反刍动物幼畜的饲养方式、饲粮营养水平和乳源生长因子都会影响其消化道的发育[1],犊牛的早期培育又是牦牛养殖中重要的环节。研究表明,通过补饲有利于犊牛胃肠道消化功能的快速建立[2],实现牦牛犊牛适时断奶培育,有效促进母牦牛早期发情和配种,进而提高牦牛养殖的生产效益,加快草畜生产高质量发展。营养丰富的开食料不仅易消化吸收,还能显著提高犊牛的采食量,促进其生长发育[3-5]。反刍动物通过瘤胃微生物将膳食纤维转化为糖原以供微生物发酵并为机体细胞提供能量[6-7],开食料中70%~85%的粗纤维和其他可消化物质都能够被反刍动物瘤胃消化利用[8],因此在宿主的生长发育和免疫中,瘤胃微生物的群落结构尤为重要[9-10]。本实验室前期研究表明,早期断奶和补饲开食料有利于提高牦牛犊牛的生长性能和消化道发育[11],但对哺乳期补饲开食料如何调控牦牛犊牛瘤胃发育的机制研究还不够深入。因此,本研究利用16S扩增子测序技术,在牦牛犊牛采食苜蓿干草的基础上,进行补饲开食料,探究早期补饲开食料对哺乳期牦牛犊牛生长性能、瘤胃形态及功能发育的影响,为牦牛犊牛断奶后的代乳培育技术研究提供重要参考。
1 材料与方法 1.1 试验设计本试验于2020年7月至11月在青海省海北藏族自治州海晏县开展,试验采用单因素设计,选取体重相近、体况良好的20头30日龄牦牛公犊牛作为试验对象,单栏饲养,并饲喂一定比例的代乳粉(代乳粉: 温水=1:5)。经过1周适应期后随机分为对照组(RA组)和试验组(RAS组),每组各10头。在饲喂代乳粉的基础上,RA组饲喂苜蓿干草(代乳粉+苜蓿草),RAS组按照苜蓿干草: 开食料=1:1的模式进行饲喂(代乳粉+苜蓿草+开食料),自由饮水。开食料组成及营养水平见表 1。代乳粉、苜蓿干草营养水平见表 2。试验期间定期对圈舍进行消毒和清洁工作。预试期14 d,正试期120 d。试验结束后,每组随机选取5头进行屠宰取样。
|
|
表 1 开食料组成及营养水平(干物质基础) Table 1 Composition and nutrient levels of the starter feed (DM basis) |
|
|
表 2 代乳粉和苜蓿干草营养水平(干物质基础) Table 2 Nutrient levels of milk replacer and alfalfa hay (DM basis) |
犊牛屠宰后,取瘤胃内容物经4层纱布过滤,采集瘤胃液立即测定pH。另采集约50 mL瘤胃液分装后经液氮运输回实验室保存,用于DNA的提取和后续分析以及挥发性脂肪酸(VFA)、氨态氮(NH3-N)浓度和微生物蛋白(MCP)含量的测定。取大小为2 cm×2 cm的瘤胃组织保存于4%多聚甲醛溶液中,用于固定组织和石蜡切片的制作。
1.3 测定指标与方法 1.3.1 干物质采食量、体重增重试验期间,每日称量并饲喂草和料,记录投喂量,第2天饲喂前分别清理剩料并记录称重。试验开始和结束前,早晨对犊牛进行称量空腹体重。试验结束后计算犊牛干物质采食量(DMI)、平均日增重(ADG)和料重比。
