2. 江西天佳 生物工程股份有限公司, 南昌 330200
2. Jiangxi Tianjia Biological Engineering Company Limited, Nanchang 330200, China
肠道不仅是消化和吸收营养物质的重要场所,也是机体重要的屏障器官,能够抵御内源性和外源性有害物质进入机体内环境[1]。许多因素如应激、感染和炎症等能导致肠黏膜损伤和功能障碍[2],但是肠道损伤的机制尚未阐明。
肠道损伤与细胞死亡密切相关。目前公认的主要细胞死亡类型有3种,即坏死、凋亡和自噬。凋亡又称“程序性死亡”,在生物体发育过程中普遍存在,是一种由基因控制的、主动的、有序性的细胞死亡方式。坏死则是由一种由物理、化学或生物等因素导致的细胞死亡方式,传统上,坏死被认为是无序的、被动的、不可逆和不可调控的[2-3]。近年来,一种新鉴定的细胞死亡方式——程序性坏死,因有别于传统的细胞死亡方式——坏死、凋亡及自噬,而成为生命医学领域的研究热点[3]。研究表明,程序性坏死是一种由基因决定的细胞主动有序的死亡方式,不依赖于半胱氨酸天冬氨酸蛋白酶(Caspase)途径,一般在凋亡被抑制的情况下发生,可最终引发邻近细胞的免疫应答[4];死亡细胞具有典型的坏死细胞的形态学特征,细胞和细胞器体积肿大,线粒体扩大崩解,溶酶体膜破坏,酶类释放到周围组织,核膜破裂,核溶解,碎核涌出细胞,会引起周围组织的炎症反应[5]。
程序性坏死信号通路的关键调控因子有受体相互作用蛋白激酶1(RIP1)、受体相互作用蛋白激酶3(RIP3)、混合系列蛋白激酶结构域样蛋白(MLKL)[6]。研究表明,程序性坏死在缺血再灌注和炎症反应等多种因素导致的组织损伤中发挥重要作用。抑制程序性坏死对这些因素诱导的组织损伤具有重要的预防和治疗作用[7]。然而,程序性坏死在脂多糖(LPS)注射诱导的仔猪肠道损伤中的作用尚未被阐明。因此,本试验采用腹膜注射LPS构建仔猪肠道损伤模型,来探究LPS刺激对断奶仔猪肠道形态及程序性坏死信号通路的影响,为揭示程序性坏死在肠道损伤中的作用奠定初步基础,最终为寻求缓解肠道损伤的措施提供新的理论依据。
1 材料与方法 1.1 试验材料LPS:大肠杆菌血清型055∶B5,购于Sigma公司;RNA提取、反转录及实时定量PCR所用试剂盒信息参见黄菲菲等[8]。
1.2 试验设计选取12头平均体重为(7.07±0.57) kg的28日龄杜×长×大三元断奶仔猪,随机分为2组,分别为对照组和LPS刺激组,每组6头。所有仔猪自由饮水、采食,预饲9 d,仔猪状态良好,体重达到(8.96±0.54) kg。LPS刺激组腹腔注射100 μg/kg BW的LPS,对照组注射等量的生理盐水。注射LPS 4 h后将仔猪麻醉屠宰,取空肠中部置于冰上,用生理盐水清洗后,剪取3 cm固定于4%多聚甲醛溶液中用于制作组织切片;另取相近肠段10 cm沿纵向剪开刮取肠黏膜后存放在液氮中,最后再转移到-80 ℃超低温冰箱保存待测。
1.3 测定指标与方法 1.3.1 空肠组织形态学分析首先将空肠组织样品进行包埋,制成5 μm的切片,然后进行苏木素-伊红(HE)染色、树脂胶封片等处理,之后在Olympus光学显微镜下观察空肠组织形态,测量绒毛高度、隐窝深度,并计算两者比值。具体方法参照陈逢[9]。
1.3.2 空肠黏膜DNA、RNA、蛋白质含量测定取空肠黏膜制备成匀浆液,然后稀释为1%匀浆液后测定DNA、RNA、蛋白质含量。蛋白质含量采用南京建成生物工程研究所生产的考马斯亮蓝试剂盒测定,DNA和RNA含量采用参照Johnson等[10]使用分光光度计法测定。根据蛋白质、DNA和RNA含量计算RNA/DNA、蛋白质/DNA值。
1.3.3 空肠炎性细胞因子和程序性坏死信号通路相关基因mRNA相对表达量测定提取空肠黏膜总RNA,然后按照Prime Script RT Reagent Kit试剂盒步骤将RNA逆转录成cDNA,荧光定量聚合酶链式反应采用SYBR® Premix Ex TaqTM(Tli RNaseH Plus)real-time PCR试剂盒。以3-磷酸甘油醛脱氢酶(GAPDH)作为参比基因,采用Livak等[11]的2-ΔΔCt法计算目的基因——炎性细胞因子[环氧合酶2(COX2)、热休克蛋白70(HSP70)、白细胞介素-6(IL-6)]和程序性坏死信号通路相关基因[RIP1、自杀相关因子死亡结构域(FADD)、Caspase8、RIP3、MLKL、磷酸甘油酸变位酶5(PAGM5)、动力相关蛋白1(Drp1)、高迁移率族蛋白1(HMGB1)]的mRNA相对表达量。引物序列见表 1。
|
|
表 1 引物序列 Table 1 Primer sequences |
