2. 湖南普菲克生物科技有限公司, 长沙 410153;
3. 长沙医学院中药生物饲料研究中心, 长沙 410219
2. Hunan Perfly Biotech Co., Ltd., Changsha 410153, China;
3. Research Center of Traditional Chinese Medicine Biological Feed, Changsha Medical University, Changsha 410219, China
肠道内栖息着一个复杂多样的微生物群落,该群落由细菌、真菌、病毒和原生动物等组成。在长期共同进化过程中,微生物群落与宿主之间形成一个相互制约且动态平衡的微生态系统,并参与宿主的营养、代谢和免疫等重要生理过程[1]。短链脂肪酸(short-chain fatty acids,SCFAs)是肠道微生物的重要代谢产物之一,可被动物肠道快速吸收,在调节宿主代谢、免疫应答、肠道微生物及改善肠道功能等方面发挥重要作用[2-3]。国内外研究表明,SCFAs作为饲料添加剂具有良好的应用前景[4-5]。因此,本文系统综述了动物体内SCFAs的产生以及影响其产生的因素,同时讨论了SCFAs在肠道免疫反应中的调控机制及其在畜禽生产中的应用,以期为SCFAs的进一步研究及其作为饲料添加剂的开发应用提供参考。
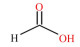
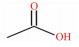
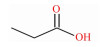
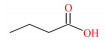
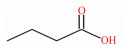
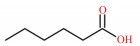
1 SCFAs的产生SCFAs又称挥发性脂肪酸,是指含有1~6个碳原子的有机脂肪酸,以直链或支链的构象存在,包括甲酸、乙酸、丙酸、丁酸、戊酸和己酸等(表 1)。抗性淀粉是指未能被小肠消化吸收,但能在结肠被微生物发酵的部分膳食淀粉。90%以上SCFAs是由抗性淀粉和膳食纤维在后肠微生物的作用下发酵产生,未被肠道微生物代谢的SCFAs通过被动扩散或单羧酸转运蛋白1(monocarboxylate transporter 1,MCT1)介导的主动转运被肠上皮细胞吸收[6]。一旦被吸收,SCFAs便成为肠上皮细胞重要的能量来源,或经血液循环供其他组织利用[7]。乙酸、丙酸、丁酸作为哺乳动物消化道含量最丰富的SCFAs,主要分布于结肠,它们被肠道吸收后,在宿主内的分布、去向及生理作用有所不同[8]。丁酸是肠上皮细胞的主要供能物质,丙酸主要被运往肝脏中代谢,而乙酸随体循环运送至宿主外周器官和组织被吸收利用[9]。在生理功能上,丁酸有促进肠黏膜上皮细胞增殖并增强结肠屏障功能的作用,还能在一定程度上减轻炎症和缓解腹泻[10];丙酸具有降低胆固醇、降血糖等生理功能,还能调节机体脂质代谢[11]。
|
|
表 1 SCFAs的化学式、结构式和摩尔质量 Table 1 Chemical formula, structural formula and molar mass of short-chain fatty acids |
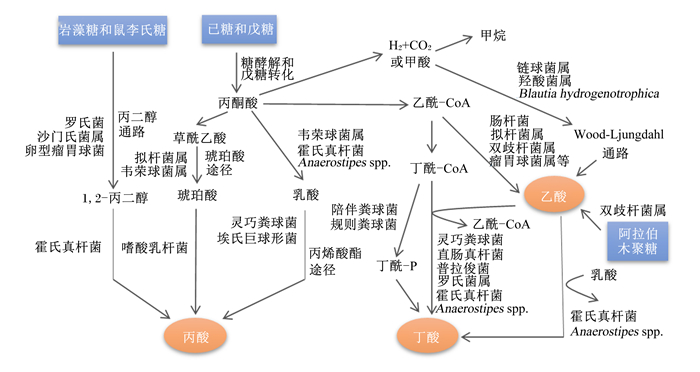
结肠中定居着丰富多样的微生物,是SCFAs产生的主要场所。乙酸、丙酸和丁酸在机消化道内的生成主要涉及的通路和参与的微生物如图 1所示。乙酸是结肠中含量最丰富的一种SCFAs,占粪便中检测到的总短链脂肪酸1/2以上。肠道中大部分乙酸是由丙酮酸在产乙酸菌的发酵作用下通过“乙酰-CoA途径”产生,而小部分乙酸则是由氢气(H2)和二氧化碳(CO2)或甲酸在链球菌属和羧酸菌属的发酵作用下通过“Wood-Ljundahl通路”合成的[12]。肠道细菌利用琥珀酸通过“琥珀酸途径”发酵产生丙酸,这是产丙酸的主要途径,此外,还可通过利用乳酸的“丙烯酸酯途径”和脱氧己糖(鼠李氏糖和岩藻糖)的“丙二醇通路”来发酵产生丙酸[13]。丁酸主要是在产丁酸菌的作用下,通过“丁酰CoA/乙酰CoA转移酶途径”和“丁酸激酶途径”2条途径发酵产生,大多数产丁酸菌利用“丁酰-CoA/乙酰-CoA转移酶途径”,而只有少量细菌(陪伴粪球菌、规则粪球菌)通过“丁酸激酶途径”[14-15]。此外,肠道中梭状芽胞杆菌属可以通过发酵氨基酸来产生乙酸和丁酸。
|
图 1 SCFAs的合成途径及其参与细菌 Fig. 1 Synthesis pathway of short-chain fatty acids and its participating bacteria[12-15] |
补充可溶性纤维能增加乙酸、丙酸、丁酸在结肠中的含量[16]。Cuerv等[17]发现,膳食纤维能增加粪便中乙酸和丙酸的含量,丁酸含量只与可溶性纤维相关。Zhao等[18]研究报道,饲喂高纤维饲粮能显著增加猪回肠总SCFAs的含量,回肠食糜中乙酸的含量与饲粮中性洗涤纤维和酸性洗涤纤维含量呈正相关,丁酸含量与可溶性纤维含量呈正相关。肉鸡饲喂高纤维饲粮也能增加盲肠食糜总SCFAs的含量,还能提高回肠寡糖的含量[19]。肠道总SCFAs含量与膳食纤维摄入量并不是线性关系。例如:用菊粉代替10%的小麦饲粮,盲肠中总SCFAs含量增加,当菊粉含量增加到20%时,盲肠总SCFAs含量却下降,同时SCFAs的组成由原来以乙酸为主转变为丙酸和丁酸为主[20]。饲粮成分的改变也是影响肠道SCFAs含量的重要因素。当饲粮中直链/支链淀粉比值增加时,育肥猪结肠食糜中丙酸和总SCFAs含量也随之增加[21]。用玉米芯代替麦麸饲喂断奶仔猪后,仔猪盲肠乙酸含量增加,但盲肠和结肠中丁酸含量显著降低[22]。Han等[23]给大鼠饲喂不同能量来源的饲粮发现,饲喂小麦饲粮的大鼠肠道中总SCFAs含量比饲喂稻谷饲粮的大鼠高,饲喂糙米和全麦的大鼠盲肠和结肠食糜中乙酸和丁酸含量远高于饲喂精米和精小麦。综上所述,膳食纤维能增加肠道总SCFAs含量,SCFAs的含量和组成由摄入膳食纤维的含量和种类决定,其原因可能是膳食纤维的摄入改变了肠道微生物区系的组成,从而影响肠道中SCFAs的生成。
2.2 肠道微生物群落的组成肠道内发酵膳食纤维的微生物组成不同,其产生SCFAs的种类及含量也不同。在猪的肠道中,结肠近端SCFAs含量高达140 mm/L,而远端最高仅40 mml/L,这是由于结肠近端含有丰富的微生物以及大量可发酵的物质[24]。Zeng等[25]将莲子抗性淀粉饲喂小鼠后,伴随着肠道微生物乳杆菌属(Lactobacillus)、梭状芽孢杆菌(Clostridium)、疣微菌科(Ruminococcaceae)和双歧杆菌属(Bifidobacterium spp.)的丰度显著增加,肠道内乙酸、丙酸和丁酸含量也随之增加,这提示SCFAs的含量与肠道菌群的丰度密切相关。可见,肠道中细菌的组成和数量是影响动物机体SCFAs产生的重要因素。此外,动物在应激条件下易导致肠道菌群失衡,伴随着肠道有益微生物丰度下降,结肠食糜中SCFAs尤其是乙酸和丁酸含量显著降低[26]。Liu等[27]给肠道失衡仔猪饲喂葛根芩连汤后,仔猪肠道微生物区系的组成得到改善,尤其是拟杆菌属、梭状芽孢杆菌、瘤胃球菌属(Ruminococcus spp.)等产SCFAs细菌的丰度显著提高,粪便中乙酸、丙酸和丁酸含量也显著增加。但目前国内外研究尚未证实微生物群落的组成是否直接影响宿主肠道内SCFAs含量。
