细胞凋亡也称细胞程序性死亡,是由多种基因决定的,细胞主动的、有秩序的死亡过程[1]。宰后成熟是提升畜禽肉品质的关键,并且细胞凋亡在此过程中有着不可忽视的作用。肌纤维是构成肌肉组织的基本单位,其特性在动物宰前就已基本确定,然而它是如何在宰后成熟过程中影响细胞凋亡进程进而影响肉品质,目前尚未有系统报道。此外,研究宰后成熟过程中不同肌肉部位的品质差异及其机制至关重要。因此,本文总结了细胞凋亡概念、细胞凋亡路径、细胞凋亡对宰后肌肉成熟过程的影响、控制宰后成熟过程中细胞凋亡的方法等,最后将细胞凋亡与肌纤维特性联系起来,为肌肉的分部位成熟提供参考依据。
1 细胞凋亡 1.1 细胞凋亡概念及形态学特征细胞凋亡是细胞停止生长和分裂的过程,是一种细胞自杀方式,最终导致细胞受控死亡,而细胞内的物质不会泄露到外界环境中。机体去除不必要细胞的有效途径是使其主动凋亡,通过活化细胞中凋亡相关基因使细胞死亡;生物生长和保持机体内环境稳定均需要细胞凋亡的发生[2-3]。
细胞如果有凋亡的预兆,首先有变化的就是形状,细胞凋亡的形态变化如图 1所示[4]。凋亡的细胞其形态特征主要表现在细胞皱缩变小,细胞核破碎,线粒体和核糖体集合在一起,细胞器发生轻微变化,染色质逐步凝结为月牙形,围绕在细胞核膜附近;随后细胞质膜和核膜出泡,出泡的部分塌陷形成凋亡小体(apoptotic bodies),且凋亡小体中有部分染色质[5-6]。
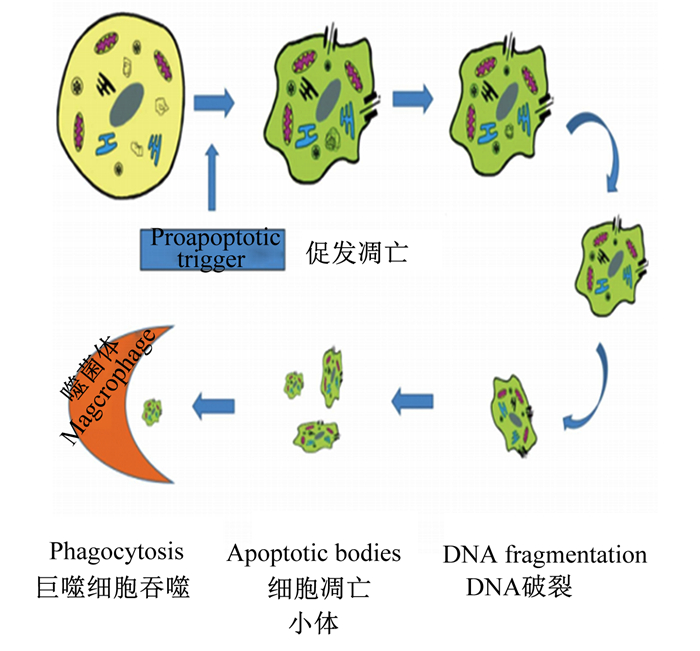
|
图 1 细胞凋亡的形态变化 Fig. 1 Morphological changes of apoptosis |
细胞凋亡路径主要包括3种:内源性线粒体途径、外源性死亡受体途径和内质网途径[7],如图 2所示。其中,线粒体途径是最主要的细胞凋亡路径[8]。相关研究表明这3条细胞凋亡途径是相互关联的,且都由同一种蛋白酶——半胱氨酸蛋白酶(cysteinyl aspartate specific proteinase,Caspase)家族连接。
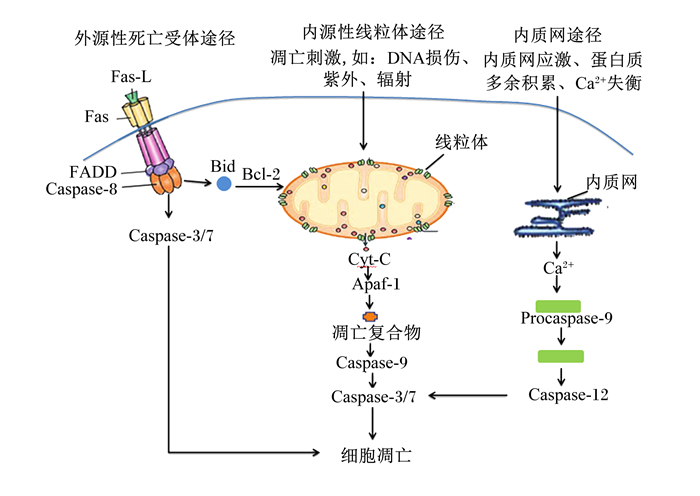
|
Fas-L:Fas配体Fas ligand;FADD:与Fas相关的死亡结构域Fas associated via death domain;Caspase-8:半胱氨酸蛋白酶-8 cysteinyl aspartate specific proteinase-8;Caspase-3/7:半胱氨酸蛋白酶-3/7 cysteinyl aspartate specific proteinase-8;Bid:BH3结构域凋亡诱导蛋白BH3 interacting domain death agonist;Bcl-2:B淋巴细胞瘤/白血病-2 B-cell lymphoma/leukemia-2;Cyt-C:细胞色素C cytochrome-C;Apaf-1:凋亡酶因子-1 apoptosis protease-activating factor-1;Caspase-9:半胱氨酸蛋白酶-9 cysteinyl aspartate specific proteinase-9;Procaspase-12:半胱氨酸蛋白酶前体-12 cysteinyl aspartate specific proteinase precursor-12;Caspase-12:半胱氨酸蛋白酶-12 cysteinyl aspartate specific proteinaser-12。 图 2 细胞凋亡路径 Fig. 2 Pathway of apoptosis[7] |
内源性线粒体途径开始于线粒体,当机体内细胞接收到凋亡刺激时将信号传递至线粒体,线粒体膜通透性转换孔(mitochondrial permeability transition pore,mPTP)打开,释放细胞色素C(cytochrome-C,Cyt-C),接着Cyt-C与凋亡酶因子-1(apoptotic protease activating factor-1,Apaf-1)相互作用,并与辅因子ATP组成凋亡复合物[9],该复合物启动细胞凋亡级联反应并激活细胞凋亡启动酶Caspase-9。经活化的Caspase-9自我剪切,进而活化细胞凋亡效应酶Caspase-3[10-11]。值得一提的是整个细胞凋亡过程受到基因家族B淋巴细胞瘤/白血病-2(B-cell lymphoma/leukemia-2,Bcl-2)家族蛋白的调控。Bcl-2家庭分为2种功能性蛋白:抗凋亡蛋白[Bcl-2、B细胞淋巴瘤/白血病-xl(Bcl-xl)]和促凋亡蛋白[B细胞淋巴瘤/白血病-2相关X蛋白(B-cell lymphoma/leukaemia-2-associated X protein,Bax)、BH3结构域凋亡诱导蛋白(BH3 interacting domain death agonist,Bid)、B淋巴细胞瘤/白血病-2拮抗/杀伤因子(Bcl-2 homologous antago-nist/killer,Bak)][12]。研究表明,Bcl-2可通过抑制mPTP的形成,维持线粒体跨膜电位,进而抑制Cyt-C的释放,也防止其他促凋亡因子从孔道释放,并且已释放到细胞质中的Cyt-C也能够被Bcl-2抑制激活细胞凋亡酶系统。Bcl-xl可与Apaf-1结合,使之不能与Cyt-C形成凋亡复合体,从而阻止下游Caspase活化[13-14]。而过表达的Bax可直接插入到线粒体膜上增加线粒体的通透性,增加Cyt-C的释放,激活Caspase家族,促进细胞凋亡发生。Bid可被Caspase-8水解,其水解产物可以触发线粒体释放Cyt-C;此外Bax可与Bcl-2和Bcl-xl结合形成同源二聚体以加速细胞凋亡,由此可知,细胞凋亡的发生及发展程度主要由Bcl-2家族蛋白调控[15-16]。
外源性死亡受体途径是由细胞表层的死亡受体(death receptors,DR)接收凋亡讯号,与死亡配体(death ligands)联合传递到细胞内,又与Caspase-2、Caspase-8和Caspase-10结合,形成凋亡复合体,进而产生下游一系列的生物学效应使细胞凋亡。目前研究最多的是Fas、Fas与Fas配体(Fas ligand,Fas-L)结合形成凋亡复合物并启动细胞凋亡级联反应[17-18]。机体内大多数组织细胞中都有Fas和其配体的存在,通过调控细胞凋亡来发挥作用[19]。
内质网途径是内质网应激、蛋白质多余积累和钙离子(Ca2+)失衡触发的细胞凋亡过程[20-21]。凋亡因子在内质网上堆集并释放Ca2+,激活前体Caspase-12,进而激活Caspase-9和Caspase-3,最终使细胞发生凋亡[22]。