1.3.2 瘤胃发酵参数瘤胃液样品在测定当天进行解冻并离心,离心后取上清液,待测样品放在4 ℃冰箱内保存。瘤胃液pH使用UB-7型(梅特勒-托利多,美国)酸度计进行测定。瘤胃NH3-N浓度采用冯宗慈等[12]改进的比色法进行测定。MCP含量采用南京建成生物工程研究所提供的试剂盒进行测定。使用岛津GC-2014气相色谱仪测定瘤胃液VFA的浓度[13],气相色谱的条件如下:载气为N2,分流比40:1,进样量为1 μL,进样孔温度、辅助箱温度和气化室温度均为250 ℃;FID检测器温度:250 ℃。恒流模式下的流量为2.1 mL/min,平均线速度38 cm/s,柱压11.3 psi(0.1 MPa),柱温箱设定为程序升温,90 ℃下1 min,120 ℃下1 min,150 ℃下运行3 min。
1.3.3 瘤胃组织形态观察在操作台上将瘤胃组织从固定液中取出,用刀片对组织进行修整后放入脱水盒内并编号,经梯度酒精脱水、浸蜡,浸蜡后的组织置于包埋机内进行包埋,蜡块修整后进行切片、展片、烤片。染色采用苏木精-伊红染色法。切片在Olympus BX53电子显微镜下观察,进行数据采集。
1.3.4 瘤胃微生物区系检测将5 mL的瘤胃液样品经干冰运输至北京诺和致源生物技术公司的Illumina MiSeq平台上对细菌扩增子进行测序,引物序列341F(5′-CCTAYGGGRBGCASCAG-3′)和806R(5′-GGACTACNNGGGTATCTAAT-3′)。文库构建使用的是TruSeq® DNA PCR-Free Sample Preparation Kit建库试剂盒。
1.4 数据处理与统计分析采用Excel 2016对数据进行整理,用SPSS 22.0软件进行数据独立性方差齐性检验,采用one-way ANOVA程序进行单因素方差分析。P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果与分析 2.1 补饲开食料对牦牛犊牛生长性能的影响由表 3可知,补饲开食料后,RAS组犊牛在试验期末体重极显著高于RA组(P < 0.01),RA组和RAS组分别增重39.62和45.70 kg。RA组干物质采食量为678.71 g/d,显著低于RAS组(P < 0.05)。
|
|
表 3 补饲开食料对牦牛犊牛干物质采食量和增重的影响 Table 3 Effects of supplementary starter feed on dry matter intake and weight gain of yak calves |
由表 4可知,RAS组犊牛早期采食开食料后,瘤胃NH3-N浓度极显著提高了33.74%(P < 0.01),RAS组瘤胃MCP含量显著提高了31.30%(P < 0.05);RA组犊牛瘤胃液乙酸浓度显著高于RAS组(P < 0.05);RAS组戊酸浓度显著高于RA组(P < 0.05)。
|
|
表 4 补饲开食料对牦牛犊牛瘤胃发酵参数的影响 Table 4 Effects of supplementary starter feed on rumen fermentation parameters of yak calves |
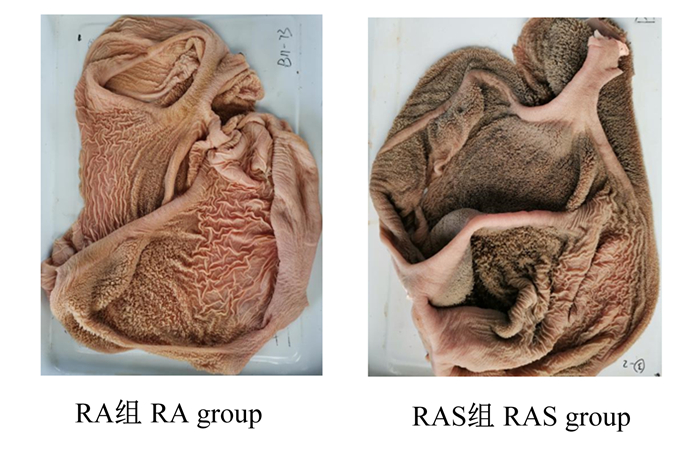
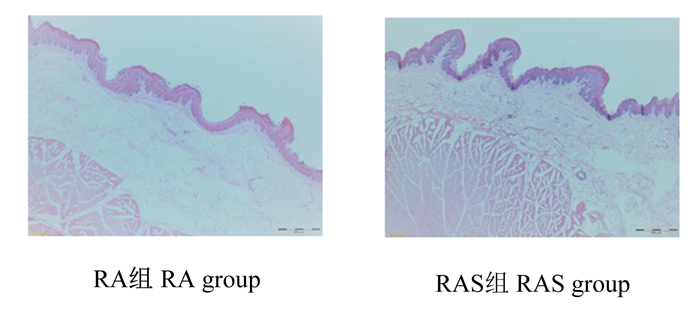