2.3 益生菌和益生元益生菌能通过调节肠道有益菌群(包括产SCFAs细菌)的组成和生长来影响肠道内SCFAs的产生。据报道,益生菌嗜酸乳杆菌DDS-1处理后的动物盲肠中有益菌(如阿克曼菌属和乳酸杆菌属)的丰度显著增加,从而提高了肠道总SCFAs含量,尤其是丁酸的含量[28]。饲粮中添加粪链球菌可以促进断奶仔猪肠道发育,提高空肠食糜中丙酸、异丁酸等SCFAs含量[29]。Peng等[30]试验发现,植物乳杆菌B1能增加肉仔鸡肠道中乳酸菌的丰度,提高盲肠中乳酸和总SCFAs的含量, 并进一步抑制肠道中有害菌的繁殖。本实验室在不同阶段猪饲粮中添加德氏乳杆菌后,研究发现断奶仔猪和育肥猪结肠中菌群组成得到改善,同时结肠食糜中丁酸的含量得到提高。益生元是一类不被宿主消化吸收却能够被肠道菌群发酵,通过选择性地促进消化道有益菌的增殖和代谢,从而改善宿主健康的有机物质,其底物的类型、发酵活性以及与该过程相关的微生物种群强烈影响发酵终产物的组成[31-32]。例如,异麦芽糖能改善肠道菌群组成,增加有益菌群的丰度,抑制病原体繁殖,提高动物粪便中丙酸、丁酸及总SCFAs含量[33]。果聚糖能改善大肠肠道环境,提高肠道内双歧杆菌属和乳杆菌属的丰度,促进肠道细菌发酵产生SCFAs[34]。饲粮中添加菊粉能增加雏鸡肠道内乳杆菌属和链球菌属的丰度,提高盲肠中乙酸和丁酸含量,但使丙酸含量降低[35]。综上所述,益生菌和益生元能通过调节肠道菌群结构,尤其是增加产SCFAs菌数量及活性来影响肠道SCFAs产生。
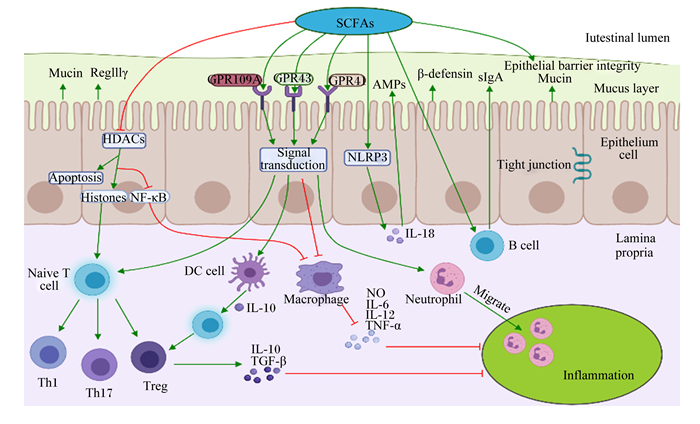
3 SCFAs对宿主肠道免疫的调控肠道是机体防御的最前线,也是最大的免疫器官。SCFAs在维持肠道稳态和调节免疫功能上发挥重要作用。免疫系统通过分泌细胞因子和介导病原体的清除来保护宿主免受病原体侵袭,但细胞因子过多分泌会导致机体免疫过激,对宿主肠道健康造成危害。SCFAs能通过调节免疫细胞的基因表达、趋化、分化、增殖和凋亡来参与机体免疫调控,这些调控过程主要通过激活G蛋白偶联受体(G protein-coupled receptors,GPCRs)和抑制组蛋白去乙酰化酶(histone deacetylases, HDACs)活性来实现(图 2)。
|
箭头表示促进;平头箭头表示抑制;红色和蓝色字符分别表示增加或减少。The narrow arrows indicate promotions; flathead arrows indicate inhibitions; red and blue characters indicate increases and decreases, respectively. SCFAs:短链脂肪酸short chain fatty acid;Intestinal lumen:肠腔;Mucus layer:黏液层;Epithelium cell:上皮细胞;Lamina propria:固有层;Naïve T cell:初始T细胞;Th:辅助性T细胞helper T cell;Macrophage:巨噬细胞;Neutrophil:中性粒细胞;DC cell:树突状细胞;B cell:B淋巴细胞;Treg:调节性T细胞regulatory T cells;Mucin:黏蛋白;β-defensin:β-防御素;IL:白细胞介素interleukin;TNF-α:肿瘤坏死因子-α tumor necrosis factor-α;AMPs:抗菌肽antimicrobial peptide;NO:一氧化氮nitric oxide;TGF-β:转化生长因子-β transforming growth factor-β;sIgA:分泌型免疫球蛋白A secretory immunoglobulin A;NLRP3:核苷酸结合寡聚化结构域样受体蛋白3 nucleotide binding oligomerization domain-like receptor protein 3;Epithelial barrier integrity:肠上皮屏障完整性;Tight junction:紧密连接;Migrate:迁移;Inflammation:炎症;Apoptosis:细胞凋亡;NF-κB:核转录因子-κB nuclear transcription factor-κB;GPR:G蛋白偶联受体G-protein-coupled receptors;HDACs:组蛋白去乙酰化酶histone deacetylase;Histones:组蛋白。 图 2 SCFAs对动物肠道免疫系统的调节 Fig. 2 Regulation of short-chain fatty acids on intestinal immune system in animals[40-41, 43-45, 51-53, 55-57] |
GPCRs涉及7个跨膜结构域,是哺乳动物最大的细胞表面受体蛋白家族,参与调节体内几乎所有的细胞和生理功能[36]。SCFAs激活的GPCRs主要是GPR41/FFAR3、GPR43/FFAR2和GPR109A/HCA2[37]。GPCRs广泛分布于多种免疫细胞和肠上皮细胞中,其中丙酸是FFAR3和FFAR2最有效的激动剂,而HCA2主要被丁酸激活[38-39]。Zhao等[40]试验发现,与野生型(WT)小鼠比较,GPR43-/-小鼠肠上皮细胞RegⅢγ和β-防御素1、3、4的表达水平均下降,随后给WT小鼠和GPR43-/-小鼠口服SCFAs,WT小鼠的RegⅢγ和β-防御素表达显著上升,而GPR43-/-小鼠无显著变化,这表明SCFAs以GPR43依赖的方式促进肠上皮细胞生成抗菌肽(antibacterial peptides,AMPs)和RegⅢγ。调节性T细胞(regulatory cells,Tregs)是一类控制体内自身免疫反应性的T细胞亚群,在调节肠道炎症中起着关键作用。丙酸处理GPR43-/-和GPR43+/+小鼠能增加GPR43+/+小鼠结肠中Tregs的数量,并促进Tregs中转录因子Foxp3和白细胞介素-10(IL-10)的表达,从而减轻了小鼠结肠炎症,但对GPR43-/-没有显著影响,表明这个调节过程依赖于GPR43[41]。丁酸盐能通过激活GPR41促进CD4+T细胞和先天淋巴样细胞(ILCs)芳基烃受体(AhR)和缺氧诱导因子1α(HIF1α)的表达来上调抗炎性因子白细胞介素-22(IL-22)的产生,保护肠道细胞免受炎性损伤[42]。丁酸钠可以缓解2,4,6-三硝基苯磺酸(2, 4, 6-trinitrobenzene sulfonic acid,TNBS)诱导的小鼠结肠炎和肠上皮屏障损伤,但对GPR109A-/-小鼠无保护作用;体外细胞试验发现,丁酸钠可以通过抑制脂多糖(lipopolysaccharide,LPS)诱导的巨噬细胞核转录因子(nuclear transcription factor,NF)-κB p65和蛋白激酶B(protein kinase B,AKT)通路磷酸化来下调肿瘤坏死因子-α(TNF-α)、白细胞介素-6(IL-6)的表达,这提示丁酸钠能通过激活GPR109A来抑制AKT和NF-κB信号通路的表达,从而改善TNBS诱导的小鼠炎症反应和肠上皮屏障功能障碍[43]。此外,乙酸能通过激活GPR43促进中性粒细胞迁移至炎症区域,并增强其细菌吞噬能力,从而缓解细菌感染引起的炎症损伤[44]。丁酸还可通过激活GPR109A促进肠上皮细胞白细胞介素-18(IL-18)的表达和产生IL-10的T细胞分化,抑制结肠炎症和结肠癌[45]。