试验数据采用SPSS 22.0软件进行独立样本t检验,结果以平均值±标准误来表示。以P≤0.01为差异极显著,P≤0.05为差异显著,0.05<P≤0.10为差异有显著趋势。
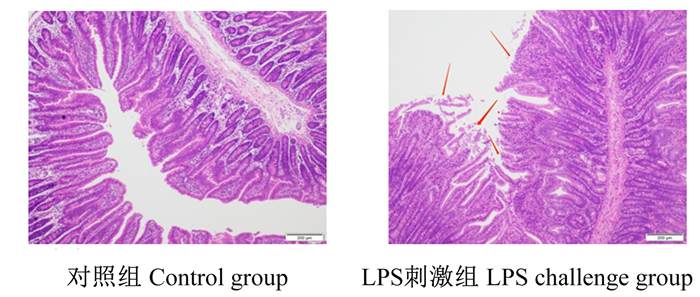
2 结果 2.1 LPS刺激对断奶仔猪空肠形态结构的影响由图 1可知,对照组空肠绒毛形态结构完整,绒毛较长;LPS刺激后空肠绒毛严重萎缩,并大量脱落。由表 2可知,与对照组相比,LPS刺激导致空肠绒毛高度和隐窝深度显著降低(P<0.05)。
|
图 1 空肠组织切片 Fig. 1 Sections of jejunal tissue (100×) |
|
|
表 2 LPS刺激对断奶仔猪空肠形态结构的影响 Table 2 Effects of LPS challenge on jejunal morphology of weaned piglets |
由表 3可知,与对照组相比,LPS刺激显著降低了空肠黏膜RNA/DNA(P<0.05),同时蛋白质含量和蛋白质/DNA有显著下降趋势(P=0.054、P=0.095)。
|
|
表 3 LPS刺激对断奶仔猪空肠黏膜蛋白质含量以及RNA/DNA和蛋白质/DNA的影响 Table 3 Effects of LPS challenge on protein content, RNA/DNA and protein/DNA in jejunum of weaned piglets |
由表 4可知,与对照组相比,LPS刺激后导致空肠HSP70和IL-6的mRNA相对表达量显著升高(P<0.05),COX2的mRNA相对表达量极显著升高(P<0.01)。
|
|
表 4 LPS刺激对断奶仔猪空肠炎性细胞因子mRNA相对表达量的影响 Table 4 Effects of LPS stimulation challenge on mRNA relative expression levels of inflammatory cytokines in jejunum of weaned piglets |
由表 5可知,与对照组相比,LPS刺激后导致空肠程序性坏死信号通路相关基因RIP1的mRNA相对表达量显著升高(P<0.05),RIP3的mRNA相对表达量极显著升高(P<0.01)。
|
|
表 5 LPS刺激对断奶仔猪空肠程序性坏死信号通路相关基因mRNA相对表达量的影响 Table 5 Effects of LPS challenge on mRNA relative expression levels of related genes of necroptotic signaling pathway in jejunum of weaned piglets |
LPS存在于所有革兰氏阴性细菌的细胞膜中,对宿主有毒性。LPS能诱导肠道形态学损伤,如黏膜下水肿、出血、黏膜坏死,并导致黏膜通透性增加和细菌移位[12]。因此,通过在猪腹膜或静脉注射一定剂量的LPS是构建仔猪肠道损伤模型的经典方式[13]。本试验采用LPS刺激仔猪,探究其对仔猪空肠形态结构、炎症反应和程序性坏死信号通路的影响。
肠道绒毛高度、隐窝深度、绒毛高度/隐窝深度常被用来评估肠道形态和结构的完整性[14]。蛋白质、RNA和DNA含量是反映肠道生长发育及损伤修复的指标,蛋白质/DNA、RNA/DNA可表明蛋白质合成能力[15]。本试验中,LPS刺激导致断奶仔猪肠道绒毛形态结构发生了显著变化,表现为绒毛萎缩、脱落;同时,LPS刺激导致空肠绒毛高度和隐窝深度显著降低,并显著降低了空肠黏膜RNA/DNA,同时蛋白质含量、蛋白质/DNA有显著下降趋势,表明LPS刺激导致断奶仔猪空肠结构和功能受损。Liu等[16]的研究结果也表明LPS刺激会导致断奶仔猪空肠和回肠黏膜结构损伤,与本研究结果一致。
研究表明,炎性细胞因子COX2、HSP70和IL-6等在炎症性肠病中起着重要作用[17]。COX2在炎症的发生和消退过程中都起到了重要作用:在炎症发生期通过合成前列腺素E2(PGE2)诱导炎症细胞释放趋化因子发挥促炎作用,在炎症消退期以合成前列腺素D2(PGD2)、15-脱氧前列腺素J2(15ΔPGJ2)发挥拮抗炎症和抑制氧化应激的作用[18]。HSP70在机体抵御各种有害刺激中发挥重要作用,可抗细胞凋亡、抗氧化,参与机体的先天免疫反应和细胞免疫[19]。IL-6则能通过加强炎性细胞因子的级联反应,促使炎性细胞因子大量产生,最终引起局部炎症反应[20]。Lang等[21]的研究结果表明,在大鼠肠道中LPS刺激会提高炎性细胞因子COX2和HSP70的mRNA相对表达量,并促使其在短时间内(1~2 h)达到峰值。本试验中,LPS刺激导致断奶仔猪空肠COX2、HSP70和IL-6的mRNA相对表达量显著提高,表明LPS刺激导致空肠炎症反应激活并使空肠受损加重。
