生产性能等方面发挥重要作用。肠道微生物与母猪繁殖性能相关,有研究表明具有健康肠道微生物群落的母猪高产仔数的可能性较大[1]。猪肠道微生物异常丰富,共有30多个属,400~500种微生物,包括需氧型、兼性厌氧型和厌氧型微生物,其数量可达1012~1013 CFU/g[2-4]。这些微生物共同维持肠道正常的生理过程,在营养物质的消化吸收等过程中发挥重要作用。妊娠期是母猪生产中最重要的一个阶段,该阶段母体各种生理和代谢机制会发生重大改变,这个过程包括复杂的生殖激素波动、体重增加、炎症反应、免疫系统变化和微生物群落的扰动[5]。有研究表明,母体肠道微生物对胎盘的发育和功能具有重要影响[6]。此外,Vuong等[7]在小鼠上研究发现,母体肠道微生物群可以通过调节其产生的代谢产物促进胎儿大脑的健康发育。可见,妊娠期母猪肠道微生物对自身正常生理和子代的发育至关重要,在妊娠周期中一个阶段的菌群状况会影响下一个阶段及以后各阶段的状况,从而影响母猪的生产性能和仔猪的生长发育[8]。影响肠道微生物群落差异的因素有很多,包括品种、年龄、生理状况、饲粮等[4, 9]。鉴于此,本试验通过研究同一品种、年龄和饲养管理水平下,不同妊娠阶段母猪肠道微生物的变化,揭示肠道微生物结构与不同生理阶段的关系,为保证妊娠正常进行及促进子代健康发育提供参考。
1 材料与方法 1.1 试验动物与饲养管理饲养试验在河北某大型猪场进行。试验选取同一圈舍内50头发情正常、体况均匀、健康状况良好、胎次为3胎的长大二元母猪,饲喂相同饲粮、自由饮水。分娩前1周转至产房,试验期为母猪配种至分娩后。饲养管理和免疫消毒程序按照本试验所在猪场常规管理程序及免疫程序进行。母猪饲粮参照我国《猪饲养标准》(NY/T 65—2004)配制,基础饲粮组成及营养水平见表 1。妊娠期和分娩后的饲喂量分别为:妊娠1~85 d饲喂量从1.5 kg/d逐渐增加至2.5 kg/d,妊娠85 d至分娩饲喂量为3.5 kg/d,分娩后饲喂量可视情况增加0.5 kg/d。
|
|
表 1 基础饲粮组成及营养水平(饲喂基础) Table 1 Composition and nutrient levels of the basal diet (as-fed basis) |
在妊娠30(D30)、70(D70)、110 d(D110)和分娩后(F)随机挑选8头母猪,采用直肠法收集母猪100 g粪便样品,将样品液氮速冻后-80 ℃保存供测定微生物多样性使用。
1.3 粪便微生物的测定 1.3.1 DNA提取和PCR扩增根据E.Z.N.A.® Soil DNA Kit(Omega,Bio-Tek, 美国)说明书提取微生物群落总DNA,使用1%的琼脂糖凝胶电泳检测DNA的提取质量,使用NanoDrop 2000测定DNA浓度和纯度。选用测序引物338F(5’-ACTCCTACGGGAGGCAGCAG-3’)和806R(5’-GGACTACHVGGGTWTCTAAT-3’)对16S rRNA基因的V3~V4可变区域进行PCR扩增。
1.3.2 Illumina MiSeq测序将同一样本的PCR产物混合后,经2%琼脂糖凝胶电泳分离条带,利用AxyPrep DNA Gel Extraction Kit(Axygen Biosciences, 美国)试剂盒进行纯化,所有样品的高通量测序工作均委托上海美吉生物技术有限公司进行。纯化后的样品利用Illumina公司的MiSeq PE250平台进行测序。
1.4 数据分析利用上海美吉生物技术有限公司提供的数据云分析平台,对原始测序序列进行质控,筛除误差数据,提高测序结果的准确性和可信度,然后对肠道微生物进行Alpha多样性分析、物种组成分析、Beta多样性分析、物种差异分析和功能预测分析等。使用SPSS 21.0软件进行one-way ANOVA单因素方差分析Alpha多样性,以P < 0.05作为显著性判断标准;使用GraphPad Prism 8.0.2软件对差异菌群丰度进行统计分析并绘制相对丰度柱形图。
1.4.1 Alpha多样性分析采用Chao1、Ace、Shannon和Simpson指数分析粪便微生物的多样性和丰富度。
1.4.2 物种组成分析Venn图分析:用于统计样本中所共有和独有的物种操作分类单元(OTU)数目。一般选用相似水平为97%的OTU或其他分类学水平的样本进行分析。
Heatmap分析:根据分析结果使高丰度和低丰度的物种分块聚集,通过颜色变化来反映不同分组(或样本)在各分类学水平上群落组成的相似性和差异性。
1.4.3 Beta多样性分析主坐标分析(principal co-ordinates analysis,PCoA)是一种非约束性的数据降维分析方法,可用来研究样本群落组成的相似性或差异性。不同的点代表不同的样本,样本之间的距离表示样本之间的相似性,距离越近,相似性越大。
1.4.4 物种差异分析线性判别分析效应量(linear discriminant analysis effect size,LEfSe)是利用非参数Kruskal-Wallis(KW)sum-rank test和Wilcoxon rank-sum test检验获得显著差异物种,然后根据线性判别分析(linear discriminant analysis, LDA)估算每个组分(物种)丰度对差异效果影响的大小,寻找核心差异物种。
1.4.5 功能预测通过PICRUSt2对16S扩增子测序结果进行功能预测分析,本研究主要根据COG数据库的信息,从eggNOG数据库中解析到各个COG的描述信息,以及其功能信息,从而得到功能丰度谱。
2 结果与分析 2.1 Alpha多样性分析在属水平上,母猪不同妊娠阶段和分娩后肠道微生物多样性指数如表 2所示。由表可知,妊娠30 d的Shannon和Simpson指数与妊娠70 d和分娩后均存在显著差异(P < 0.05),Ace和Chao1指数差异不显著(P>0.05)。妊娠30与110 d的Simpson、Ace和Chao1指数存在显著差异(P < 0.05)。妊娠70和110 d时Shannon、Simpson、Ace和Chao1指数均无显著差异(P>0.05)。通过Shannon、Simpson、Ace、Chao1指数分析,表明不同妊娠阶段肠道微生物组成存在显著差异。随着妊娠的进行,肠道微生物多样性降低,丰富度升高。在妊娠30 d时,母猪肠道微生物多样性高于妊娠70、110 d和分娩后;妊娠110 d时,母猪肠道微生物丰富度显高于妊娠30 d和分娩后,同时妊娠70 d时高于分娩后。但妊娠70和110 d时母猪肠道微生物多样性和丰富度差异不显著。
|
|
表 2 母猪不同妊娠阶段和分娩后肠道微生物α多样性指数 Table 2 Alpha diversity indices of gut microbiota of sows at different stages of pregnancy and after delivery |
母猪不同妊娠阶段和分娩后肠道微生物物种组成分析如图 1所示。Venn图(图 1-A)显示,妊娠30、70、110 d和分娩后共有的OTU为594个,妊娠30、70、110 d和分娩后特有的OTU分别为80、77、105和124个。妊娠30和70 d共有89个OTU,妊娠70和110 d共有82个OTU,妊娠110 d和分娩后共有60个OTU。不同妊娠阶段母猪肠道微生物注释到7个菌门,其中优势菌门为厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidetes),共占总菌数的70%以上。随着妊娠的进行,变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、梭杆菌门(Fusobacteria)和Patescibacteria的相对丰度均升高,螺旋体门(Spirochaetes)的相对丰度降低(表 3)。相对丰度排名前10的菌属见表 4,丰度前50的菌群Heatmap(图 1-B)显示,母猪不同妊娠阶段和分娩后肠道优势菌属不同,但妊娠70和110 d的肠道菌群结构较为相似。随着妊娠的进行,普雷沃氏菌属(Prevotella)、卟啉单胞菌属(Porphyromonas)、梭杆菌属(Fusobacterium)、狭窄梭菌属1(Clostridium_sensu_stricto_1)、拟杆菌属(Bacteroides)、大肠埃希菌-志贺菌属(Escherichia-Shigella)的相对丰度升高,乳杆菌属(Lactobacillus)、密螺旋体属(Treponema)的相对丰度降低。
