肠道干细胞(intestinal stem cells,ISCs)及其分化的吸收细胞、杯状细胞、肠内分泌细胞和潘氏细胞等组成的单层肠上皮是机体与外界环境进行物质、信息交换的重要场所[1-2]。分子信号驱动的肠上皮生态系统的良性循环,包括Kelch样环氧氯丙烷相关蛋白1(Keap1)/核因子E2相关因子2(Nrf2)介导的抗氧化系统及Wnt和Notch介导的ISCs增殖分化系统,维持着肠上皮细胞的正常状态及其对营养物质的快速消化吸收[3-4]。然而,饲料源毒素、温热环境、有害菌、病毒等刺激因子的持续攻击会导致细胞线粒体内活性氧(reactive oxygen species,ROS)含量的急剧增加,扰乱肠道氧化还原系统平衡,诱导细胞凋亡,阻碍隐窝绒毛轴有序更新,损伤机体健康[5-9]。通过某些药物或营养素干预Keap1/Nrf2信号通路,提高抗氧化酶和解毒酶水平,可有效清除肠细胞内累积的ROS,促进ISCs再生和伤口愈合。随着肠类器官培养技术、基因编辑和系谱示踪技术的建立,深入剖析Keap1/Nrf2发挥抗氧化作用的活性位点,结合分子对接技术筛选饲料组分精准靶向该信号的机制,可能为营养素功能的挖掘和肠道健康调控剂的开发提供新思路。
1 Keap1/Nrf2信号通路的级联反应Keap1/Nrf2信号通路是机体抵抗氧化应激最重要的防御机制[10]。机体或细胞未受任何有害刺激时,细胞质中Keap1二聚体上的BTB区域分别与Nrf2上的DLG和ETGE 2个位点结合,同时Keap1与泛素连接酶CULLIN3(CUL3)形成泛素E3连接酶复合体并不断泛素化降解Nrf2,致使胞质中游离的Nrf2维持在较低水平[11-14],Nrf2活性被Keap1抑制,Keap1/Nrf2信号总是处于“非活跃”的状态。
当机体处于氧化应激状态时,Keap1上BTB区域的半胱氨酸关键位点(Cys 151、Cys 273、Cys 288)被修饰,导致Nrf2的DLG从BTB区域解离(结合力较弱的位点),Nrf2无法被泛素蛋白酶体识别,与Keap1的结合处于饱和状态;新合成的Nrf2转而进入细胞核与Maf蛋白形成异二聚体,识别并结合抗氧化反应元件(ARE),启动超氧化物歧化酶(superoxide dismutase,SOD)、血红素氧合酶-1(heme oxygenase-1,HO-1)、γ-谷氨酰半胱胺酸合成酶(γ-glutamylcysteine synthetase,γ-GCS)和醌氧化还原酶-1[NAD(P)H: quinone oxidoreductase-1,NQO-1]等靶基因的转录。其中SOD可与超氧自由基发生歧化反应生成过氧化氢(H2O2)和水,H2O2再经过氧化氢酶降解[15];HO-1既能抑制血液中游离的血红素发生氧化反应,又可与其酶解产物胆红素和一氧化碳协同发挥抗氧化作用[16];γ-GCS通过调节谷胱甘肽(glutathione, GSH)的合成速度调节抗氧化系统功能[17];NQO-1催化醌及其衍生物降解[18]。这些抗氧化酶和Ⅱ相解毒酶协同清除ROS,抵抗氧化应激,维持肠道氧化还原平衡[19-21]。
2 Keap1/Nrf2信号通路调控肠道抗氧化能力Nrf2对肠道抗氧化作用的重要性在Nrf2基因敲除小鼠模型中充分体现。Cheung等[22]用葡聚糖硫酸钠盐(DSS)诱导结肠炎小鼠模型,发现相较于野生型小鼠,Nrf2-/-小鼠肠道抗氧化应激能力降低,炎症因子水平显著升高;进一步给野生型小鼠注射32 mg/kg BW的奥沙利铂(Nrf2激活剂)后,SOD和HO-1的表达量显著升高,而奥沙利铂给药对Nrf2-/-小鼠抗氧化酶表达量没有显著影响[23]。值得注意的是,某些功能性物质与奥沙利铂有相似的功能,可靶向Keap1/Nrf2,调节肠道抗氧化的作用。筛选此类功能性物质可能为畜牧生产中预防机体氧化应激提供有效的营养干预措施。
2.1 酚类化合物调控Keap1/Nrf2信号通路大多数酚类化合物具有的不饱和化学结构被证实具有抗氧化活性。阿魏酸是发现于植物阿魏中的一种多酚天然化合物[24-25],可作用于Keap1,激活Nrf2,提高热暴露状态下大鼠肠上皮细胞(IEC-6细胞)的SOD活性,从而降低ROS和丙二醛(malondialdehyde,MDA)的含量[26]。鼠尾草酸是一种存在于鼠尾草和迷迭香中的酚类化合物,作为一种强效抗氧化剂,具有高效清除ROS的功效[27]。鼠尾草酸通过激活Keap1/Nrf2信号,提高肠道中GSH含量和SOD活性,降低MDA含量,抑制炎症因子的产生,并缓解DSS诱导的小鼠结肠炎和氧化损伤[28]。姜黄素是来源于姜黄中的一种具有多酚结构的抗氧化物,饲粮添加800 mg/kg的姜黄素饲喂1日龄樱桃谷肉鸭,21 d后雏鸭空肠中Nrf2、SOD、过氧化氢酶(catalase,CAT)等抗氧化酶基因的mRNA丰度显著增加[29]。这些酚类化合物具有α, β-不饱和羰基结构可能与Keap1上巯基发生迈克尔加成反应[30],导致Keap1结构变化,Nrf2泛素化被抑制,抗氧化酶表达增加。上述研究中阿魏酸、鼠尾草酸和姜黄素的用法和剂量范围可为畜禽生产提供参考。