在病理条件下,细胞程序性坏死和炎症互相影响[22]。目前,研究较为广泛的是肿瘤坏死因子(TNF)与死亡受体肿瘤坏死因子受体1(TNFR1)结合所介导的程序性坏死信号通路。TNF与TNFR1结合可引起后者构象变化,随后募集胞内的TNF受体相关信号分子肿瘤坏死因子受体相关死亡域蛋白(TRADD)、RIP1等形成复合体Ⅰ。随后,复合体Ⅰ上的TRADD和RIP1会解离并发生磷酸化且提供新的结合位点,这些结合位点又会募集FADD、Caspase8、RIP1形成新的复合体Ⅱ[23-25]。当Caspase8活性被抑制时,复合体Ⅱ上的RIP1、RIP3、MLKL发生磷酸化,执行程序性坏死功能。本试验检测了程序性坏死信号通路相关基因的mRNA相对表达量变化,结果表明,LPS刺激显著提高了断奶仔猪空肠程序性坏死信号通路相关基因RIP1、RIP3的mRNA相对表达量,说明LPS刺激导致程序性坏死信号通路激活。因此,我们推测,仔猪空肠的损伤和炎症反应与程序性坏死信号通路激活密切相关。
4 结论LPS刺激损伤了断奶仔猪的空肠形态结构,激活了炎症反应和程序性坏死信号通路。
| [1] |
LI H H, LI Y P, ZHU Q, et al. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88[J]. Journal of Applied Microbiology, 2018, 125(4): 964-975. DOI:10.1111/jam.13936 |
| [2] |
BLIKSLAGER A T, MOESER A J, GOOKIN J L, et al. Restoration of barrier function in injured intestinal mucosa[J]. Physiological Reviews, 2007, 87(2): 545-564. DOI:10.1152/physrev.00012.2006 |
| [3] |
詹雅清, 赖汉津, 沈建通, 等. 程序性坏死的发生机制及其在缺血损伤性疾病中的研究进展[J]. 中华普通外科学文献(电子版), 2021, 15(3): 224-228. ZHAN Y Q, LAI H J, SHEN J T, et al. Advances in the molecular mechanisms of necroptosis and its progression in organ ischemic injuries[J]. Chinese Archives of General Surgery (Electronic Edition), 2021, 15(3): 224-228 (in Chinese). DOI:10.3877/cma.j.issn.1674-0793.2021.03.015 |
| [4] |
LI Z, SCOTT M J, FAN E K, et al. Tissue damage negatively regulates LPS-induced macrophage necroptosis[J]. Cell Death and Differentiation, 2016, 23(9): 1428-1447. DOI:10.1038/cdd.2016.21 |
| [5] |
DEGTEREV A, HUANG Z H, BOYCE M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury[J]. Nature Chemical Biology, 2005, 1(2): 112-119. DOI:10.1038/nchembio711 |
| [6] |
李先根. 细胞程序性坏死在脂多糖诱导的仔猪肝脏损伤中的作用[D]. 硕士学位论文. 武汉: 武汉轻工大学, 2019. LI X G. The role of necroptosis on lipopolysaccharide-induced liver injury in piglets [D]. Master's Thesis. Wuhan: Wuhan Polytechnic University, 2019. (in Chinese) |
| [7] |
WANG Z C, FENG J N, YU J Y, et al. FKBP12 mediates necroptosis by initiating RIPK1-RIPK3-MLKL signal transduction in response to TNF receptor 1 ligation[J]. Journal of Cell Science, 2019, 132(10): jcs227777. |