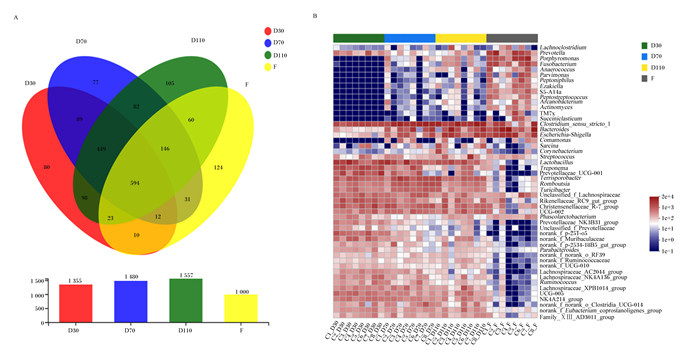
|
Venn图(图A)重叠部分表示多个样本分组中共有OTU,没有重叠的部分表示该样本分组所特有的OTU,数字表示对应的数目。Heatmap(图B)横坐标为样本名,纵坐标为物种名,通过色块颜色梯度来展示样本中不同物种的丰度变化情况,图中右侧为颜色梯度代表的数值。 D30:妊娠30 d 30 d of pregnancy;D70:妊娠70 d 70 d of pregnancy;D110:妊娠110 d 110 d of pregnancy;F: 分娩后after delivery。下图同the same as below。 Lachnoclostridium:毛螺菌属;Prevotella:普雷沃氏菌属;Porphyromonas:卟啉单胞菌属;Fusobacterium:梭杆菌属;Anaerococcus:厌氧菌属;Parvimonas:单孢菌属;Peptoniphilus:嗜血杆菌属;Peptostreptococcus:消化链球菌属;Arcanobacterium:隐秘杆菌属;Actinomyces:放线菌属;Succiniclasticum:琥珀酸菌属;Clostridium_sensu_stricto_1:狭窄梭菌属1;Bacteroides:拟杆菌属;Escherichia-Shigella:大肠埃希菌-志贺菌属;Comamonas:丛毛单胞菌属;Sarcina:八联球菌属;Corynebacterium:棒状杆菌属;Streptococcus:链球菌属;Lactobacillus:乳杆菌属;Treponema:密螺旋体属;Prevotellaceae_UCG-001:普雷沃氏菌科UCG-001;Terrisporobacter:土孢杆菌属;Turicibacter:图利杆菌属;Unclassified_f_Lachnospiraceae:未分类的毛螺菌科;Rikenellaceae_RC9_gut_group:理研氏菌科RC9肠群;Christensenellaceae_R-7_group:克里斯滕菌科R-7群;Phascolarctobacterium:考拉杆菌属;Prevotellaceae_NK3B31_group:普雷沃氏菌科NK3B31群;Unclassified_f_Prevotellaceae:未分类的普雷沃氏菌科;norank_f_p-251-o5:未分类的p-251-o5;norank_f_Muribaculaceae:未分类的绒毛杆菌科;norank_f_p-2534-18B5_gut_group:未分类的p-2534-18B5肠群;Parabacteroides:副拟杆菌属;norank_f_norank_o_RF39:未分类的RF39;norank_f_Ruminococcaceae:未分类的瘤胃球菌科;norank_f_UCG-010:未分类的UCG-010;Lachnospiraceae_AC2044_group:毛螺菌科AC2044群;Lachnospiraceae_NK4A136_group:毛螺菌科NK4A136群;Ruminococcus:瘤胃球菌属;Lachnospiraceae_XPB1014_group:毛螺菌科XPB1014群;NK4A214_group:NK4A214群;norank_f_norank_o_Clostridia_UCG-014:未分类的梭菌纲UCG-014;norank_f_Eubacterium_coprostanoligenes_group:未分类的真杆菌属coprostanoligenes群;Family_ⅩⅢ_AD3011_group:ⅩⅢ科AD3011群。图 3同the same as Fig. 3。 The overlapping part of the Venn diagram (figure A) indicated that the OTU was shared among multiple sample groups, the non-overlapping part indicated the unique OTU of the sample group, and the number indicated the corresponding OUT number. The abscissa of Heatmap (figure B) was the name of the sample, and the vertical axis was the name of the genera. The color gradient of the color block was used to show the abundance changes of different species in the sample. The right side of the figure is the value represented by the color gradient. 图 1 母猪不同妊娠阶段和分娩后肠道微生物的Venn图和Heatmap Fig. 1 Venn diagram and Heatmap of gut microbiome of sows at different stages of pregnancy and after delivery |
|
|
表 3 母猪肠道微生物在门水平上的相对丰度 Table 3 Relative abundances of gut microbiota at phylum level of sows |
|
|
表 4 母猪肠道中排名前10的菌属的相对丰度 Table 4 Relative abundances of top 10 bacterial genus in gut of sows |
基于Bray-Curtis距离的PCoA(图 2)显示,在属水平上,妊娠30 d和分娩后的菌群结构与妊娠70和110 d具有较大差异,但妊娠70和110 d的菌群结构存在较高的相似性(R=0.572 8,P=0.001 0)。
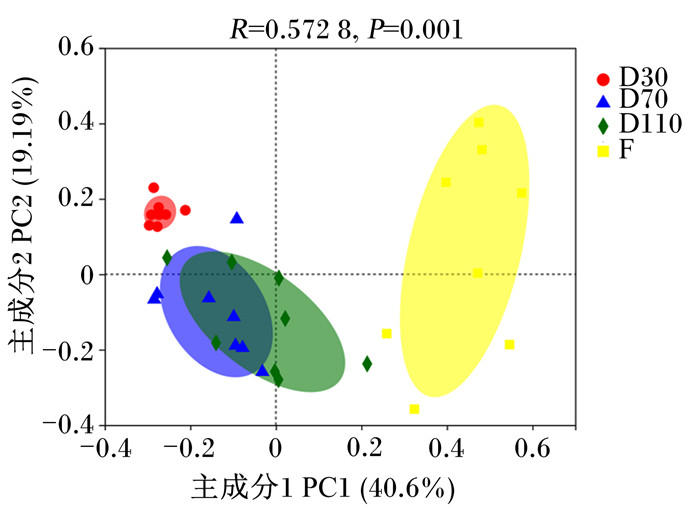
|
图中不同的颜色代表不同的组别,点代表样本,样本之间的距离表示样本之间的相似性,距离越近,相似性越大。 In the figure, different colors represented different groups, points represented samples, and the distance between samples represented the similarity between samples. The closer distance, the greater similarity. 图 2 属水平上肠道微生物群落PCoA图 Fig. 2 PCoA graph of gut microbiome community at genus level |