1.3 细胞凋亡对宰后成熟过程的影响及其调控手段 1.3.1 细胞凋亡对宰后成熟过程及肉嫩度的影响动物屠宰后,机体内营养物质供给缺失,血液循环的中断使肌细胞处于无氧环境,当机体内细胞处于这样恶劣的环境中,细胞会发出凋亡信号,促使肌细胞强制性死亡,即细胞凋亡。宰后成熟是提升畜禽肉品质的重要手段,其中钙蛋白酶系统、由Caspase转导的细胞凋亡酶系统和热休克蛋白家族(HSP)等对肉品质的形成至关重要。目前越来越多的研究发现,细胞凋亡在宰后成熟过程的作用尤为显著[23-25]。Zhang等[11]研究发现,随着成熟时间的延长,牛肉的pH逐渐降低,线粒体膜通透性增加,Cyt-C大量释放,促凋亡蛋白Bax含量增加,抗凋亡蛋白HSP27含量降低,且细胞凋亡启动酶和效应酶的活性均发生变化,证明了宰后成熟过程发生了细胞凋亡现象[26]。Huang等[27]和孙志昶[28]分别试验证明,用DEVD-CHO(一种Caspase-3的特异性抑制剂)处理鸡胸和耗牛肉可显著抑制宰后成熟过程中肌肉蛋白质的降解,这一结果说明畜禽宰后成熟过程中,细胞凋亡是重要参与过程。王琳琳等[29]以耗牛背最长肌为研究对象,通过向肌肉中注射环孢菌素A(cyclosporin A,CsA,mPTP的专一性抑制剂)后发现,CsA显著抑制了mPTP的开放程度和Cyt-C的释放,降低了Caspase-3活性,成熟过程有所减缓,并且随着成熟时间不断增长,CsA活性降低,成熟过程随之增加。Chen等[30]利用细胞凋亡诱导剂处理鸡肉,结果表明,肌原纤维蛋白降解产物显著增加,Caspase-3活性显著增强;进一步通过试验发现,在肌原纤维蛋白的降解过程中,钙蛋白酶和Caspase-3起协同作用[31]。综上所述,细胞凋亡参与了畜禽宰后成熟过程并直接影响了肉品的嫩化程度。
1.3.2 控制宰后成熟过程细胞凋亡的方法细胞凋亡对宰后成熟过程有着重要的调控作用,宰后成熟又与肉品质密切相关,因此深入研究控制宰后成熟过程中细胞凋亡的措施,对控制整个成熟过程及改善肉品质至关重要。
国内外学者的研究表明,当前有不少方法均可以控制细胞凋亡。如李文东等[32]以不同冷却方式处理耗牛背最长肌,发现快速冷却处理较常规冷却处理减缓了pH的下降速率,抑制了Caspase-9、Caspase-3活性,进而影响了细胞凋亡进程。王琳琳等[33]通过在耗牛背最长肌中注射茶多酚探究其对细胞凋亡的影响,发现肌肉中注射茶多酚抑制了Cyt-C释放和Caspase-3活性,进而抑制了细胞凋亡的发生。张爽[34]使用细胞凋亡诱导剂氯化钙(CaCl2)和茶多酚研究其对宰后山羊肌肉细胞凋亡的影响,发现CaCl2和茶多酚显著增加了肌肉Caspase-3活性和肌原纤维蛋白的降解程度,可有效改善山羊肌肉的嫩度。Chen等[35]在研究氧化应激对牛肉嫩度的影响时发现,随着活性氧(ROS)含量的增加,线粒体膜通透性也随之增加,细胞凋亡程度增强,牛肉的成熟过程加快,嫩度得以改善。陈琳[36]研究表明,Ca2+处理可显著提高宰后成熟过程鸡胸肉中Caspase-3活性。Zhang等[23]研究表明,溶酶体铁增加了宰后肌肉ROS含量和线粒体膜通透性,降低了线粒体膜电位,增强了Cyt-C释放,加快了宰后成熟过程的细胞凋亡进程。
2 细胞凋亡与畜禽肌纤维的关联机制肌纤维是一种肌细胞,是构成骨骼肌的基本单位,占骨骼肌总体积的75%~90%,由细胞器、外膜、肌红蛋白、肌球蛋白和肌动蛋白等部分组成[37]。肌纤维根据颜色、收缩速度、代谢特性可分为以下几类:红肌纤维(Ⅰ型肌纤维,为慢收缩氧化型肌纤维)、白肌纤维(Ⅱ型肌纤维,为快收缩酵解型肌纤维)和中间型肌纤维(Ⅱx型肌纤维)[38]。目前关于哪种肌纤维更易发生凋亡的问题,国内外学者发表了不同观点。Libera等[39]对大鼠进行试验研究结果显示,与大鼠Ⅱ型肌纤维相比,Ⅰ型肌纤维的凋亡速度更慢。Yamada等[40]的试验结果与上述试验结果一致,表明Ⅱ型肌纤维中存在Fas(促凋亡蛋白)且含量丰富,而没有Bcl-2(抗凋亡蛋白)。而周婕等[41]试验结果却表明,中等强度和高强度运动时,与大鼠的胫骨前肌(Ⅱ型肌纤维)相比,比目鱼肌(Ⅰ型肌纤维)的凋亡率显著升高。从以上研究可以发现,肌纤维的构成差异确实可影响细胞凋亡。因此,研究肌纤维如何影响细胞凋亡的机制十分必要。而据相关文献报道,其关联机制可能与凋亡蛋白含量、线粒体含量、一氧化氮合成酶(NOS)含量及活性、蛋白质亚硝基化和pH等有关。
2.1 凋亡蛋白凋亡蛋白是调节细胞凋亡级联反应的关键蛋白,不同类型肌纤维其凋亡蛋白含量存在差异。Riva等[42]试验结果显示,大鼠腓肠肌(Ⅱ型肌纤维为主)的凋亡速率要显著低于比目鱼肌(Ⅰ型肌纤维为主),进一步研究发现,Bax(促凋亡蛋白)在大鼠比目鱼肌中含量高于腓肠肌,而Bcl-2(抗凋亡蛋白)在大鼠腓肠肌中含量较高,因此凋亡蛋白和抗凋亡蛋白含量很可能是引起肌纤维凋亡出现差异的重要原因。曹锦轩[43]研究证明,牛肉氧化型肌肉(Ⅰ型肌纤维)中Bax和Bcl-2含量高于酵解型肌肉(Ⅱ型肌纤维),而Bid蛋白表达量表现为氧化型肌肉(Ⅰ型肌纤维)低于酵解型肌肉(Ⅱ型肌纤维),因此2个部位的凋亡速率不一致。HSP家族能够抑制细胞凋亡,其中HSP27是调控细胞凋亡的关键因子,它能够影响Cyt-C释放,减少凋亡小体形成,进而抑制凋亡发生;此外,HSP27可使Bax失活,达到抑制细胞凋亡的作用[44-45]。与快肌(Ⅱ型肌纤维)相比,慢肌(Ⅰ型肌纤维)中HSP27和HSP40的蛋白表量较高[46],因此快肌的凋亡速率要高于慢肌。上述出现的2种不同结论,究其原因可能是物种差异导致。但无论是哪种肌纤维表现出更快速率的凋亡,凋亡蛋白含量在其中的作用至关重要。
2.2 线粒体线粒体是细胞的能量制造厂,控制着细胞的能量代谢,也是线粒体凋亡通路中的关键部位[47]。此外,该细胞器还控制着凋亡因子的释放,Cyt-C就是由线粒体释放到细胞质中并参与线粒体凋亡路径[48]。大量试验结论已证明,线粒体含量可能是连接肌纤维和细胞凋亡的关键枢纽。Cramer等[49]研究结果显示,氧化型肌纤维的线粒体和Cyt-C含量更多,与之对应的是细胞凋亡更易发生在氧化型肌纤维中。Ke等[50]和张佳莹[51]研究认为,酵解型肌纤维比氧化型肌纤维中的线粒体含量低,不易发生线粒体功能障碍,即线粒体膜通透性的改变和Cyt-C释放。