瘤胃组织发育形态观察如图 1所示,RAS组犊牛瘤胃颜色加深,乳头分布密集。图 2为瘤胃上皮组织切片在倒置电子显微镜下成像图。统计结果如表 5所示,RAS组犊牛瘤胃乳头的长度极显著高于RA组(P < 0.01),瘤胃乳头宽度和肌肉层厚度组间无显著差异(P>0.05)。
|
图 1 牦牛犊牛瘤胃上皮外观形态观察 Fig. 1 Morphological observation of rumen epithelium in yak calves |
|
图 2 牦牛犊牛瘤胃上皮组织切片 Fig. 2 Section of rumen epithelium tissue of yak calves (50×) |
|
|
表 5 补饲开食料对牦牛犊牛瘤胃上皮形态发育的影响 Table 5 Effects of supplementary starter feed on rumen morphology development of yak calves |
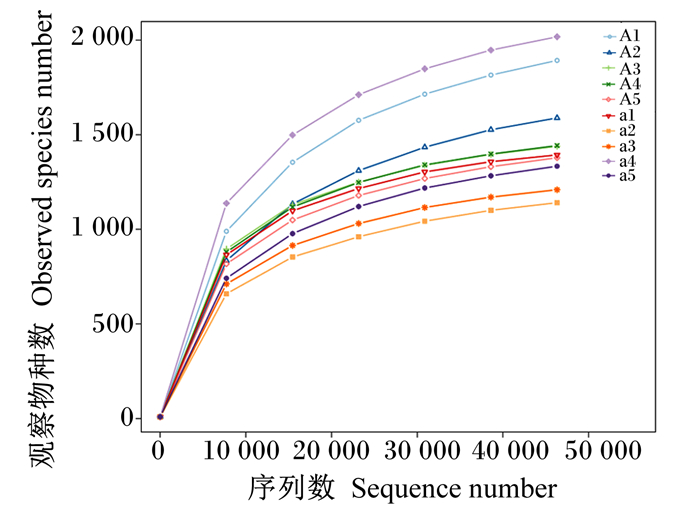
根据16S rRNA基因测序所得数据,绘制出的犊牛瘤胃液微生物样本的稀释曲线如图 3,稀疏曲线具有97%的相似度,说明样品有足够的测序覆盖率,能够代表每个样本中大多数微生物信息。
|
RA组包括犊牛样本:A1、A2、A3、A4、A5;RAS组包括犊牛样本:a1、a2、a3、a4、a5。 The RA group included yak calve samples: A1, A2, A3, A4, A5; the RAS group included yak calve samples: a1, a2, a3, a4, a5. 图 3 牦牛犊牛瘤胃微生物稀释曲线图 Fig. 3 Rumen microbial dilution curve of yak calves |
本试验从10头犊牛瘤胃液中获得925 748条有效序列,在3%的距离上,10个样本共获得1 591个OTU,平均每个样本有502.90个OTU,所有样本共有的OTU数目为402个。由表 6可知,样本的覆盖率达到99%以上,说明测序深度足够。其中,RA组的OTU数目显著高于RAS组(P < 0.05),而2组的ACE指数和Chao1指数、香农指数和辛普森指数均无显著差异(P>0.05)。
|
|
表 6 牦牛犊牛瘤胃微生物OTU数目和Alpha多样性指数分析 Table 6 Analysis of OTU number and alpha diversity indexes of rumen bacteria of yak calves |
分析瘤胃液菌群在门水平的组成比例及变化,共检测到69个菌门。由表 7可知,在门水平上,RA组和RAS组的优势菌群一致,但相对丰度不同。其中,RA组拟杆菌门相对丰度极显著高于RAS组(P < 0.01);RAS组变形菌门相对丰度极显著高于RA组(P < 0.01)。
|
|
表 7 补饲开食料对牦牛犊牛瘤胃微生物优势菌门相对丰度的影响 Table 7 Effects of supplementary starter feed on relative abundance of rumen microbial at phylum level of yak calves |