利用LEfSe分析鉴定母猪不同妊娠阶段和分娩后肠道中的差异微生物。结果表明,母猪妊娠30 d时显著富集Lactobacillus、普雷沃氏菌科UCG-001(Prevotellaceae_UCG-001)、Treponema、未分类的p-251-o5(norank_f_p-251-o5)等菌群(P < 0.05);妊娠70 d时显著富集土孢杆菌属(Terrisporobacter)、Romboutsia、图利杆菌属(Turicibacter)等菌群(P < 0.05);妊娠110 d时显著富集Clostridium_sensu_stricto_1、UCG-002等菌群(P < 0.05);分娩后显著富集Bacteroides、Escherichia-Shigella、Porphyromonas、Prevotella、Fusobacterium等菌群(P < 0.05)(图 3)。
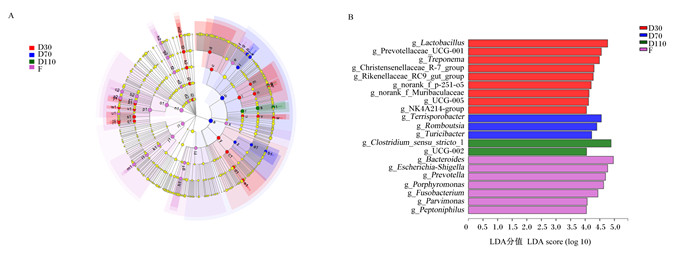
|
LEfSe多级物种层级树图(图A)从内到外表示从域到属的系统发育水平。不同颜色节点表示在对应组别中显著富集且对组间差异存在显著影响的微生物类群(P < 0.05);淡黄色节点表示在不同分组中均无显著差异或对组间差异无显著影响的微生物类群(P>0.05)。LDA判别图(图B)中不同的颜色代表不同的组别,纵坐标表示多组中具有显著差异的微生物,横坐标(LDA分值)表示物种丰度,LDA分值越大,代表物种丰度对差异效果的影响越大。 a: 厚壁菌门p_Firmicutes;b: 梭状芽胞杆菌纲c_Clostridia;c: 毛螺菌目o_Lachnospirales;d: 毛螺菌科f_Lachnospiraceae;e: f_norank_o_Peptostreptococcales-Tissierellales;f: 单孢菌属g_Parvimonas;g: 厌氧菌属g_Anaerococcus;h: 嗜血杆菌属g_Peptoniphilus;i: 链球菌科f_Peptostreptococcaceae;j: 罗姆布茨菌属g_Romboutsia;k: 锥孢杆菌属g_Terrisporobacter;l: 颤螺旋菌目o_Oscillospirales;m: 瘤胃球菌科f_Ruminococcaceae;n: 颤螺旋菌科f_Oscillospiraceae;o: g_UCG-005;p: g_UCG-002;q: NK4A214群g_NK4A214_group;r: 梭状芽胞杆菌目o_Clostridiales;s: 梭状芽胞杆菌科f_Clostridiaceae;t: 狭窄梭菌属_1 g_Clostridium_sensu_stricto_1;u: 克里斯滕菌目o_Christensenellales;v: 克里斯滕菌科f_Christensenellaceae;w: 克里斯滕菌科_R-7群g_Christensenellaceae_R-7_group;x: 厚壁菌纲c_Negativicutes;y: 杆菌纲c_Bacilli;z: 丹毒丝菌目o_Erysipelotrichales;a1:丹毒丝菌科f_Erysipelotrichaceae;b1:图利杆菌属g_Turicibacter;c1:乳酸杆菌目o_Lactobacillales;d1:乳酸杆菌科f_Lactobacillaceae;e1:乳酸杆菌属g_Lactobacillus;f1:放线菌纲c_Actinobacteria;g1:放线菌目o_Actinomycetales;h1:放线菌科f_Actinomycetaceae;i1:变形菌门p_Proteobacteria;j1:伽玛变形杆菌纲c_Gammaproteobacteria;k1:肠杆菌目o_Enterobacterales;l1:肠杆菌科f_Enterobacteriaceae;m1:大肠埃希菌-志贺菌属g_Escherichia-Shigella;n1:拟杆菌门p_Bacteroidota;o1:拟杆菌纲c_Bacteroidia;p1:拟杆菌目o_Bacteroidales;q1:f_p-251-o5;r1:g_norank_f_p-251-o5;s1:普雷沃氏菌科f_Prevotellaceae;t1:普雷沃氏菌科UCG-001 g_Prevotellaceae_UCG-001;u1:普氏菌属g_Prevotella;v1:木犀科f_Muribaculaceae;w1:木犀科未命名属g_norank_f_Muribaculaceae;x1:理研氏菌科f_Rikenellaceae;y1:理研氏菌科RC9肠道群g_Rikenellaceae_RC9_gut_group;z1:拟杆菌科f_Bacteroidaceae;a2:拟杆菌属g_Bacteroides;b2:卟啉单胞菌科f_Porphyromonadaceae;c2:卟啉单胞菌属g_Porphyromonas;d2:梭杆菌门p_Fusobacteriota;e2:梭杆菌纲c_Fusobacteriia;f2:梭杆菌目o_Fusobacteriales;g2:梭杆菌科f_Fusobacteriaceae;h2:梭杆菌属g_Fusobacterium;i2:螺旋体门p_Spirochaetota;j2:螺旋体纲c_Spirochaetia;k2:螺旋体目o_Spirochaetales;l2:螺旋体科f_Spirochaetaceae;m2:密螺旋体属g_Treponema。 The LEfSe multi-level species hierarchy tree diagram (figure A) showed the phylogeny level from the phylum to the genus from the inside to the outside. Nodes with different colors indicated microbial groups that were significantly enriched in the corresponding groups and had a significant impact on the differences of groups (P < 0.05); light yellow nodes indicated microbial groups that had no significant differences in different groups or had no significant impact on the differences of groups (P>0.05). The different colors in the LDA discriminant chart (B) represented different groups, the vertical axis represented microorganisms with significant differences among groups, and the horizontal axis (LDA score) represented species abundance. The larger the LDA score, the greater the effect of species abundance. 图 3 母猪不同妊娠阶段和分娩后肠道微生物物种差异分析 Fig. 3 Analysis of differences in gut microbial species of sows at different stages of pregnancy and after delivery |