2.3 NOSNOS是诱导细胞发生凋亡的重要调节因子,研究表明,NOS通过影响一氧化氮(NO)浓度进而调控细胞凋亡。NO可调节机体内多种生物代谢活动,具有抑制或诱导细胞凋亡的双重功能,是多种线粒体途径的重要信号分子。低浓度NO具有抗凋亡作用,而高浓度NO则具有诱导细胞凋亡作用[52-53]。NOS是产生NO的主要酶,研究表明,不同类型肌纤维中NOS的含量和活性存在较大差异,因此有学者认为,NOS是连接肌纤维和细胞凋亡的又一重要“桥梁”,该信号分子很可能通过影响NO浓度进而影响细胞凋亡[54]。Planitzer等[55]在小鼠的Ⅱ型肌纤维中发现NOS大量富集,并且Ⅱa型肌纤维比Ⅱb型肌纤维的NOS含量更高。Liu等[56]研究表明,神经源性一氧化氮合酶(nNOS)的表达与Ⅱa/Ⅱx型肌纤维含量呈正相关,与Ⅱb型肌纤维含量呈负相关。
2.4 蛋白质亚硝基化蛋白质亚硝基化即NO与蛋白质的半胱氨酸巯基结合生成S-亚硝基硫醇(SNO),是作为信号分子的主要NO通路。由NO诱导的蛋白质亚硝基化可使细胞凋亡酶失活,进而影响细胞凋亡进程[57-59]。Mannick等[60]和Hou等[61]以牛的半膜肌为试验对象,通过比较蛋白质亚硝基化的肌肉与无蛋白质亚硝基化的肌肉细胞凋亡程度,发现蛋白质亚硝基化的肌肉Caspase-3和Caspase-9活性大大降低,进而影响了Cyt-C释放。Zhang等[62]研究报道,牛肉中Ⅱa/Ⅱx型肌纤维的蛋白质亚硝基化强度更高,所以抑制了Ⅱ型肌纤维的细胞凋亡。但截止到目前,关于比较不同类型肌纤维蛋白质亚硝基化程度的相关研究较少,因此,以蛋白质亚硝基化为基点探究细胞凋亡和肌纤维的关系还有待进一步摸索。
2.5 pHpH反映了宰后动物肌肉内环境的酸碱程度,也是肌肉能量代谢的基本特征。有研究显示,pH的变化和细胞凋亡密切相关,即细胞内pH降低,使得肌肉内环境酸化,更容易激活细胞凋亡酶[63]。贾青[64]研究指出,肌纤维中糖原无氧酵解产生的乳酸越多,pH越低,且低pH环境更有利于细胞凋亡的发生以及Caspase的活化。这说明肌肉pH的改变可极大程度地影响细胞凋亡。不同类型肌纤维的糖原含量、代谢方式等均存在差异,王勇峰[65]和Ryu等[66]试验结果表明,酵解型肌纤维的代谢方式主要是无氧酵解,因此pH下降速度更快,pH更低。因此,pH的变化可能是解释酵解型肌纤维凋亡较快的又一重要原因。综合以上研究可以发现,不同类型肌纤维由于其代谢方式的差异进而影响了pH的高低,而pH作为活化Caspase的重要因子,可直接影响细胞凋亡进程。
3 小结肉品质是影响消费者是否购买肉品的重要因素,主要包括色泽、风味和嫩度等指标。肌纤维是构成骨骼肌的重要组成部分,与肉品质密切相关。大量研究表明氧化型肌纤维比例越高,肉品色泽、嫩度越好,对风味也有积极作用。宰后成熟是改善肉品质的重要途径,而成熟过程其实就是肌细胞的凋亡过程,因此通过控制细胞凋亡进程可以调控和改善肉品的各项参数,提升肉品质。大量文献已经证实不同类型肌纤维由于其凋亡蛋白含量、线粒体含量、NOS含量及活性、蛋白质亚硝基化和pH的不同而直接影响凋亡进程,进而对肉品特性产生较大影响,但具体作用机制以及哪种类型肌纤维更易发生凋亡还有待更深入的研究。此外,不同类型的肌肉其凋亡信号传导途径也可能存在较大差异,因此有必要对其进行更深入的探讨。但不可否认的是掌握细胞凋亡与肌纤维之间的关联机制,将为控制宰后不同部位肌肉的成熟过程和提升肉品质提供理论依据和行之有效的调控手段。
| [1] |
KAUR A P, AGRAWAL S. A review of the molecular mechanism of apoptosis and its role in pathological conditions[J]. International Journal of Pharma and Bio Sciences, 2019, 10(2): 124-131. |
| [2] |
XIA P, LIU Y N, CHENG Z K. Signaling pathways in cardiac myocyte apoptosis[J]. BioMed Research International, 2016, 2016: 9583268. |
| [3] |
D'ARCY M S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy[J]. Cell Biology International, 2019, 43(6): 582-592. DOI:10.1002/cbin.11137 |
| [4] |
MUSUMECI G, IMBESI R, SZYCHLINSKA M A, et al. Apoptosis and skeletal muscle in aging[J]. Open Journal of Apoptosis, 2015, 4(2): 41-46. DOI:10.4236/ojapo.2015.42004 |
| [5] |
GRUNEWALD S, FITZL G, SPRINGSGUTH C. Induction of ultra-morphological features of apoptosis in mature and immature sperm[J]. Asian Journal of Andrology, 2017, 19(5): 533-537. DOI:10.4103/1008-682X.180974 |
| [6] |
ASHKENAZI A, DIXIT V M. Death receptors: signaling and modulation[J]. Science, 1998, 281(5381): 1305-1308. DOI:10.1126/science.281.5381.1305 |
| [7] |
GUPTA S, GOLLAPUDI S. Susceptibility of naïve and subsets of memory T cells to apoptosis via multiple signaling pathways[J]. Autoimmunity Reviews, 2007, 6(7): 476-481. DOI:10.1016/j.autrev.2007.02.005 |
| [8] |
THORNTON C, LEAW B, MALLARD C, et al. Cell death in the developing brain after hypoxia-ischemia[J]. Frontiers in Cellular Neuroscience, 2017, 11: 248. DOI:10.3389/fncel.2017.00248 |
| [9] |