3.2 通过抑制HDACs途径调节免疫应答SCFAs可直接通过抑制HDACs活性来调节免疫反应。众所周知,组蛋白乙酰化有利于各种转录因子和协同因子与DNA结合位点特异性结合,从而激活转录[46]。而HDACs通过去除与染色质结合的组蛋白赖氨酸乙酰基,导致染色质卷曲和转录沉默,进而抑制基因转录[47-48]。SCFAs作为HDACs抑制剂,能下调许多宿主防御基因的表达来调节机体免疫,包括受体识别、激酶、转录因子和细胞因子等[49]。据报道,丁酸盐能通过抑制结肠细胞和免疫细胞中HDACs活性,促进与信号转导相关的组蛋白超乙酰化,从而有利于基因表达和细胞分化[12]。体外细胞试验发现,丙酸和丁酸能通过下调LPS对外周单核细胞NF-κB通路的激活,抑制细胞因子TNF-α的分泌来调节细胞内的促炎反应,该过程与SCFAs抑制HDACs活性密切相关[50]。在不同细胞因子环境下,SCFAs能通过抑制HDACs调节T细胞分化所需mTOR-S6K通路中p70 S6激酶的乙酰化和rS6的磷酸化,从而促进初始T细胞分化为产生白细胞介素-17(IL-17)、IL-10或干扰素-γ(IFN-γ)的T细胞[51]。Furusawa等[52]报道,肠道共生微生物发酵产生的丁酸能抑制HDACs诱导结肠Tregs的分化,特别是CD4+T细胞亚群,具有缓解小鼠结肠炎的作用,其机制可能是SCFAs增强了Foxp3基因位点启动子和保守非编码区增强子的组蛋白H3乙酰化,从而上调了Foxp3基因的表达。Chang等[53]用丁酸处理肠道巨噬细胞后下调了LPS诱导的促炎因子一氧化氮(nitric oxide,NO)、IL-6和IL-12的表达,但不影响TNFα和单核细胞趋化蛋白-1(MCP-1)的分泌,且这些效应不依赖于Toll样受体(TLR)信号通路和GPCRs的激活,这表明SCFAs还能通过抑制HDACs来调节巨噬细胞的功能。此外,SCFAs对HDACs的抑制作用还与SCFAs的含量密切相关,大多数SCFAs(除乙酸外)在含量大于10 mmol/L时能有效抑制HDACs,且SCFAs的含量越高抑制效果越明显。值得注意的是,丁酸的抑制效果尤为突出,当丁酸浓度仅1 mmol/L时便能对HDACs有40%的抑制效果,而经2 mmol/L丁酸处理后,HDACs近乎被完全抑制[54]。
3.3 其他途径SCFAs可增强肠黏膜屏障功能,参与肠道免疫调节,其具体机制是通过增加肠上皮细胞跨膜电阻,促进紧密连接蛋白形成,抑制LPS诱导的核苷酸结合寡聚化结构域样受体蛋白3(nucleotide binding oligomerization domain-like receptor protein,NLRP3)炎症小体活化和自噬,从而刺激肠上皮屏障的形成,保护肠黏膜屏障免受炎症损伤[55]。SCFAs还参与其他免疫细胞功能的调节。一方面,SCFAs能通过调节树突状细胞(dendritic cell,DC)免疫抑制酶吲哚胺2,3-双加氧酶1(IDO1)和醛脱氢酶(Aldh1A2)的合成,促进初始T细胞转化为Foxp3+的Tregs,抑制产生炎性IFN-γ的T细胞分化,从而在预防结肠炎症中发挥关键作用[56];另一方面,丁酸能刺激肠黏膜中B细胞分泌免疫球蛋白A(secretory immunoglobulin A,sIgA),sIgA不仅能直接与细菌、病毒等病原体表面抗原结合,阻断其在黏膜上皮细胞表面的附着和定植,而且还能中和病原体所产生的酶和毒素等致病物质,是防止病原体入侵机体的第1道防线,在感染的早期即可对感染的发生和发展产生重要影响[57]。
4 SCFAs在畜禽生产中的应用在动物体内,SCFAs作为肠道微生物及肠上皮细胞的能量来源,具有维持水和电解质平衡、改善肠道菌群结构、调节机体免疫、降低炎症反应等多种生物学功能,对畜禽的健康生长起着至关重要的作用。通过查阅国内外文献发现,饲粮中添加SCFAs对不同畜禽各阶段的生长性能均有较好的促进作用。表 2总结了近年来给猪、鸡、牛在不同阶段饲喂SCFAs后对生长性能的影响,发现SCFAs在提高平均日增重方面的效果尤为显著。究其原因,一方面在于SCFAs能促进养分消化吸收,改善肠道菌群结构,提高肠道屏障功能,从而改善肠道健康。张瑞阳等[67]报道,饲粮中添加1 000或1 500 g/t丁酸钠均能显著提高断奶仔猪粗蛋白质、粗脂肪的表观消化率,降低仔猪粪便中大肠杆菌的丰度,增加乳酸杆菌的丰度。Wu等[68]在肉鸡试验中也发现,饲粮中添加800 mg/kg丁酸钠能增加回肠绒毛高度,刺激空肠和回肠绒毛杯状细胞的生长,并提高盲肠内毛螺菌科(Lachnospiraceae)的丰度,降低肠杆菌科(Enterobacteriaceae)的丰度;另一方面可能与其具有调节细胞因子分泌,提高抗体水平及免疫器官指数,进而增强免疫性能,提高抗病能力有关。Chen等[69]给生长猪静脉滴注10 mL(200 mmol/L)丁酸钠溶液后发现,结肠中促炎细胞因子IL-6、IL-8和TNF-α的基因表达显著降低,而sIgA的含量和抗炎细胞因子IL-10的表达显著增加。Luo等[70]研究表明,饲粮中添加400 mg/kg包被丁酸钠能增加肉仔鸡胸腺和法氏囊指数,提高血液中免疫球蛋白G(immunoglobulin G,IgG)和免疫球蛋白M(immunoglobulin M,IgM)含量。
|
|
表 2 SCFAs对畜禽生长性能的影响 Table 2 Effects of short-chain fatty acids on growth performance of livestock and poultry |
SCFAs(尤其是乙酸、丙酸和丁酸)作为肠道微生物发酵的主要代谢产物,因其具有维持肠道健康、提高机体免疫力、促进动物生长等作用,现已成为一种新型的绿色饲料添加剂。然而,SCFAs在畜禽生产中具有广阔的应用前景的同时,尚有诸多问题亟待解决,例如:1)SCFAs存在特殊气味,直接添加易导致采食量降低,在畜禽饲粮中的添加方式有待优化;2)SCFAs在畜禽生产中的应用研究还较少,如何与其他添加剂进行合理配伍及其添加水平仍需深入研究;3)SCFAs在调节宿主免疫功能、抑制肠道疾病发生中的作用机制仍不明确,是否与微生物存在互作效应值得进一步探讨。上述问题的解决有助于SCFAs在养殖生产上得到更广泛的应用,也可为疾病的预防和治疗提供新的思路。