根据数据库注释结果,利用PICRUSt2对试验母猪肠道菌群进行COG功能预测分析。如图 4可知,不同妊娠阶段和分娩后粪便样本中细菌功能变化不大。在妊娠30 d时,丰度前5的COG功能包括翻译、核糖体结构和生物发生(translation, ribosomal structure and biogenesis),氨基酸的运输和代谢(amino acid transport and metabolism),转录(transcription),碳水化合物的转运和代谢(carbohydrate transport and metabolism),细胞壁/膜/包膜生物发生(cell wall/membrane/envelope biogenesis);妊娠70 d和110 d丰度前5的COG功能均为氨基酸的运输和代谢(amino acid transport and metabolism),翻译、核糖体结构和生物发生(translation, ribosomal structure and biogenesis),碳水化合物的转运和代谢(carbohydrate transport and metabolism),转录(transcription),无机离子的转运和代谢(inorganic ion transport and metabolism);分娩后丰度前5的COG功能包括氨基酸的运输和代谢(amino acid transport and metabolism),翻译、核糖体结构和生物发生(translation, ribosomal structure and biogenesis),细胞壁/膜/包膜生物发生(cell wall/membrane/envelope biogenesis),碳水化合物的转运和代谢(carbohydrate transport and metabolism),复制、重组、修复(replication, recombination and repair)。在这几个时期,菌群功能相似,但在相对丰度上存在一定的差异,其中妊娠70和110 d的差异较小。
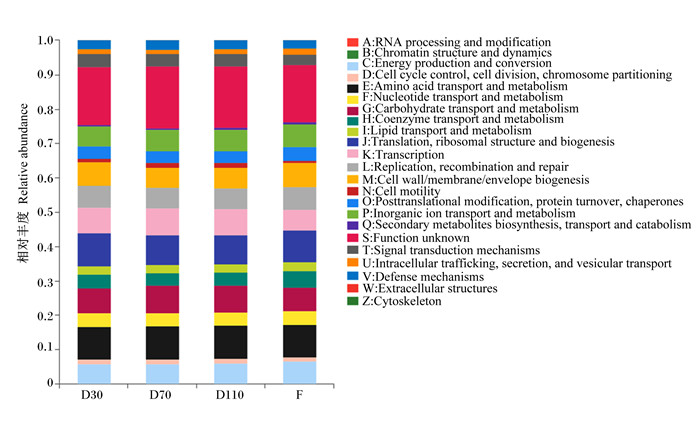
|
不同颜色代表不同的COG功能,颜色的多少代表相对丰度的大小。 RNA processing and modification:RNA加工和修饰;Chromatin structure and dynamics:染色质结构和动力学;Energy production and conversion:能源生产与转换;Cell cycle control, cell division, chromosome partitioning:细胞周期控制、细胞分裂、染色体分割;Amino acid transport and metabolism:氨基酸运输与代谢;Nucleotide transport and metabolism:核苷酸运输与代谢;Carbohydrate transport and metabolism:碳水化合物运输与代谢;Coenzyme transport and metabolism:辅酶运输与代谢;Lipid transport and metabolism:脂质运输与代谢;Translation, ribosomal structure and biogenesis:翻译、核糖体结构和生物发生;Transcription:转录;Replication, recombination and repair:复制、重组和修复;Cell wall/membrane/envelope biogenesis:细胞壁/膜/包膜生物发生;Cell motility:细胞运动性;Posttranslational modification, protein turnover, chaperones:转译后修饰、蛋白质周转、伴侣蛋白;Inorganic ion transport and metabolism:无机离子运输与代谢;Secondary metabolites biosynthesis, transport and catabolism:次生代谢产物生物合成、运输和分解代谢;Function unknown:功能未知;Signal transduction mechanisms:信号转导机制;Intracellular trafficking, secretion, and vesicular transport:细胞内运输、分泌和囊泡运输;Defense mechanisms:防御机制;Extracellular structures:细胞外结构;Cytoskeleton:细胞支架。 Different colors represented different COG functions, and the number of colors represented the size of relative abundance. 图 4 肠道微生物COG功能注释柱形图 Fig. 4 COG functional annotation bar chart of gut microbiome |
肠道中的微生物与宿主的健康和生理活动密不可分,在维持宿主健康的过程中发挥重要作用。研究表明,肠道微生物群是宿主代谢的中心调节器,能够调节宿主体内的许多代谢过程,包括能量稳态、葡萄糖代谢和脂质代谢等[10-11]。正常情况下,母猪肠道微生物群处于平衡状态,共同调节机体的健康。当生理状态、饲粮、环境等发生变化时,肠道微生物的平衡被打破,引起菌群失调,继而引发母猪免疫力下降、体况变差、便秘、厌食等,甚至表现出病理状态[12]。妊娠期是一个复杂的生理过程,在此期间母体会发生一系列变化,以适应胎儿生长发育需要,分娩后母体又逐渐恢复到未孕状态,机体会通过自身调控来维持母体健康。本试验发现,妊娠期间母体肠道微生物会发生重大变化,这与之前的研究结果[13-16]一致,但也有研究表明母体肠道微生物在妊娠期内并未发生显著性变化,而是处于相对稳定的状态[17-18],这可能与遗传、环境、饲粮等因素有关[19]。据此可推测,妊娠期母体通过调控肠道微生物保证自身健康和胎儿发育,而胎儿发育也会影响母体肠道微生物组成,具体的作用机制还有待研究。
本试验中,肠道微生物的多样性和丰富度随妊娠阶段的变化而变化,随着妊娠的进行,肠道微生物的多样性降低,丰富度升高。与之前研究结果相似,从妊娠前期至妊娠后期,母体肠道微生物多样性下降,但在丰富度变化上还存在差异[14-15, 20]。造成这种差异的原因可能与研究对象、遗传背景及肠道微生物的动态变化有关。分娩后肠道的多样性和丰富度均低于妊娠期,这与Cheng等[21]的研究结果一致。本试验中,整个妊娠期和分娩后母猪肠道微生物中优势菌门均为Firmicutes和Bacteroidetes,与前人的研究结果[22-23]一致。在妊娠30 d时,母猪肠道微生物中优势菌门为Firmicutes、Bacteroidetes和Spirochaetes,随着妊娠的进行,Spirochaetes的相对丰度降低,Proteobacteria和Actinobacteria的相对丰度升高。Koren等[14]也发现在妊娠后期Proteobacteria和Actinobacteria的相对丰度有升高的趋势。母猪分娩后母猪肠道微生物中Fusobacteria和Patescibacteria的相对丰度高于妊娠期。Cheng等[21]的研究也表明,从妊娠后期到泌乳前期,长白母猪肠道中Fusobacteria的相对丰度增加。在属水平上,随着妊娠的进行,Clostridium_sensu_stricto_1、Bacteroides、Escherichia-Shigella等菌群的相对丰度增加,Lactobacillus、Treponema、Prevotellaceae_UCG-001等菌属的相对丰度降低。分娩后,Clostridium_sensu_stricto_1、Terrisporobacter和克里斯滕森菌科R-7群(Christensenellaceae_R-7_group)的相对丰度降低,Bacteroides、Escherichia-Shigella、Prevotella、Porphyromonas和Fusobacterium的相对丰度升高,并且Bacteroides、Escherichia-Shigella成为分娩后肠道微生物中的优势菌属。刘红宾[24]、解培峰[25]、Cheng等[21]的研究也表明在妊娠期内Clostridium_sensu_stricto_1与Escherichia-Shigella的相对丰度随着妊娠的进行显著升高,Lactobacillus的相对丰度明显降低;在分娩后以Bacteroides、Escherichia-Shigella和Fusobacterium的相对丰度较高。