2.2 类胡萝卜素类化合物调控Keap1/Nrf2信号通路常见的类胡萝卜素类化合物有番茄红素和虾青素,它们具有相似的化学结构式,其众多单双键混排结构可与Keap1中的巯基发生反应,激活Keap1/Nrf2信号通路。Saada等[31]研究表明,番茄红素预处理可提高肠道SOD和GSH等抗氧化酶的活性,降低辐射诱导的大鼠氧化应激损伤;Rajput等[32]用番茄红素灌喂呕吐毒素损伤的小鼠,证实番茄红素可激活Nrf2信号通路,保护肠上皮完整性。Akduman等[33]以100 mg/kg BW虾青素治疗结肠炎小鼠,小鼠肠道中Nrf2及其下游抗氧化酶的表达显著上升,改善了肠细胞凋亡和肠道损伤的状况。类胡萝卜素类物质发挥抗氧化作用的机制可能与其化学结构中多个—C=C—存在有关,这种多不饱和结构的存在加大了与Keap1巯基相互作用的可能[34]。类胡萝卜素类化合物具有高效抗氧化的特点,被广泛应用于制药、食品等行业,安全性被普遍验证。推进该类化合物进入饲料添加剂的行列或许能有效应对畜禽生产中的氧化应激问题。
2.3 苷类化合物调控Keap1/Nrf2信号通路芦丁又名芸香苷,可激活Keap1/Nrf2信号通路,降低小鼠肠道ROS含量,增加SOD、HO-1等蛋白质的表达水平,缓解辐射诱导的肠道损伤[35]。人参皂苷是从人参中提取出的一类抗氧化物,小鼠静脉注射15 mg/kg BW人参皂苷Rb1增加p-Nrf2表达,增加SOD等抗氧化酶活性,缓解肠道缺血再灌注(intestinal ischemia reperfusion,IIR)损伤[36]。萝卜硫苷由西兰花提取而来,凭借其特殊官能团结构(—N=C=S)成为一种强的亲电试剂,激活Keap1/Nrf2信号通路,增加下游抗氧化酶的表达水平,清除肠道内过量ROS,以抵抗非甾体抗炎药诱导的肠道氧化应激损伤[37]。这些苷类物质的亲电结构决定了其在化学反应中的高活性,更易与Keap1上半胱氨酸巯基发生反应[38],介导抗氧化效果。饲料添加苷类抗氧化物同样可为畜禽生产提供预防或治疗肠道氧化应激作用。
2.4 其他常见营养素调控Keap1/Nrf2信号通路小肽类物质肌肽是由β-丙氨酸和L-组氨酸组成,具有调节抗氧化酶、中和自由基的特性。本课题组研究表明,肌肽与Keap1存在结合位点,促进Nrf2入核,上调HO-1和NQO-1表达,增强ISCs抗氧化能力,加速呕吐毒素暴露下肠上皮损伤修复进程[39]。含硒化合物硒化氨基多糖可激活Keap1/Nrf2信号,增加猪肠上皮细胞IPEC-1细胞中抗氧化酶和Ⅱ相解毒酶基因丰度,缓解H2O2诱导的猪肠上皮细胞氧化应激[40]。花生四烯酸代谢产物抗脂素包含单双键交替排列的不饱和结构能够修饰Keap1蛋白质上的关键半胱氨酸残基,放大Nrf2信号,抑制ROS的产生[41]。抗脂素A4处理IIR大鼠模型可激活Nrf2,提高HO-1的表达水平,缓解IIR引起的肠道损伤[42]。
上述调控肠道抗氧化能力的营养素均具有不饱和的化学结构(表 1),大多以修饰Keap1蛋白质上的关键半胱氨酸残基(如Cys 151、Cys 273、Cys 288),导致Keap1的构象发生改变,释放Nrf2进入细胞核而发挥作用[43]。除了上述3个关键修饰位点,Keap1蛋白质上的Cys 226、Cys 434和Cys 613等半胱氨酸残基,也同样被证明具有调控Keap1和Nrf2结合的作用[44-45]。然而,目前尚不清楚上述抗氧化剂与这些半胱氨酸残基能否结合,有待进一步的研究确证[46]。
|
|
表 1 调控Keap1/Nrf2信号通路的营养素及其潜在的调控机制 Table 1 Nutrients regulating Keap1/Nrf2 signaling pathway and their regulatory mechanisms |
此外,研究发现,通过营养素的干预激活Keap1/Nrf2信号通路提升肠道抗氧化能力,能够逆转受损肠道中氧化还原系统的不平衡,肠道抗氧化酶表达水平总是与ISCs标志物Lgr5的表达量相一致[47-49],说明肠道抗氧化能力影响ISCs活力,Keap1/Nrf2信号参与ISCs命运调控。
3 Keap1/Nrf2信号与ISCs命运调控ISCs参与肠道损伤修复的过程与其所处的氧化还原状态密切相关(图 1)。在果蝇ISCs中,Nrf2的缺失会导致ROS的积累,刺激ISCs增殖和分化[50]。这一过程被认为是果蝇ISCs应对氧化应激和炎症反应的调控机制。但是这种增殖和分化活动一旦失控,将加速果蝇肠上皮的退化[51]。然而,Keap1/Nrf2对哺乳动物ISCs的调控作用与果蝇相反,即Nrf2信号缺失将导致哺乳动物ISCs抗氧化能力大幅下降。研究发现,在高水平的ROS环境下,小鼠ISCs的标志蛋白质Lgr5的表达量显著下降,ISCs增殖和分化进程被阻滞[52]。进一步的研究证实,Keap1/Nrf2信号通路的激活显著降低了肠道中的ROS含量,为肠道细胞营造了一个适宜的ROS含量,增强了Lgr5标记的ISCs的活性[49]。
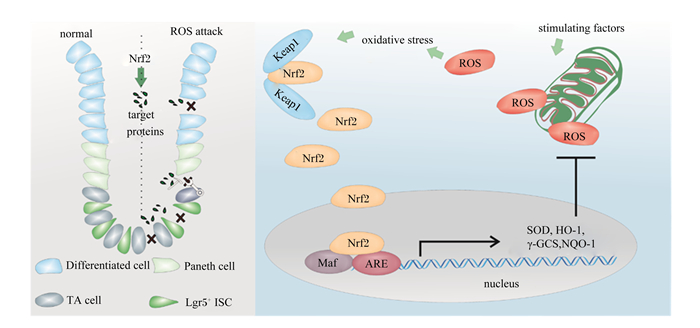
|