| [1] |
SOMMER F, BÄCKHED F. The gut microbiota—masters of host development and physiology[J]. Nature Reviews Microbiology, 2013, 11(4): 227-238. DOI:10.1038/nrmicro2974 |
| [2] |
VAN DER HEE B, WELLS J M. Microbial regulation of host physiology by short-chain fatty acids[J]. Trends in Microbiology, 2021, 29(8): 700-712. DOI:10.1016/j.tim.2021.02.001 |
| [3] |
AKHTAR M, CHEN Y, MA Z Y, et al. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation[J]. Animal Nutrition, 2021. DOI:10.1016/j.aninu.2021.11.005 |
| [4] |
LIU W H, LA A L T Z, EVANS A, et al. Supplementation with sodium butyrate improves growth and antioxidant function in dairy calves before weaning[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 2. DOI:10.1186/s40104-020-00521-7 |
| [5] |
JIAO A R, DIAO H, YU B, et al. Infusion of short chain fatty acids in the ileum improves the carcass traits, meat quality and lipid metabolism of growing pigs[J]. Animal Nutrition, 2021, 7(1): 94-100. DOI:10.1016/j.aninu.2020.05.009 |
| [6] |
HALESTRAP A P, WILSON M C. The monocarboxylate transporter family-role and regulation[J]. IUBMB Life, 2012, 64(2): 109-119. DOI:10.1002/iub.572 |
| [7] |
TOPPING D L, CLIFTON P M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides[J]. Physiological Reviews, 2001, 81(3): 1031-1064. DOI:10.1152/physrev.2001.81.3.1031 |
| [8] |
YAO Y, CAI X Y, FEI W D, et al. The role of short-chain fatty acids in immunity, inflammation and metabolism[J]. Critical Reviews in Food Science and Nutrition, 2022, 62(1): 1-12. DOI:10.1080/10408398.2020.1854675 |
| [9] |
SLEETH M L, THOMPSON E L, FORD H E, et al. Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation[J]. Nutrition Research Reviews, 2010, 23(1): 135-145. DOI:10.1017/S0954422410000089 |
| [10] |
BEDFORD A, GONG J. Implications of butyrate and its derivatives for gut health and animal production[J]. Animal Nutrition, 2018, 4(2): 151-159. DOI:10.1016/j.aninu.2017.08.010 |
| [11] |
ZHOU H, YU B, SUN J, et al. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 61. DOI:10.1186/s40104-021-00581-3 |
| [12] |
LOUIS P, HOLD G L, FLINT H J. The gut microbiota, bacterial metabolites and colorectal cancer[J]. Nature Reviews Microbiology, 2014, 12(10): 661-672. DOI:10.1038/nrmicro3344 |
| [13] |
REICHARDT N, DUNCAN S H, YOUNG P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota[J]. The ISME Journal, 2014, 8(6): 1323-1335. DOI:10.1038/ismej.2014.14 |
| [14] |
FLINT H J, DUNCAN S H, SCOTT K P, et al. Links between diet, gut microbiota composition and gut metabolism[J]. The Proceedings of the Nutrition Society, 2015, 74(1): 13-22. DOI:10.1017/S0029665114001463 |
| [15] |
DUNCAN S H, BARCENILLA A, STEWART C S, et al. Acetate utilization and butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine[J]. Applied and Environmental Microbiology, 2002, 68(10): 5186-5190. DOI:10.1128/AEM.68.10.5186-5190.2002 |
| [16] |
GUERIN-DEREMAUX L, RINGARD F, DESAILLY F, et al. Effects of a soluble dietary fibre NUTRIOSE® on colonic fermentation and excretion rates in rats[J]. Nutrition Research and Practice, 2010, 4(6): 470-476. DOI:10.4162/nrp.2010.4.6.470 |