MONDAL J, DAS J, SHAH R, et al. A homeopathic nosode, hepatitis C 30 demonstrates anticancer effect against liver cancer cells in vitro by modulating telomerase and topoisomerase Ⅱ activities as also by promoting apoptosis via intrinsic mitochondrial pathway[J]. Journal of Integrative Medicine, 2016, 14(3): 209-218. DOI:10.1016/S2095-4964(16)60251-0 |
| [10] |
YANG S P, ZHANG Y G, LUO Y, et al. Hinokiflavone induces apoptosis in melanoma cells through the ROS-mitochondrial apoptotic pathway and impairs cell migration and invasion[J]. Biomedicine & Pharmacotherapy, 2018, 103: 101-110. |
| [11] |
ZHANG J Y, YU Q L, HAN L, et al. Study on the apoptosis mediated by cytochrome c and factors that affect the activation of bovine longissimus muscle during postmortem aging[J]. Apoptosis, 2017, 22(6): 777-785. DOI:10.1007/s10495-017-1374-2 |
| [12] |
PATWARDHAN G A, BEVERLY L J, SISKIND L J, et al. Sphingolipids and mitochondrial apoptosis[J]. Journal of Bioenergetics and Biomembranes, 2016, 48: 153-168. DOI:10.1007/s10863-015-9602-3 |
| [13] |
PHANEUF S, LEEUWENBURGH C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age[J]. American Journal of Physiology.Regulatory, Integrative and Comparative Physiology, 2002, 282(2): R423-R430. DOI:10.1152/ajpregu.00296.2001 |
| [14] |
吉木斯, 李存保. Bcl-2家族在线粒体细胞凋亡途径中的作用[J]. 内蒙古医科大学学报, 2013, 35(2): 152-157. JI M S, LI C B. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway[J]. Journal of Inner Mongolia Medical University, 2013, 35(2): 152-157 (in Chinese). DOI:10.3969/j.issn.1004-2113.2013.02.014 |
| [15] |
ADAMS J M, CORY S. The Bcl-2 protein family: arbiters of cell survival[J]. Science, 1998, 281(5381): 1322-1326. DOI:10.1126/science.281.5381.1322 |
| [16] |
卢晓晔, 钟雪云. Caspases与细胞凋亡(综述)[J]. 暨南大学学报(自然科学与医学版), 2000, 21(6): 120-124. LU X Y, ZHONG X Y. Caspases and apoptosis[J]. Journal of Jinan University (Natural Science & Medicine Edition), 2000, 21(6): 120-124 (in Chinese). DOI:10.3969/j.issn.1000-9965.2000.06.047 |
| [17] |
LIU G, YUAN Y, LONG M F, et al. Beclin-1-mediated autophagy protects against cadmium-activated apoptosis via the Fas/Fasl pathway in primary rat proximal tubular cell culture[J]. Scientific Reports, 2017, 7(1): 977. DOI:10.1038/s41598-017-00997-w |
| [18] |
KOUTSOGIANNAKI S, HOU L F, BABAZADA H, et al. The volatile anesthetic sevoflurane reduces neutrophil apoptosis via Fas death domain-Fas-associated death domain interaction[J]. The FASEB Journal, 2019, 33(11): 12668-12679. DOI:10.1096/fj.201901360R |
| [19] |
CAI G D, SI M X, LI X, et al. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway[J]. Environmental Toxicology, 2019, 34(4): 424-433. DOI:10.1002/tox.22696 |
| [20] |
BHAT T A, CHAUDHARY A K, KUMAR S, et al. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer[J]. Biochimica et Biophysica Acta (BBA): Reviews on Cancer, 2017, 1867(1): 58-66. DOI:10.1016/j.bbcan.2016.12.002 |