Lgr5+: 基底柱状细胞basal columnar cells;Paneth cell: 潘氏细胞;TA cell: 瞬时扩增细胞transiently expanded cells;Differentiated cell:分化细胞;Keap1:Kelch样环氧氯丙烷相关蛋白1 Kelch-like ECH-associated protein 1;Nrf2:核因子NF-E2相关因子2 nuclearfactor erythroidderived 2-like 2;ROS:活性氧自由基reactive oxygen species;Maf: 肌肉腱膜纤维肉瘤癌基因同源物musculoaponeurotic fibrosarcoma oncogene homolog;ARE: 抗氧化反应元件antioxidant response elements;SOD: 超氧化物歧化酶superoxide dismutase;γ-GCS:γ-谷氨酰半胱胺酸合成酶γ-glutamylcysteine synthase;HO-1:血红素氧合酶-1 heme oxygenase-1;NQO-1:醌氧化还原酶-1 quinone oxidoreductase-1。 图 1 Keap1/Nrf2信号通过调节氧化还原平衡调控肠细胞增殖分化 Fig. 1 Keap1/Nrf2 signaling regulates intestinal cell proliferation and differentiation by regulating redox balance |
此外,Keap1/Nrf2信号可能与Notch信号通路协同作用决定ISCs增殖分化活动。有研究发现,小鼠Notch1基因的功能调节区位于Nrf2启动子的近端,可能随Nrf2的激活而被激活。在敲除小鼠胚胎成纤维细胞中的Nrf2基因之后,Notch信号表达减弱[53]。通过抑制Keap1以激活Nrf2的表达,Nrf2负调控Notch下游效应器Math1,从而扰乱了Notch的级联反应,使小鼠肠道干细胞增殖分化进程加快[54]。
4 小结与展望Keap1/Nrf2信号在维持肠道干细胞微环境稳态中发挥重要作用,通过维持ISCs生态位氧化还原平衡维持肠上皮更新与再生。外源营养素靶向Keap1/Nrf2信号,对抗氧化应激损伤,已成为一种调节动物肠道健康的新途径。借助分子对接分析技术分析饲料添加剂与Keap1/Nrf2信号受体分子的相互作用[55],利用小分子数据库,结合分子对接和动物试验,可以筛选出更多Keap1/Nrf2信号通路的激动剂,为探寻新型的抗氧化剂提供参考[56]。
然而,目前针对Keap1/Nrf2信号调节肠道干细胞增殖分化的研究多集中于果蝇和小鼠等少数几个模式动物上,在畜禽上的研究相对较少。此外,大多数研究局限于单一信号的作用,对于Keap1/Nrf2信号通路及其与其他信号通路之间协同调控ISCs命运机制几乎没有涉及。借助肠道类器官、分子对接、靶基因敲除或抑制等技术,进一步探索Keap1/Nrf2信号调控ISCs命运的作用机制,逐步构筑基于Keap1/Nrf2信号的营养评价体系,并推进其应用于生产实际,或是解决上述问题的有效方法。
| [1] |
TAKAHASHI T, FUJISHIMA K, KENGAKU M. Modeling intestinal stem cell function with organoids[J]. International Journal of Molecular Sciences, 2021, 22(20): 10912. DOI:10.3390/ijms222010912 |
| [2] |
LIU Z H, XIE W W, ZAN G X, et al. Lauric acid alleviates deoxynivalenol-induced intestinal stem cell damage by potentiating the Akt/mTORC1/S6K1 signaling axis[J]. Chemico-Biological Interactions, 2021, 348: 109640. DOI:10.1016/j.cbi.2021.109640 |
| [3] |
LIU X Y, LI T M, LIU Y P, et al. Nuclear factor erythroid 2-related factor 2 potentiates the generation of inflammatory cytokines by intestinal epithelial cells during hyperoxia by inducing the expression of interleukin 17D[J]. Toxicology, 2021, 457: 152820. DOI:10.1016/j.tox.2021.152820 |
| [4] |
DENG F, HU J J, YANG X, et al. Interleukin-10 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and Notch signaling[J]. Biochemical and Biophysical Research Communications, 2020, 533(4): 1330-1337. DOI:10.1016/j.bbrc.2020.10.014 |
| [5] |