| [17] |
CUERVO A, SALAZAR N, RUAS-MADIEDO P, et al. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly[J]. Nutrition Research, 2013, 33(10): 811-816. DOI:10.1016/j.nutres.2013.05.016 |
| [18] |
ZHAO J B, BAI Y, TAO S Y, et al. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model[J]. Journal of Functional Foods, 2019, 57: 266-274. DOI:10.1016/j.jff.2019.04.009 |
| [19] |
LIN Y, OLUKOSI O A. Qualitative and quantitative profiles of jejunal oligosaccharides and cecal short-chain fatty acids in broiler chickens receiving different dietary levels of fiber, protein and exogenous enzymes[J]. Journal of the Science of Food and Agriculture, 2021, 101(12): 5190-5201. DOI:10.1002/jsfa.11165 |
| [20] |
DEN BESTEN G, VAN EUNEN K, GROEN A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research, 2013, 54(9): 2325-2340. DOI:10.1194/jlr.R036012 |
| [21] |
WANG H J, PU J N, CHEN D W, et al. Effects of dietary amylose and amylopectin ratio on growth performance, meat quality, postmortem glycolysis and muscle fibre type transformation of finishing pigs[J]. Archives of animal nutrition, 2019, 73(3): 1-14. |
| [22] |
CARNEIRO M S C, LORDELO M M, CUNHA L F, et al. Effects of dietary fibre source and enzyme supplementation on faecal apparent digestibility, short chain fatty acid production and activity of bacterial enzymes in the gut of piglets[J]. Animal Feed Science and Technology, 2008, 146(1/2): 124-136. |
| [23] |
HAN F, WANG Y, HAN Y Y, et al. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats[J]. Journal of Agricultural and Food Chemistry, 2018, 66(25): 6326-6335. DOI:10.1021/acs.jafc.8b01891 |
| [24] |
MARTIN L J M, DUMON H J W, CHAMP M M J. Production of short-chain fatty acids from resistant starch in a pig model[J]. Journal of the Science of Food and Agriculture, 1998, 77(1): 71-80. DOI:10.1002/(SICI)1097-0010(199805)77:1<71::AID-JSFA3>3.0.CO;2-H |
| [25] |
ZENG H L, HUANG C C, LIN S, et al. Lotus seed resistant starch regulates gut microbiota and increases short-chain fatty acids production and mineral absorption in mice[J]. Journal of Agricultural and Food Chemistry, 2017, 65(42): 9217-9225. DOI:10.1021/acs.jafc.7b02860 |
| [26] |
GAO R, TIAN S Y, WANG J, et al. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets[J]. Journal of Animal Science and Biotechnology, 2021, 12(1): 92. DOI:10.1186/s40104-021-00612-z |
| [27] |
LIU C S, LIANG X, WEI X H, et al. Gegen Qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids[J]. Frontiers in Microbiology, 2019, 10: 825. DOI:10.3389/fmicb.2019.00825 |
| [28] |
VEMURI R, GUNDAMARAJU R, SHINDE T, et al. Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice[J]. Nutrients, 2019, 11(6): 1297. DOI:10.3390/nu11061297 |
| [29] |
王汉星, 虎千力, 杨建涛, 等. 饲粮中添加粪链球菌与枯草芽孢杆菌对断奶仔猪生长性能和肠道健康的影响[J]. 动物营养学报, 2020, 32(5): 2074-2086. WANG H X, HU Q L, YANG J T, et al. Effects of dietary Streptococcus faecalis and Bacillus subtilis on growth performance and intestinal health of weaned piglets[J]. Chinese Journal of Animal Nutrition, 2020, 32(5): 2074-2086 (in Chinese). DOI:10.3969/j.issn.1006-267x.2020.05.016 |