| [21] |
方向楠, 秦彦文, 岳卫东. 内质网应激与缺血性脑血管病的研究进展[J]. 中华老年心脑血管病杂志, 2016, 18(4): 441-443. FANG X N, QIN Y W, YUE W D. Research progress of endoplasmic reticulum stress and ischemic cerebrovascular disease[J]. Chinese Journal of Geriatric Heart Brain and Vessel Diseases, 2016, 18(4): 441-443 (in Chinese). DOI:10.3969/j.issn.1009-0126.2016.04.030 |
| [22] |
ADAMS J M. Ways of dying: multiple pathways to apoptosis[J]. Genes & Development, 2003, 17(20): 2481-2495. |
| [23] |
ZHANG J Y, YU Q L, HAN L, et al. Effects of lysosomal iron involvement in the mechanism of mitochondrial apoptosis on postmortem muscle protein degradation[J]. Food Chemistry, 2020, 328: 127174. DOI:10.1016/j.foodchem.2020.127174 |
| [24] |
WANG L L, HAN L, MA X L, et al. Effect of mitochondrial apoptotic activation through the mitochondrial membrane permeability transition pore on yak meat tenderness during postmortem aging[J]. Food Chemistry, 2017, 234: 323-331. DOI:10.1016/j.foodchem.2017.04.185 |
| [25] |
杨致昊, 刘畅, 窦露, 等. Caspases的研究进展及其与肉嫩度的关联机制[J]. 食品与发酵工业, 2021, 47(17): 277-282. YANG Z H, LIU C, DOU L, et al. Research progress of caspases and its association mechanism with meat tenderness[J]. Food and Fermentation Industries, 2021, 47(17): 277-282 (in Chinese). |
| [26] |
HUANG F, HUANG M, ZHANG H, et al. Changes in apoptotic factors and caspase activation pathways during the postmortem aging of beef muscle[J]. Food Chemistry, 2016, 190: 110-114. DOI:10.1016/j.foodchem.2015.05.056 |
| [27] |
HUANG M, HUANG F, XU X L, et al. Influence of caspase3 selective inhibitor on proteolysis of chicken skeletal muscle proteins during post mortem aging[J]. Food Chemistry, 2009, 115(1): 181-186. DOI:10.1016/j.foodchem.2008.11.095 |
| [28] |
孙志昶. 宰后牦牛肉成熟过程中细胞凋亡的发生及其对肉品质与微观结构变化的影响[D]. 博士学位论文. 兰州: 甘肃农业大学, 2015. SUN Z C. The research in the mechanism of apoptosis occurrence on yak meat quality and microstructure changes during postmortem aging[D]. Ph. D. Thesis. Lanzhou: Gansu Agricultural University, 2015. (in Chinese) |
| [29] |
王琳琳, 马君义, 余群力, 等. 宰后牦牛肉细胞凋亡对肌肉内环境与嫩度的影响[J]. 农业机械学报, 2017, 48(7): 317-324. WANG L L, MA J Y, YU Q L, et al. Effects of apoptosis on muscle internal environment and tenderness during yak meat postmortem aging[J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(7): 317-324 (in Chinese). |
| [30] |
CHEN L, FENG X C, LU F, et al. Effects of camptothecin, etoposide and Ca2+ on caspase-3 activity and myofibrillar disruption of chicken during postmortem ageing[J]. Meat Science, 2011, 87(3): 165-174. DOI:10.1016/j.meatsci.2010.10.002 |
| [31] |
CHEN L, FENG X C, ZHANG W G, et al. Effects of inhibitors on the synergistic interaction between calpain and caspase-3 during post-mortem aging of chicken meat[J]. Journal of Agricultural and Food Chemistry, 2012, 60(34): 8465-8472. DOI:10.1021/jf300062n |