WANG S, WU K T, XUE D F, et al. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: insights from mitochondrial dysfunction[J]. Food and Chemical Toxicology, 2021, 153: 112214. DOI:10.1016/j.fct.2021.112214 |
| [6] |
TANG M, YUAN D X, LIAO P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets[J]. Environmental Pollution, 2021, 289: 117865. DOI:10.1016/j.envpol.2021.117865 |
| [7] |
LI L, TAN H P, ZOU Z M, et al. Preventing necroptosis by scavenging ROS production alleviates heat stress-induced intestinal injury[J]. International Journal of Hyperthermia, 2020, 37(1): 517-530. DOI:10.1080/02656736.2020.1763483 |
| [8] |
WU A M, YU B, ZHANG K Y, et al. Transmissible gastroenteritis virus targets Paneth cells to inhibit the self-renewal and differentiation of Lgr5 intestinal stem cells via Notch signaling[J]. Cell Death & Disease, 2020, 11(1): 40. |
| [9] |
陈丽霏, 张世倡, 肖林, 等. 炎症性肠病中活性氧及抗氧化的研究进展[J]. 中国当代医药, 2020, 27(9): 24-27. CHEN L F, ZHANG S C, XIAO L, et al. Research progress of reactive oxygen species and anti-oxidation in inflammatory bowel diseases[J]. China Modern Medicine, 2020, 27(9): 24-27 (in Chinese). DOI:10.3969/j.issn.1674-4721.2020.09.008 |
| [10] |
姚娟, 吴平安, 李芸, 等. Keap1-Nrf2-ARE信号通路及其激活剂的研究进展[J]. 中国药理学通报, 2019, 35(10): 1342-1346. YAO J, WU P, LI Y, et al. Research progress of small molecule activators in Keap1-Nrf2-ARE signaling pathway[J]. Chinese Pharmacological Bulletin, 2019, 35(10): 1342-1346 (in Chinese). DOI:10.3969/j.issn.1001-1978.2019.10.003 |
| [11] |
ADAMS J, KELSO R, COOLEY L. The kelch repeat superfamily of proteins: propellers of cell function[J]. Trends in Cell Biology, 2000, 10(1): 17-24. DOI:10.1016/S0962-8924(99)01673-6 |
| [12] |
KANG M I, KOBAYASHI A, WAKABAYASHI N, et al. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(7): 2046-2051. DOI:10.1073/pnas.0308347100 |
| [13] |
VELICHKOVA M, HASSON T. Keap1 in adhesion complexes[J]. Cell Motility and the Cytoskeleton, 2003, 56(2): 109-119. DOI:10.1002/cm.10138 |
| [14] |
DINKOVA-KOSTOVA A T, HOLTZCLAW W D, WAKABAYASHI N. Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein[J]. Biochemistry, 2005, 44(18): 6889-6899. DOI:10.1021/bi047434h |
| [15] |
RUBIOLO J A, MITHIEUX G, VEGA F V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes[J]. European Journal of Pharmacology, 2008, 591(1/2/3): 66-72. |