| [30] |
PENG Q, ZENG X F, ZHU J L, et al. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens[J]. Poultry Science, 2016, 95(4): 893-900. DOI:10.3382/ps/pev435 |
| [31] |
GIBSON G R, ROBERFROID M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics[J]. The Journal of Nutrition, 1995, 125(6): 1401-1412. DOI:10.1093/jn/125.6.1401 |
| [32] |
SLAVIN J. Fiber and prebiotics: mechanisms and health benefits[J]. Nutrients, 2013, 5(4): 1417-1435. DOI:10.3390/nu5041417 |
| [33] |
YANG Z D, GUO Y S, HUANG J S, et al. Isomaltulose exhibits prebiotic activity, and modulates gut microbiota, the production of short chain fatty acids, and secondary bile acids in rats[J]. Molecules, 2021, 26(9): 2464. DOI:10.3390/molecules26092464 |
| [34] |
HAN Y, MA H R, LIU Y L, et al. Effects of goat milk enriched with oligosaccharides on microbiota structures, and correlation between microbiota and short-chain fatty acids in the large intestine of the mouse[J]. Journal of Dairy Science, 2021, 104(3): 2773-2786. DOI:10.3168/jds.2020-19510 |
| [35] |
SONG J, LI Q H, EVERAERT N, et al. Dietary inulin supplementation modulates short-chain fatty acid levels and cecum microbiota composition and function in chickens infected with Salmonella[J]. Frontiers in Microbiology, 2020, 11: 584380. DOI:10.3389/fmicb.2020.584380 |
| [36] |
WOOTTEN D, CHRISTOPOULOS A, MARTI-SOLANO M, et al. Mechanisms of signalling and biased agonism in G protein-coupled receptors[J]. Nature Reviews Molecular Cell Biology, 2018, 19(10): 638-653. DOI:10.1038/s41580-018-0049-3 |
| [37] |
NATARAJAN N, PLUZNICK J L. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology[J]. American Journal of Physiology.Cell Physiology, 2014, 307(11): C979-C985. DOI:10.1152/ajpcell.00228.2014 |
| [38] |
LE POUL E, LOISON C, STRUYF S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation[J]. Journal of Biological Chemistry, 2003, 278(28): 25481-25489. DOI:10.1074/jbc.M301403200 |
| [39] |
BLAD C C, TANG C, OFFERMANNS S. G protein-coupled receptors for energy metabolites as new therapeutic targets[J]. Nature Reviews Drug Discovery, 2012, 11(8): 603-619. DOI:10.1038/nrd3777 |
| [40] |
ZHAO Y, CHEN F D, WU W, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3[J]. Mucosal Immunology, 2018, 11(3): 752-762. DOI:10.1038/mi.2017.118 |
| [41] |
SMITH P M, HOWITT M R, PANIKOV N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis[J]. Science, 2013, 341(6145): 569-573. DOI:10.1126/science.1241165 |
| [42] |
YANG W J, YU T M, HUANG X S, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity[J]. Nature Communications, 2020, 11(1): 4457. DOI:10.1038/s41467-020-18262-6 |
| [43] |
CHEN G X, RAN X, LI B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model[J]. EBioMedicine, 2018, 30: 317-325. DOI:10.1016/j.ebiom.2018.03.030 |
| [44] |
GALVÃO I, TAVARES L P, CORRÊA R O, et al. The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung[J]. Frontiers in Immunology, 2018, 9: 142. DOI:10.3389/fimmu.2018.00142 |