| [32] |
李文东, 韩玲, 宋仁德, 等. 快速冷却对宰后牦牛肉成熟过程中细胞凋亡酶活力与嫩度的影响[J]. 现代食品科技, 2019, 35(3): 73-79. LI W D, HAN L, SONG R D, et al. The effect of rapid chilling on tenderness and apoptotic activity during yak meat postmortem aging[J]. Modern Food Science & Technology, 2019, 35(3): 73-79 (in Chinese). |
| [33] |
王琳琳, 陈炼红, 韩玲, 等. 茶多酚对宰后牦牛肉线粒体细胞凋亡和肌肉嫩度的影响[J]. 农业机械学报, 2019, 50(10): 352-359, 366. WANG L L, CHEN L H, HAN L, et al. Effects of tea polyphenols on mitochondrial apoptosis and meat tenderness in post-mortem yak meat[J]. Transactions of the Chinese Society for Agricultural Machinery, 2019, 50(10): 352-359, 366 (in Chinese). DOI:10.6041/j.issn.1000-1298.2019.10.041 |
| [34] |
张爽. 细胞凋亡诱导剂对羊肉成熟过程Caspase-3激活通路的机制研究[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2018. ZHANG S. The mechanism of apoptosis inducers on caspase-3 activation pathway during matutation of lamb[D]. Master's Thesis. Yangling: Northwest A&F University, 2018. (in Chinese) |
| [35] |
CHEN C, ZHANG J Y, GUO Z B, et al. Effect of oxidative stress on AIF-mediated apoptosis and bovine muscle tenderness during postmortem aging[J]. Journal of Food Science, 2020, 85(1): 77-85. DOI:10.1111/1750-3841.14969 |
| [36] |
陈琳. Caspase-3在鸡肉成熟过程中的作用以及与calpain的交互关系研究[D]. 博士学位论文. 南京: 南京农业大学, 2011. CHEN L. Study on the effects of caspase-3 and its association with calpain during postmortem ageing of chicken meat[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2011. (in Chinese) |
| [37] |
尹靖东. 动物肌肉生物学与肉品科学[M]. 北京: 中国农业大学出版社, 2011. YIN J D. Animal muscle biology and meat quality[M]. Beijing: China Agricultural University Press, 2011 (in Chinese). |
| [38] |
章杰, 李学伟, 勇立. 肌纤维与肉质的关系[J]. 猪业科学, 2013, 30(3): 116-118. ZHANG J, LI X W, YONG L. Relationship between muscle fiber and meat quality[J]. Swine Industry Science, 2013, 30(3): 116-118 (in Chinese). DOI:10.3969/j.issn.1673-5358.2013.03.029 |
| [39] |
LIBERA L D, ZENNARO R, SANDRI M, et al. Apoptosis and atrophy in rat slow skeletal muscles in chronic heart failure[J]. American Journal of Physiology, 1999, 277(5): C982-C986. DOI:10.1152/ajpcell.1999.277.5.C982 |
| [40] |
YAMADA H, NAKAGAWA M, HIGUCHI I, et al. Type Ⅱ muscle fibers are stained by anti-Fas antibody[J]. Journal of the Neurological Sciences, 1995, 134(1/2): 115-118. |
| [41] |
周婕, 汤长发, 李善妮, 等. 不同强度运动对大鼠骨骼肌细胞凋亡的影响[J]. 体育科学, 2005(5): 55-58. ZHOU J, TANG C F, LI S N, et al. Influence of different training intensity on skeletal muscle apoptosis in rats[J]. China Sport Science, 2005(5): 55-58 (in Chinese). DOI:10.3969/j.issn.1000-677X.2005.05.013 |
| [42] |
RIVA C, CHEVRIER C, PASQUAL N, et al. Bcl-2/Bax protein expression in heart, slow-twitch and fast-twitch muscles in young rats growing under chronic hypoxia conditions[J]. Molecular and Cellular Biochemistry, 2001, 226(1/2): 9-16. DOI:10.1023/A:1012772931313 |