肠道微生物组成的变化可能与妊娠生理有关,在妊娠期,母体新陈代谢、生殖激素、免疫功能等急剧变化。研究发现,妊娠前期与未妊娠时肠道微生物组成相似,但随着妊娠的进行,肠道微生物组成发生变化[5]。在妊娠前期胚胎发育较慢,能量需要较低,主要用于维持母体组织的生长;中期和后期由于母猪内分泌活动增强、母体增重加快、胎儿体积增加、乳腺组织生长等,能量需求增加[26]。肠道微生物(Firmicutes、Bacteroidetes和Fusobacteria)能够产生短链脂肪酸(SCFA)为机体提供能量[27-28];并且SCFA能够抵抗炎症发生和抑制有害物质的积累,对母体和胎儿健康具有积极的影响[29-30]。同时,肠道中的Clostridium_sensu_stricto_1[31-33]、Bacteroides[34]、Treponema[35]、Prevotella[36]等能够降解低聚糖、多糖、木质纤维素及难以消化的物质,为母猪和胎儿提供能量。在本研究中,母猪妊娠后期肠道微生物中Clostridium_sensu_stricto_1、Bacteroides、Prevotella等与能量相关的菌群相对丰度高于妊娠前期,为妊娠后期胎儿快速发育提供能量支持[20, 35, 37]。
肠道微生物还通过影响胆汁酸的代谢来调节脂质代谢[38-40]。有研究表明,肠道微生物与胆汁酸具有双向调控作用,与此相关的微生物有Lactobacillus、Clostridium_sensu_stricto_1、Bacteroides等[40]。本研究中,母猪肠道微生物中Clostridium_sensu_stricto_1、Bacteroides的相对丰度随着妊娠的进行而升高,可能影响了脂质代谢,从而促进胎儿生长[41],但具体的作用机制还有待验证。激素也与母体肠道微生物的变化有关,雌激素水平能引起肠道菌群丰度发生变化;反之,肠道菌群的丰度和功能也能影响雌激素水平[42-43]。雌激素和孕酮(progesterone, P4)是维持妊娠和调节生殖中各种功能的关键激素[44-45],其合成与Firmicutes、Actinobacteria、Proteobacteria有关[46]。研究发现,随着胚胎的发育,母体孕酮和雌二醇(estradiol, E2)的含量升高,而P4与双歧杆菌、E2与Clostridium_sensu_stricto_1的丰度存在正相关[46-47],E2与普雷沃氏菌科(Prevotellaceae)的丰度存在负相关[48],Clostridium_sensu_stricto_1、Prevotellaceae和双歧杆菌分别属于Firmicutes、Bacteroidetes、Actinobacteria。这与本研究结果一致,即随着妊娠的进行,母猪肠道微生物中Firmicutes、Actinobacteria的相对丰度呈现升高的趋势,Bacteroidetes的相对丰度呈现降低的趋势。此外,炎症是影响母猪健康状况的主要因素之一,随着妊娠的进行,与炎症反应相关的肠道菌群的丰度增加,血清中促炎细胞因子的含量增多[21, 49-50]。在妊娠后期和分娩前后,促炎因子白细胞介素-6(IL-6)的水平升高,抗炎因子白细胞介素-10(IL-10)的水平降低,而高丰度的Proteobacteria、Actinobacteria和Fusobacteria会增加炎症发生的可能[21, 37]。本研究也表明,随着妊娠的进行,母猪肠道微生物中Proteobacteria、Actinobacteria和Fusobacteria的相对丰度升高,可能促进了炎症反应的发生。
综上所述,肠道微生物与母体生理状态密切相关,能够参与母体能量代谢、脂质代谢、激素分泌和调节葡萄糖稳态等,并且在抵抗炎症、加强机体的免疫屏障等方面具有积极作用。本研究揭示了妊娠期母猪肠道微生物的变化规律,为后续开展饲料添加剂或营养因子通过改变肠道微生物多样性影响母猪繁殖性能的相关研究奠定了基础。
4 结论本研究发现,随着妊娠日龄的增加,母猪肠道微生物丰富度升高、多样性降低。肠道微生物中优势菌门为Firmicutes和Bacteroidetes,妊娠期优势菌属为Clostridium_sensu_stricto_1、Lactobacillus、Bacteroides等,且菌群随妊娠的进行动态变化;分娩后微生物多样性和丰富度均降低,优势菌属为Bacteroides、Escherichia-Shigella、Porphyromonas等。母猪妊娠70和110 d时肠道微生物结构相似,并且与妊娠30 d和分娩后明显不同。
| [1] |
翁善钢, 陈琳, BRUTSAERT B, 等. 母猪肠道越健康, 窝重越大[J]. 国外畜牧学(猪与禽), 2015, 35(3): 21-22. WENG S G, CHEN L, BRUTSAERT B, et al. Healthy sow gut-higher litter weight[J]. Animal Science Abroad (Pigs and Poultry), 2015, 35(3): 21-22 (in Chinese). |
| [2] |
LEY R E, PETERSON D A, GORDON J I. Ecological and evolutionary forces shaping microbial diversity in the human intestine[J]. Cell, 2006, 124(4): 837-848. DOI:10.1016/j.cell.2006.02.017 |
| [3] |
CASTILLO M, MARTÍN-OR U ' E S M, ANGUITA M, et al. Adaptation of gut microbiota to corn physical structure and different types of dietary fibre[J]. Livestock Science, 2007, 109(1/3): 149-152. |
| [4] |
杨伟平, 王建刚, 曹斌云. 猪肠道微生物群落组成变化及其影响因素[J]. 中国畜牧杂志, 2017, 53(1): 12-16. YANG W P, WANG J G, CAO B Y. Composition and influence factors of pigs' gut microbial community[J]. Chinese Journal of Animal Science, 2017, 53(1): 12-16 (in Chinese). |
| [5] |
NURIEL-OHAYON M, NEUMAN H, KOREN O. Microbial changes during pregnancy, birth, and infancy[J]. Frontiers in Microbiology, 2016, 7: 1031. |