| [16] |
KONGPETCH S, KUKONGVIRIYAPAN V, PRAWAN A, et al. Crucial role of heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to chemotherapeutic agents[J]. PLoS One, 2012, 7(4): e34994. DOI:10.1371/journal.pone.0034994 |
| [17] |
江刚, 戴爱国. PI3K/Akt-aPKCι/ζ-Nrf2调控大鼠气道上皮细胞γ-谷氨酰半胱氨酸合成酶表达[J]. 中国应用生理学杂志, 2011, 27(1): 115-119. JIANG G, DAI A G. The pathway of PI3K/Akt-aPKCι/ξ-Nrf2 regulating the expression of γ-glutamylcysteine synthetase in the bronchial epithelial cells of rats[J]. Chinese Journal of Applied Physiology, 2011, 27(1): 115-119 (in Chinese). |
| [18] |
EIZIRIK D L, FLODSTRÖM M, KARLSEN A E, et al. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells[J]. Diabetologia, 1996, 39(8): 875-890. DOI:10.1007/BF00403906 |
| [19] |
BELLEZZA I, GIAMBANCO I, MINELLI A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress[J]. Biochimica et Biophysica Acta-Molecular Cell Research, 2018, 1865(5): 721-733. DOI:10.1016/j.bbamcr.2018.02.010 |
| [20] |
YANG Y, TIAN Z Y, DING Y K, et al. EGFR-targeted immunotoxin exerts antitumor effects on esophageal cancers by increasing ROS accumulation and inducing apoptosis via inhibition of the Nrf2-Keap1 pathway[J]. Journal of Immunology Research, 2018, 2018: 1090287. |
| [21] |
窦彩霞, 王倩, 安建勇, 等. Keap1-Nrf2/ARE信号通路及其在畜禽抗氧化中的研究进展[J]. 中国畜牧兽医, 2019, 46(9): 2567-2574. DOU C X, WANG Q, AN J Y, et al. The reasearch progress of Keap1-Nrf2/ARE signaling pathway and its role in the antioxidation of livestock and poultry[J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(9): 2567-2574 (in Chinese). |
| [22] |
CHEUNG K L, LEE J H, KHOR T O, et al. Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation[J]. Molecular Carcinogenesis, 2014, 53(1): 77-84. DOI:10.1002/mc.21950 |
| [23] |
WANG X J, LI Y Y, LUO L, et al. Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs[J]. Free Radical Biology and Medicine, 2014, 70: 68-77. DOI:10.1016/j.freeradbiomed.2014.02.010 |
| [24] |
ALAM M A. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action[J]. Frontiers in Nutrition, 2019, 6: 121. DOI:10.3389/fnut.2019.00121 |
| [25] |
MANCUSO C, SANTANGELO R. Ferulic acid: pharmacological and toxicological aspects[J]. Food and Chemical Toxicology, 2014, 65: 185-195. DOI:10.1016/j.fct.2013.12.024 |