| [8] |
黄菲菲, 张阳, 汪洋, 等. 脂多糖刺激后不同时间点断奶仔猪回肠和结肠中转化生长因子β1及Smads信号通路的动态变化[J]. 中国畜牧杂志, 2020, 56(12): 104-108. HUANG F F, ZHANG Y, WANG Y, et al. Developmental changes of TGF-β1 and smads signaling pathway in ileum and colon of piglets at different timepoints after LPS challenge[J]. Chinese Journal of Animal Science, 2020, 56(12): 104-108 (in Chinese). |
| [9] |
陈逢. 鱼油通过TLR4和NOD信号通路对脂多糖诱导的仔猪肠道、肝脏损伤和肌肉蛋白质降解的调控作用[D]. 硕士学位论文. 武汉: 武汉轻工大学, 2013. CHEN F. Regulative role of fish oil on intestinal and liver injury, and muscle protein degradation of piglets after lipopolysaccharide challenge through TLR4 and NOD signaling pathway[D]. Master's Thesis. Wuhan: Wuhan Polytechnic University, 2013. (in Chinese) |
| [10] |
JOHNSON L R, CHANDLER A M. RNA and DNA of gastric and duodenal mucosa in antrectomized and gastrin-treated rats[J]. The American Journal of Physiology, 1973, 224(4): 937-940. DOI:10.1152/ajplegacy.1973.224.4.937 |
| [11] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [12] |
PI D A, LIU Y L, SHI H F, et al. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge[J]. The Journal of Nutritional Biochemistry, 2014, 25(4): 456-462. DOI:10.1016/j.jnutbio.2013.12.006 |
| [13] |
朱惠玲, 谢小利, 刘玉兰, 等. 脂多糖应激对断奶仔猪肠黏膜免疫屏障的影响[J]. 中国畜牧杂志, 2009, 45(5): 14-17. ZHU H L, XIE X L, LIU Y L, et al. Effect of lipopolysaccharide challenge on intestinal mucosal immune barrier in weanling pigs[J]. Chinese Journal of Animal Science, 2009, 45(5): 14-17 (in Chinese). |
| [14] |
CHEN Y X, XIE Y N, ZHONG R Q, et al. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets[J]. Journal of Animal Science, 2021, 99(7): skab183. DOI:10.1093/jas/skab183 |
| [15] |
刘坚, 侯永清, 丁斌鹰, 等. α-酮戊二酸对脂多糖应激断奶仔猪空肠黏膜蛋白合成和抗氧化能力的影响[J]. 中国畜牧杂志, 2010, 46(11): 35-38. LIU J, HOU Y Q, DING B Y, et al. Effects of α-ketoglutaric acid on protein synthesis and antioxidative capacity in jejunal mucosa of weaned pigs chronically challenged with lipopolysaccharide[J]. Chinese Journal of Animal Science, 2010, 46(11): 35-38 (in Chinese). |