| [45] |
SINGH N, GURAV A, SIVAPRAKASAM S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis[J]. Immunity, 2014, 40(1): 128-139. DOI:10.1016/j.immuni.2013.12.007 |
| [46] |
HE J, ZHANG P W, SHEN L Y, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism[J]. International Journal of Molecular Sciences, 2020, 21(17): 6356. DOI:10.3390/ijms21176356 |
| [47] |
HINNEBUSCH B F, MENG S F, WU J T, et al. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation[J]. The Journal of Nutrition, 2002, 132(5): 1012-1017. DOI:10.1093/jn/132.5.1012 |
| [48] |
ALENGHAT T, ARTIS D. Epigenomic regulation of host-microbiota interactions[J]. Trends in Immunology, 2014, 35(11): 518-525. DOI:10.1016/j.it.2014.09.007 |
| [49] |
ROGER T, LUGRIN J, LE ROY D, et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection[J]. Blood, 2011, 117(4): 1205-1217. DOI:10.1182/blood-2010-05-284711 |
| [50] |
USAMI M, KISHIMOTO K, OHATA A, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells[J]. Nutrition Research, 2008, 28(5): 321-328. DOI:10.1016/j.nutres.2008.02.012 |
| [51] |
PARK J, KIM M, KANG S G, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway[J]. Mucosal Immunology, 2015, 8(1): 80-93. DOI:10.1038/mi.2014.44 |
| [52] |
FURUSAWA Y, OBATA Y, FUKUDA S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells[J]. Nature, 2013, 504(7480): 446-450. DOI:10.1038/nature12721 |
| [53] |
CHANG P V, HAO L M, OFFERMANNS S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(6): 2247-2252. DOI:10.1073/pnas.1322269111 |
| [54] |
WALDECKER M, KAUTENBURGER T, DAUMANN H, et al. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon[J]. Journal of Nutritional Biochemistry, 2008, 19(9): 587-593. DOI:10.1016/j.jnutbio.2007.08.002 |
| [55] |
FENG Y H, WANG Y, WANG P, et al. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy[J]. Cellular Physiology and Biochemistry, 2018, 49(1): 190-205. DOI:10.1159/000492853 |
| [56] |
GURAV A, SIVAPRAKASAM S, BHUTIA Y D, et al. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions[J]. Biochemical Journal, 2015, 469(2): 267-278. DOI:10.1042/BJ20150242 |
| [57] |
MURAKOSHI S, FUKATSU K, OMATA J, et al. Effects of adding butyric acid to PN on gut-associated lymphoid tissue and mucosal immunoglobulin A levels[J]. Journal of Parenteral and Enteral Nutrition, 2011, 35(4): 465-472. DOI:10.1177/0148607110387610 |
| [58] |
LU H, SU S, AJUWON K M. Butyrate supplementation to gestating sows and piglets induces muscle and adipose tissue oxidative genes and improves growth performance[J]. Journal of Animal Science, 2012, 90(Suppl.4): 430-432. |
| [59] |
寇莎莎, 王诏升, 徐德旺, 等. 日粮中添加不同水平丁酸钠对断奶仔猪生长性能、腹泻率及血液生化指标的影响[J]. 中国畜牧兽医, 2018, 45(7): 1841-1848. KOU S S, WANG Z S, XU D W, et al. Effects of adding different levels of sodium butyrate on growth performance, diarrhea rate and blood biochemical indexes of weaned piglets[J]. China Animal Husbandry & Veterinary Medicine, 2018, 45(7): 1841-1848 (in Chinese). |
| [60] |