| [6] |
AL-ASMAKH M, HEDIN L, PETTERSSON S. Maternal microbiota regulate glucocorticoids levels and placental development in mice[J]. Endocrine Abstracts, 2015, 37: OC5.1. |
| [7] |
VUONG H E, PRONOVOST G N, WILLIAMS D W, et al. The maternal microbiome modulates fetal neurodevelopment in mice[J]. Nature, 2020, 586(7828): 281-286. DOI:10.1038/s41586-020-2745-3 |
| [8] |
王津, 马蕊, 韩烨, 等. 母猪繁殖周期肠道微生物的研究[J]. 饲料研究, 2016(20): 30-34. WANG J, MA R, HAN Y, et al. Study on intestinal microflora of sows during reproductive cycle[J]. Feed Research, 2016(20): 30-34 (in Chinese). |
| [9] |
LYNCH S V, PEDERSEN O. The human intestinal microbiome in health and disease[J]. The New England Journal of Medicine, 2016, 375(24): 2369-2379. DOI:10.1056/NEJMra1600266 |
| [10] |
SCHOELER M, CAESAR R. Dietary lipids, gut microbiota and lipid metabolism[J]. Reviews in Endocrine & Metabolic Disorders, 2019, 20(4): 461-472. |
| [11] |
SONNENBURG J L, BÄCKHED F. Diet-microbiota interactions as moderators of human metabolism[J]. Nature, 2016, 535(7610): 56-64. DOI:10.1038/nature18846 |
| [12] |
古金元, 彭涛, 胡东方, 等. 浅谈妊娠母猪生理特性及发酵饲料对其影响[J]. 猪业科学, 2017, 34(7): 94-96. GU J Y, PENG T, HU D F, et al. Discussion on physiological characteristics of pregnant sows and effects of fermented feed on them[J]. Swine Industry Science, 2017, 34(7): 94-96 (in Chinese). DOI:10.3969/j.issn.1673-5358.2017.07.040 |
| [13] |
HUANG X C, GAO J, ZHAO Y Z, et al. Dramatic remodeling of the gut microbiome around parturition and its relationship with host serum metabolic changes in sows[J]. Frontiers in Microbiology, 2019, 10: 2123. DOI:10.3389/fmicb.2019.02123 |
| [14] |
KOREN O, GOODRICH J K, CULLENDER T C, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy[J]. Cell, 2012, 150(3): 470-480. DOI:10.1016/j.cell.2012.07.008 |
| [15] |
KONG X F, JI Y J, LI H W, et al. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: effects of food composition at different times of pregnancy[J]. Scientific Reports, 2016, 6: 37224. DOI:10.1038/srep37224 |
| [16] |
LIU H B, HOU C L, LI N, et al. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation[J]. The FASEB Journal, 2019, 33(3): 4490-4501. DOI:10.1096/fj.201801221RR |
| [17] |
BISANZ J E, ENOS M K, PRAYGOD G, et al. Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of Moringa-supplemented probiotic yogurt[J]. Applied and Environmental Microbiology, 2015, 81(15): 4965-4975. DOI:10.1128/AEM.00780-15 |
| [18] |
GOHIR W, WHELAN F J, SURETTE M G, et al. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother's periconceptional diet[J]. Gut Microbes, 2015, 6(5): 310-320. DOI:10.1080/19490976.2015.1086056 |
| [19] |
李扬. 纤维营养对妊娠母猪繁殖性能和肠道菌群的影响[D]. 博士学位论文. 雅安: 四川农业大学, 2019. LI Y. Effects of dietary fiber in gestation diet on sow reproductive performance and gut microbiota[D]. Ph. D. Thesis. Ya'an: Sichuan Agricultural University, 2019. (in Chinese) |
| [20] |
王仁杰, 贺宇, 张云常, 等. 妊娠后期与泌乳期母猪肠道微生物多样性研究[J]. 动物营养学报, 2021, 33(1): 537-545. WANG R J, HE Y, ZHANG Y C, et al. A study on intestinal microbiota diversity of sows during late gestation and lactation periods[J]. Chinese Journal of Animal Nutrition, 2021, 33(1): 537-545 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.01.054 |
| [21] |