| [16] |
LIU Y L, CHEN F, ODLE J, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge[J]. The Journal of Nutrition, 2012, 142(11): 2017-2024. DOI:10.3945/jn.112.164947 |
| [17] |
GLOBIG A M, HENNECKE N, MARTIN B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease[J]. Inflammatory Bowel Diseases, 2014, 20(12): 2321-2329. DOI:10.1097/MIB.0000000000000210 |
| [18] |
谢翔, 应炜阳, 金胜威. COX-2在炎症消退中的研究进展[J]. 生命的化学, 2016, 36(4): 461-464. XIE X, YING W Y, JIN S W. Research progress of cyclooxygenase-2 in the resolution of inflammation[J]. Chemistry of Life, 2016, 36(4): 461-464 (in Chinese). |
| [19] |
刘嘉敏, 赵元莙. HSP70的研究进展及其在生物医学中的应用[J]. 教育教学论坛, 2017(50): 61-62. LIU J M, ZHAO Y J. Research progress for HSP70 and its application in biomedicine study[J]. Education Forum, 2017(50): 61-62 (in Chinese). DOI:10.3969/j.issn.1674-9324.2017.50.028 |
| [20] |
梁婵华, 黄妍, 莫敏敏, 等. 元宝枫籽油改善脂多糖诱导的小鼠肠道炎症[J]. 现代食品科技, 2021, 37(10): 37-45, 6. LIANG C H, HUANG Y, MO M M, et al. Acer truncatum bunge seed oil attenuated the lipopolysaccharide-induced intestinal inflammation in mice[J]. Modern Food Science & Technology, 2021, 37(10): 37-45, 6 (in Chinese). |
| [21] |
LANG C H, SILVIS C, DESHPANDE N, et al. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle[J]. Shock, 2003, 19(6): 538-546. DOI:10.1097/01.shk.0000055237.25446.80 |
| [22] |
NEWTON K, MANNING G. Necroptosis and inflammation[J]. Annual Review of Biochemistry, 2016, 85: 743-763. DOI:10.1146/annurev-biochem-060815-014830 |
| [23] |
郭双, 邢栋, 吕勃. 细胞凋亡及细胞程序性坏死和细胞焦亡的研究进展[J]. 中华实用诊断与治疗杂志, 2021, 35(3): 321-324. GUO S, XING D, LYU B. Advances in apoptosis, necroptosis and pyroptosis[J]. Journal of Chinese Practical Diagnosis and Therapy, 2021, 35(3): 321-324 (in Chinese). |
| [24] |
HÄCKER H, KARIN M. Regulation and function of IKK and IKK-related kinases[J]. Science's STKE, 2006, 2006(357): re13. |
| [25] |
CHO Y S, CHALLA S, MOQUIN D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation[J]. Cell, 2009, 137(6): 1112-1123. |