| [26] |
HE S S, GUO Y H, ZHAO J X, et al. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway[J]. International Journal of Hyperthermia, 2019, 35(1): 112-121. |
| [27] |
BIRTIĆ S, DUSSORT P, PIERRE F X, et al. Carnosic acid[J]. Phytochemistry, 2015, 115: 9-19. DOI:10.1016/j.phytochem.2014.12.026 |
| [28] |
YANG N, XIA Z L, SHAO N Y, et al. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway[J]. Scientific Reports, 2017, 7(1): 11036. DOI:10.1038/s41598-017-11408-5 |
| [29] |
RUAN D, ZHU Y W, FOUAD A M, et al. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes[J]. Poultry Science, 2019, 98(9): 3705-3714. DOI:10.3382/ps/pez058 |
| [30] |
NOUREDDIN S A, EL-SHISHTAWY R M, AL-FOOTY K O. Curcumin analogues and their hybrid molecules as multifunctional drugs[J]. European Journal of Medicinal Chemistry, 2019, 182: 111631. DOI:10.1016/j.ejmech.2019.111631 |
| [31] |
SAADA H N, REZK R G, ELTAHAWY N A. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress[J]. Phytotherapy Research, 2010, 24(Suppl.2): S204-S208. |
| [32] |
RAJPUT S A, LIANG S J, WANG X Q, et al. Lycopene protects intestinal epithelium from deoxynivalenol-induced oxidative damage via regulating Keap1/Nrf2 signaling[J]. Antioxidants, 2021, 10(9): 1493. DOI:10.3390/antiox10091493 |
| [33] |
AKDUMAN H, TAYMAN C, KORKMAZ V, et al. Astaxanthin reduces the severity of intestinal damage in a neonatal rat model of necrotizing enterocolitis[J/OL]. American Journal of Perinatology. https://pubmed.ncbi.nlm.nih.gov/33853144/. DOI: 10.1055/s-0041-1727156.
|
| [34] |
RAO A V, RAO L G. Carotenoids and human health[J]. Pharmacological Research, 2007, 55(3): 207-216. DOI:10.1016/j.phrs.2007.01.012 |
| [35] |
DUTTA A, GUPTA M L, KALITA B. The combination of the active principles of Podophyllum hexandrum supports early recovery of the gastrointestinal system via activation of Nrf2-HO-1 signaling and the hematopoietic system, leading to effective whole-body survival in lethally irradiated mice[J]. Free Radical Research, 2015, 49(3): 317-330. DOI:10.3109/10715762.2015.1004328 |
| [36] |
CHEN S F, LI X, WANG Y L, et al. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway[J]. Molecular Medicine Reports, 2019, 19(5): 3633-3641. |
| [37] |