CHENG C S, WEI H K, YU H C, et al. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites[J]. Frontiers in Microbiology, 2018, 9: 1989. DOI:10.3389/fmicb.2018.01989 |
| [22] |
WANG H, JI Y C, YIN C, et al. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation[J]. Frontiers in Microbiology, 2018, 9: 1665. DOI:10.3389/fmicb.2018.01665 |
| [23] |
ZHAO J B, LIU P, HUANG C F, et al. Effect of wheat bran on apparent total tract digestibility, growth performance, fecal microbiota and their metabolites in growing pigs[J]. Animal Feed Science and Technology, 2018, 239: 14-26. DOI:10.1016/j.anifeedsci.2018.02.013 |
| [24] |
刘红宾. 母猪微生物垂直传递影响仔猪肠道的微生物定植与功能发育[D]. 博士学位论文. 北京: 中国农业大学, 2018. LIU H B. Vertical transmission of sows' microbiota affects microbial colonization and functional development of intestine in piglets[D]. Ph. D. Thesis. Beijing: China Agricultural University, 2018. (in Chinese) |
| [25] |
解培峰. 妊娠环江香猪繁殖性能及其肠道微生物代谢特性研究[D]. 硕士学位论文. 长沙: 湖南农业大学, 2018. XIE P F. Study on reproductive performance and intestinal microbial metabolic characteristics of pregnant Huanjiang mini-pigs[D]. Master's Thesis. Changsha: Hunan Agricultural University, 2018. (in Chinese) |
| [26] |
杨友成. 关于猪机体对能量需要量的分析[J]. 饲料博览, 2018(6): 80. YANG Y C. Analysis of energy requirement of pig body[J]. Feed Review, 2018(6): 80 (in Chinese). DOI:10.3969/j.issn.1001-0084.2018.06.041 |
| [27] |
DAHIYA D K, RENUKA, PUNIYA M, et al. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review[J]. Frontiers in Microbiology, 2017, 8: 563. DOI:10.3389/fmicb.2017.00563 |
| [28] |
刘壮. 猪肠道微生物与机体营养代谢[J]. 饲料博览, 2017(8): 16-22. LIU Z. The pigs intestinal microbiota and host nutrient metabolism[J]. Feed Review, 2017(8): 16-22 (in Chinese). DOI:10.3969/j.issn.1001-0084.2017.08.005 |
| [29] |
FURUSAWA Y, OBATA Y, FUKUDA S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells[J]. Nature, 2013, 504(7480): 446-450. DOI:10.1038/nature12721 |
| [30] |
田威龙, 李月月, 吕冬玲, 等. 猪不同生长阶段肠道微生物组的变化及其影响因素[J]. 中国畜牧兽医, 2021, 48(2): 509-515. TIAN W L, LI Y Y, LV D L, et al. Changes of intestinal microbiome and its influencing factors at different growth stages in pigs[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(2): 509-515 (in Chinese). |
| [31] |
WLODARSKA M, WILLING B P, BRAVO D M, et al. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection[J]. Scientific Reports, 2015, 5: 9253. DOI:10.1038/srep09253 |
| [32] |
ZHOU S H, GENG B, LI M J, et al. Comprehensive analysis of environmental factors mediated microbial community succession in nitrogen conversion and utilization of ex situ fermentation system[J]. The Science of the Total Environment, 2021, 769: 145219. DOI:10.1016/j.scitotenv.2021.145219 |
| [33] |
LIU T, SUN L, MVLLER B, et al. Importance of inoculum source and initial community structure for biogas production from agricultural substrates[J]. Bioresource Technology, 2017, 245. |
| [34] |
POWER S E, O'TOOLE P W, STANTON C, et al. Intestinal microbiota, diet and health[J]. British Journal of Nutrition, 2014, 111(3): 387-402. DOI:10.1017/S0007114513002560 |
| [35] |
郭秀兰. 猪肠道硬壁菌门和拟杆菌门数量的检测及其相对丰度与脂肪沉积的相关性研究[D]. 博士学位论文. 雅安: 四川农业大学, 2008. GUO X L. Detection of Firmicutes and Bacteroidetes in the pig gut and the correlation between their abundance and fat deposit[D]. Ph. D. Thesis. Ya'an: Sichuan Agricultural University, 2008. (in Chinese) |
| [36] |