沈建明, 唐亮, 周学光, 等. 包膜丁酸钠在保育仔猪生产中的应用研究[J]. 中国畜牧杂志, 2010, 46(10): 64-67. SHEN J M, TANG L, ZHOU X G, et al. Application research of the coated sodium butyrate on the piglets[J]. Chinese Journal of Animal Science, 2010, 46(10): 64-67 (in Chinese). |
| [61] |
董冠, 杨维仁, 杨在宾, 等. 不同丁酸钠对生长猪生产性能的影响及其比较研究[J]. 饲料工业, 2012(S1): 56-58. DONG G, YANG W R, YANG Z B, et al. Effects of different sodium butyrates on growth performance of growing pigs and their comparative studies[J]. Feed Industry, 2012(S1): 56-58 (in Chinese). |
| [62] |
SUN W, SUN J, LI M, et al. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs[J]. Journal of Applied Microbiology, 2020, 128(6): 1613-1623. DOI:10.1111/jam.14612 |
| [63] |
LEVY A W, KESSLER J W, FULLER L, et al. Effect of feeding an encapsulated source of butyric acid (ButiPEARL) on the performance of male Cobb broilers reared to 42 d of age[J]. Poultry Science, 2015, 94(8): 1864-1870. DOI:10.3382/ps/pev130 |
| [64] |
邹杨, 杨在宾, 杨维仁, 等. 不同剂型丁酸钠与抗生素对肉仔鸡生产性能、肠道pH及挥发性脂肪酸含量的影响[J]. 动物营养学报, 2010, 22(3): 675-681. ZOU Y, YANG Z B, YANG W R, et al. Effects of different preparations of sodium butyrate and antibiotics on performance, intestinal pH and concentration of volatile fatty acids in broilers[J]. Chinese Journal of Animal Nutrition, 2010, 22(3): 675-681 (in Chinese). DOI:10.3969/j.issn.1006-267x.2010.03.023 |
| [65] |
俞文靓. 开食料中添加丁酸钠对哺乳期犊牛生长性能、瘤胃发酵和胃肠道发育的影响[D]. 硕士学位论文. 南宁: 广西大学, 2020. YU W L. Effects of sodium butyrate on growth performance, rumen fermentation and gastrointestional development of lactating calves[D]. Master's Thesis. Nanning: Guangxi University, 2020. (in Chinese) |
| [66] |
赵会利, 高艳霞, 李建国, 等. 丁酸钠对断奶犊牛生长、血液生化指标及胃肠道发育的影响[J]. 畜牧兽医学报, 2013, 44(10): 1600-1608. ZHAO H L, GAO Y X, LI J G, et al. Effect of sodium butyrate on growth, serum biochemical parameters and gastrointestinal development of weaning calves[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(10): 1600-1608 (in Chinese). DOI:10.11843/j.issn.0366-6964.2013.10.013 |
| [67] |
张瑞阳, 孟玲, 李方方, 等. 包被丁酸钠对断奶仔猪生长性能、血清生化指标、养分表观消化率和粪便微生物菌群的影响[J]. 动物营养学报, 2019, 31(5): 2296-2302. ZHANG R Y, MENG L, LI F F, et al. Effects of coated sodium butyrate on growth performance, serum biochemical indices, nutrient apparent digestibility and fecal microflora population of weaning piglets[J]. Chinese Journal of Animal Nutrition, 2019, 31(5): 2296-2302 (in Chinese). DOI:10.3969/j.issn.1006-267x.2019.05.036 |
| [68] |
WU W, XIAO Z B, AN W Y, et al. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers[J]. PLoS One, 2018, 13(5): e0197762. DOI:10.1371/journal.pone.0197762 |
| [69] |
CHEN X, XU J M, SU Y, et al. Effects of intravenous infusion with sodium butyrate on colonic microbiota, intestinal development- and mucosal immune-related gene expression in normal growing pigs[J]. Frontiers in Microbiology, 2018, 9: 1652. DOI:10.3389/fmicb.2018.01652 |
| [70] |
LUO D, LI J L, XING T, et al. Combined effects of xylo-oligosaccharides and coated sodium butyrate on growth performance, immune function, and intestinal physical barrier function of broilers[J]. Animal Science Journal, 2021, 92(1): e13545. |