| [43] |
曹锦轩. 宰后牛肉成熟过程中肌细胞死亡生理研究[D]. 博士学位论文. 南京: 南京农业大学, 2010. CAO J X. The research on the physiology of the death of myocytes in beef during postmortem conditioning[D]. Ph. D. Thesis. Nanjing: Nanjing Agricultural University, 2010. (in Chinese) |
| [44] |
SHAN R T, LIU N, YAN Y Y, et al. Apoptosis, autophagy and atherosclerosis: relationships and the role of Hsp27[J]. Pharmacological Research, 2021, 166: 105169. DOI:10.1016/j.phrs.2020.105169 |
| [45] |
李婕, 师希雄, 韩玲, 等. 热休克蛋白对宰后成熟过程中肉品质影响的研究进展[J]. 食品工业科技, 2016, 37(1): 392-395, 399. LI J, SHI X X, HAN L, et al. Advances in research of the effect of heat shock proteins on meat quality during postmortem aging[J]. Science and Technology of Food Idustry, 2016, 37(1): 392-395, 399 (in Chinese). |
| [46] |
GUILLEMIN N, JURIE C, CASSAR-MALEK I, et al. Variations in the abundance of 24 protein biomarkers of beef tenderness according to muscle and animal type[J]. Animal, 2011, 5(6): 885-894. DOI:10.1017/S1751731110002612 |
| [47] |
BOCK F J, TAIT S W G. Mitochondria as multifaceted regulators of cell death[J]. Nature Reviews Molecular Cell Biology, 2020, 21(2): 85-100. DOI:10.1038/s41580-019-0173-8 |
| [48] |
KOPEINA G S, PROKHOROVA E A, LAVRIK I N, et al. Alterations in the nucleocytoplasmic transport in apoptosis: caspases lead the way[J]. Cell Proliferation, 2018, 51(5): e12467. DOI:10.1111/cpr.12467 |
| [49] |
CRAMER T, PENICK M L, WADDELL J N, et al. A new insight into meat toughness of callipyge lamb loins-the relevance of anti-apoptotic systems to decreased proteolysis[J]. Meat Science, 2018, 140: 66-71. DOI:10.1016/j.meatsci.2018.03.002 |
| [50] |
KE Y L, MITACEK R M, ABRAHAM A, et al. Effects of muscle-specific oxidative stress on cytochrome c release and oxidation-reduction potential properties[J]. Journal of Agricultural and Food Chemistry, 2017, 65(35): 7749-7755. DOI:10.1021/acs.jafc.7b01735 |
| [51] |
张佳莹. 线粒体通路信号介导细胞凋亡机制及对宰后牛肉嫩化影响[D]. 博士学位论文. 兰州: 甘肃农业大学, 2020. ZHANG J Y. The mechanism of effects of mitochondrial pathway signaling mediated apoptosis on bovine muscle tenderization during postmortem aging[D]. Ph. D. Thesis. Lanzhou: Gansu Agricultural University, 2020. (in Chinese) |
| [52] |
RODRIGUES G S, GODINHO R O, KIYOMOTO B H, et al. Integrated analysis of the involvement of nitric oxide synthesis in mitochondrial proliferation, mitochondrial deficiency and apoptosis in skeletal muscle fibres[J]. Scientific Reports, 2016, 6: 20780. DOI:10.1038/srep20780 |
| [53] |
GAO W X, ZHAO J, GAO Z H, et al. Synergistic interaction of light alcohol administration in the presence of mild iron overload in a mouse model of liver injury: involvement of triosephosphate isomerase nitration and inactivation[J]. PLoS One, 2017, 12(1): e0170350. DOI:10.1371/journal.pone.0170350 |
| [54] |