陈宝剑, 吴永绍, 覃兆鲜, 等. 猪不同发育阶段肠道微生物菌群特征分析[J]. 中国畜牧杂志, 2021, 57(1): 101-108. CHEN B J, WU Y S, QIN Z X, et al. Characteristics of intestinal microflora in different developmental stages of pigs[J]. Chinese Journal of Animal Science, 2021, 57(1): 101-108 (in Chinese). |
| [37] |
ZHANG J H, MENG H, KONG X C, et al. Combined effects of polyethylene and organic contaminant on zebrafish (Danio rerio): accumulation of 9-nitroanthracene, biomarkers and intestinal microbiota[J]. Environmental Pollution, 2021, 277: 116767. DOI:10.1016/j.envpol.2021.116767 |
| [38] |
NEEDHAM B D, KADDURAH-DAOUK R, MAZMANIAN S K. Gut microbial molecules in behavioural and neurodegenerative conditions[J]. Nature Reviews Neuroscience, 2020, 21(12): 717-731. DOI:10.1038/s41583-020-00381-0 |
| [39] |
程雅婷, 孔祥峰. 短链脂肪酸的生理作用及其在母猪生产中的应用[J]. 动物营养学报, 2021, 33(10): 5435-5440. CHENG Y T, KONG X F. Physiological functions of short-chain fatty acids and their application in sow production[J]. Chinese Journal of Animal Nutrition, 2021, 33(10): 5435-5440 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.10.004 |
| [40] |
YU Y J, RAKA F, ADELI K. The role of the gut microbiota in lipid and lipoprotein metabolism[J]. Journal of Clinical Medicine, 2019, 8(12): 2227. DOI:10.3390/jcm8122227 |
| [41] |
彭健. 妊娠母猪背膘过厚影响繁殖性能的机制及其营养调控技术研究[J]. 饲料工业, 2018, 39(19): 1-8. PENG J. Mechanism of declining reproductive performance by excessive backfat of sows during gestation and its nutritional regulation techniques[J]. Feed Industry, 2018, 39(19): 1-8 (in Chinese). |
| [42] |
HUANG G N, XU J, LEFEVER D E, et al. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis[J]. Toxicology and Applied Pharmacology, 2017, 332: 138-148. DOI:10.1016/j.taap.2017.04.009 |
| [43] |
DI SIMONE N, SANTAMARIA ORTIZ A, SPECCHIA M, et al. Recent insights on the maternal microbiota: impact on pregnancy outcomes[J]. Frontiers in Immunology, 2020, 11: 528202. DOI:10.3389/fimmu.2020.528202 |
| [44] |
LIU Y, JIANG J, LEPIK B, et al. Subdomain 2, not the transmembrane domain, determines the dimerization partner of growth hormone receptor and prolactin receptor[J]. Endocrinology, 2017, 158(10): 3235-3248. DOI:10.1210/en.2017-00469 |
| [45] |
SAWYER G, WEBSTER D, NARAYAN E. Measuring wool cortisol and progesterone levels in breeding maiden Australian merino sheep (Ovis aries)[J]. PLoS One, 2019, 14(4): e0214734. DOI:10.1371/journal.pone.0214734 |
| [46] |
HUSSAIN T, MURTAZA G, KALHORO D H, et al. Relationship between gut microbiota and host-metabolism: emphasis on hormones related to reproductive function[J]. Animal Nutrition, 2021, 7(1): 1-10. DOI:10.1016/j.aninu.2020.11.005 |
| [47] |
薛云. 孕酮和雌二醇对猪粪细菌区系的影响[D]. 硕士学位论文. 南京: 南京农业大学, 2017. XUE Y. Effects of progesterone & estradiol on the fecal microflora in pigs[D]. Master's Thesis. Nanjing: Nanjing Agricultural University, 2017. (in Chinese) |
| [48] |
SANTOS-MARCOS J A, RANGEL-ZUÑIGA O A, JIMENEZ-LUCENA R, et al. Influence of gender and menopausal status on gut microbiota[J]. Maturitas, 2018, 116: 43-53. DOI:10.1016/j.maturitas.2018.07.008 |
| [49] |
SANTACRUZ A, COLLADO M C, GARCÍA-VALDÉS L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women[J]. British Journal of Nutrition, 2010, 104(1): 83-92. DOI:10.1017/S0007114510000176 |
| [50] |
CANI P D, OSTO M, GEURTS L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity[J]. Gut Microbes, 2012, 3(4): 279-288. DOI:10.4161/gmic.19625 |