YANAKA A. Role of sulforaphane in protection of gastrointestinal tract against H. pylori and NSAID-induced oxidative stress[J]. Current Pharmaceutical Design, 2017, 23(27): 4066-4075. |
| [38] |
HALKIER B A, GERSHENZON J. Biology and biochemistry of glucosinolates[J]. Annual Review of Plant Biology, 2006, 57: 303-333. DOI:10.1146/annurev.arplant.57.032905.105228 |
| [39] |
ZHOU J Y, LIN H L, QIN Y C, et al. L-carnosine protects against deoxynivalenol-induced oxidative stress in intestinal stem cells by regulating the Keap1/Nrf2 signaling pathway[J]. Molecular Nutrition & Food Research, 2021, 65(17): e2100406. |
| [40] |
WEN Z S, MA L, XIANG X W, et al. Protective effect of low molecular-weight seleno-aminopolysaccharides against H2O2-induecd oxidative stress in intestinal epithelial cells[J]. International Journal of Biological Macromolecules, 2018, 112: 745-753. DOI:10.1016/j.ijbiomac.2018.01.191 |
| [41] |
WU S H, WANG M J, LV J, et al. Signal transduction involved in lipoxin A4-induced protection of tubular epithelial cells against hypoxia/reoxygenation injury[J]. Molecular Medicine Reports, 2017, 15(4): 1682-1692. DOI:10.3892/mmr.2017.6195 |
| [42] |
HAN X, YAO W F, LIU Z P, et al. Lipoxin A4 preconditioning attenuates intestinal ischemia reperfusion injury through Keap1/Nrf2 pathway in a lipoxin A4 receptor independent manner[J]. Oxidative Medicine and Cellular Longevity, 2016, 2016: 9303606. |
| [43] |
程茂军, 郭杰, 刘婧, 等. Nrf2及天然产物导向的Nrf2激活剂研究进展[J]. 天然产物研究与开发, 2021, 33(1): 165-177. CHENG M J, GUO J, LIU J, et al. Research progress of Nrf2 and natural product-oriented Nrf2 activator[J]. Natural Product Research and Development, 2021, 33(1): 165-177 (in Chinese). |
| [44] |
FOURQUET S, GUEROIS R Ã, BIARD D, et al. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation[J]. The Journal of Biological Chemistry, 2010, 285(11): 8463-8471. DOI:10.1074/jbc.M109.051714 |
| [45] |
MCMAHON M, LAMONT D J, BEATTIE K A, et al. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(44): 18838-18843. DOI:10.1073/pnas.1007387107 |
| [46] |
MADDEN S K, ITZHAKI L S. Structural and mechanistic insights into the Keap1-Nrf2 system as a route to drug discovery[J]. Biochimica et Biophysica Acta (BBA): Proteins and Proteomics, 2020, 1868(7): 140405. DOI:10.1016/j.bbapap.2020.140405 |