MONCADA S, ERUSALIMSKY J D. Does nitric oxide modulate mitochondrial energy generation and apoptosis?[J]. Nature Reviews Molecular Cell Biology, 2002, 3(3): 214-220. DOI:10.1038/nrm762 |
| [55] |
PLANITZER G, MIETHKE A, BAUM O. Nitric oxide synthase-1 is enriched in fast-twitch oxidative myofibers[J]. Cell and Tissue Research, 2001, 306(2): 325-333. DOI:10.1007/s004410100449 |
| [56] |
LIU R, LI Y P, ZHANG W G, et al. Activity and expression of nitric oxide synthase in pork skeletal muscles[J]. Meat Science, 2015, 99: 25-31. DOI:10.1016/j.meatsci.2014.08.010 |
| [57] |
ZHANG L L, LIU R, CHENG Y P, et al. Effects of protein S-nitrosylation on the glycogen metabolism in postmortem pork[J]. Food Chemistry, 2019, 272: 613-618. DOI:10.1016/j.foodchem.2018.08.103 |
| [58] |
WANG Y Y, LIU R, TIAN X N, et al. Comparison of activity, expression, and S-nitrosylation of calcium transfer proteins between pale, soft, and exudative and red, firm, and non-exudative pork during post-mortem aging[J]. Journal of Agricultural and Food Chemistry, 2019, 67(11): 3242-3248. DOI:10.1021/acs.jafc.8b06448 |
| [59] |
LIU R, WARNER R D, ZHOU G H, et al. Contribution of nitric oxide and protein S-nitrosylation to variation in fresh meat quality[J]. Meat Science, 2018, 144: 135-148. DOI:10.1016/j.meatsci.2018.04.027 |
| [60] |
MANNICK J B, SCHONHOFF C, PAPETA N, et al. S-nitrosylation of mitochondrial caspases[J]. Journal of Cell Biology, 2001, 154(6): 1111-1116. DOI:10.1083/jcb.200104008 |
| [61] |
HOU Q, LIU R, TIAN X N, et al. Involvement of protein S-nitrosylation in regulating beef apoptosis during postmortem aging[J]. Food Chemistry, 2020, 326: 126975. DOI:10.1016/j.foodchem.2020.126975 |
| [62] |
ZHANG W G. Involvement of protein degradation, calpain autolysis and protein nitrosylation in fresh meat quality during early postmortem refrigerated storage[D]. Ph. D. Thesis. Ames, Iowa: Iowa State University, 2009.
|
| [63] |
赵康涛, 海春旭. H2O2调控下HepG2细胞pH值与Ca2+的剂量效应关系[J]. 毒理学杂志, 2009, 23(4): 278-281. ZHAO K T, HAI C X. Dose-effect relationship between pH and Ca2+ in HepG2 cell induced by H2O2[J]. Journal of Health Toxicology, 2009, 23(4): 278-281 (in Chinese). |
| [64] |
贾青. 细胞凋亡酶-3及其抑制剂对宰后牦牛肉品质变化的影响[D]. 硕士学位论文. 兰州: 甘肃农业大学, 2016. JIA Q. Influence of caspases-3 and the inhibitors on yak meat quality changes[D]. Master's Thesis. Lanzhou: Gansu Agricultural University, 2016. (in Chinese) |
| [65] |
王勇峰. 肌纤维类型差异对牛肉品质及细胞凋亡酶3影响研究[D]. 硕士学位论文. 北京: 中国农业科学院, 2017. WANG Y F. Effects of different muscle fiber type on beef quality and caspase-3[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2017. (in Chinese) |
| [66] |
RYU Y C, KIM B C. Comparison of histochemical characteristics in various pork groups categorized by postmortem metabolic rate and pork quality[J]. Journal of Animal Science, 2006, 84(4): 894-901. DOI:10.2527/2006.844894x |