| [47] |
LI X J, WANG X X, MIAO L F, et al. Synthesis and radioprotective effects of novel hybrid compounds containing edaravone analogue and 3-n-butylphthalide ring-opening derivatives[J]. Journal of Cellular and Molecular Medicine, 2021, 25(12): 5470-5485. DOI:10.1111/jcmm.16557 |
| [48] |
ZHU X Z, TIAN X, YANG M L, et al. Procyanidin B2 promotes intestinal injury repair and attenuates colitis-associated tumorigenesis via suppression of oxidative stress in mice[J]. Antioxidants & Redox Signaling, 2021, 35(2): 75-92. |
| [49] |
LI Y, MA S S, ZHANG Y T, et al. (-)-epicatechin mitigates radiation-induced intestinal injury and promotes intestinal regeneration via suppressing oxidative stress[J]. Free Radical Research, 2019, 53(8): 851-864. DOI:10.1080/10715762.2019.1635692 |
| [50] |
HOCHMUTH C E, BITEAU B, BOHMANN D, et al. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila[J]. Cell Stem Cell, 2011, 8(2): 188-199. DOI:10.1016/j.stem.2010.12.006 |
| [51] |
SCHMIDLIN C J, DODSON M B, MADHAVAN L, et al. Redox regulation by NRF2 in aging and disease[J]. Free Radical Biology & Medicine, 2019, 134: 702-707. |
| [52] |
娄文静, 刘冬妍. 高氧降低不同日龄新生大鼠肠道干细胞Bmi1和Lgr5的表达[J]. 基础医学与临床, 2019, 39(1): 22-26. LOU W J, LIU D Y. Hyperoxia reduces the expression of Bmi1 and Lgr5 in intestinal stem cells of neonatal rats[J]. Basic & Clinical Medicine, 2019, 39(1): 22-26 (in Chinese). DOI:10.3969/j.issn.1001-6325.2019.01.007 |
| [53] |
WAKABAYASHI N, CHARTOUMPEKIS D V, KENSLER T W. Crosstalk between Nrf2 and Notch signaling[J]. Free Radical Biology & Medicine, 2015, 88(Pt.B): 158-167. |
| [54] |
YAGISHITA Y, MCCALLUM M L, KENSLER T W, et al. Constitutive activation of Nrf2 in mice expands enterogenesis in small intestine through negative regulation of math1[J]. Cellular and Molecular Gastroenterology and Hepatology, 2021, 11(2): 503-524. DOI:10.1016/j.jcmgh.2020.08.013 |
| [55] |
DE VRIES S J, VAN DIJK M, BONVIN A M J J. The HADDOCK web server for data-driven biomolecular docking[J]. Nature Protocols, 2010, 5(5): 883-897. DOI:10.1038/nprot.2010.32 |
| [56] |
UNNI S, DESHMUKH P, KRISHNAPPA G, et al. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1[J]. FEBS Journal, 2021, 288(5): 1599-1613. DOI:10.1111/febs.15485 |