分析RA组和RAS组瘤胃微生物在属水平的组成和变化,共计检测到519个菌属。根据表 8可知,RAS组甲烷短杆菌属和瘤胃球菌属相对丰度显著高于RA组(P < 0.05),RA组奎因氏菌属相对丰度极显著高于RAS组(P < 0.01)。
|
|
表 8 补饲开食料对牦牛犊牛瘤胃微生物优势菌属相对丰度的影响 Table 8 Effects of supplementary starter feed on relative abundance of rumen microbial at genus level of yak calves |
在高原地区,营养的匮乏和恶劣的环境严重影响了牦牛犊牛的成活率。随着技术的进步,科学的补饲开食料不仅能够提高母畜的繁殖率,还能改善天然牧草的载畜量,促进畜牧业健康持久地发展[14]。对放牧牦牛进行补饲开食料能够促进其生长性能,提高经济效益[15],但如何提高放牧牦牛犊牛的生长性能以及如何科学补饲的机制亟待完善,因此,本试验开展对哺乳期牦牛犊牛补饲开食料的深入探究,结果发现,补饲开食料后,犊牛增重达到45.7 kg,极显著提高了犊牛的体重,这与完玛措[16]和黄文植等[17]冷季对牦牛犊牛补饲提高增重的结果一致。断奶前后犊牛采食颗粒饲料可以提高犊牛的干物质消化率,提高生产性能[18]。有研究显示,代乳+补饲培育方式能显著提高牦牛犊牛的干物质采食量,有助于犊牛增重[19],本研究结果与之相同。在饲养管理和环境条件一致的情况下,RA组犊牛体重较低可能是由于采食营养单一的苜蓿干草,且苜蓿干草纤维水平较高,适口性差,进而影响了其生长发育。
3.2 补饲开食料对牦牛犊牛瘤胃发育和发酵参数的影响犊牛出生时瘤胃发育迟缓,其生长发育主要依靠皱胃和肠道的消化吸收。随着日龄的增长和固体饲粮的采食,瘤胃内微生物发酵产生的VFA刺激瘤胃迅速发育,消化系统也逐步建立免疫代谢功能[20-22]。研究发现,采用代乳粉和开食料的培育方式能够缓解断奶应激并提高瘤胃发育水平[23]。本试验中也发现,采食开食料刺激了犊牛瘤胃的组织形态发育,RAS组瘤胃乳头长度显著增加,这与Xie等[24]对荷斯坦犊牛补饲开食料的研究结果相似。
在早期培育方式的影响下,苜蓿干草和开食料均能够改变反刍动物消化道的健康和免疫[25-26]。瘤胃作为反刍动物体内的发酵罐,其发酵产生的VFA直接刺激着瘤胃上皮的发育进程[27]。瘤胃pH是反映瘤胃是否健康发酵的综合指标,本试验的瘤胃液pH在7.23~7.47,在瘤胃健康发酵的范围内[28]。NH3-N浓度和MCP含量是反映反刍动物瘤胃的发酵状态,NH3-N浓度在瘤胃中处于动态平衡,为反刍动物合成MCP提供原料,是检测瘤胃微生物活性的指标[29]。此外,微生物对NH3-N的利用主要受饲粮中非纤维性碳水化合物的影响,随着饲粮中非纤维性碳水化合物含量的增加,瘤胃微生物会提高对NH3-N利用[30],本研究中RA组犊牛瘤胃内NH3-N浓度显著较低,这与Agle等[31]研究发现,高比例粗饲料饲粮会降低奶牛瘤胃中NH3-N浓度的结果相同,说明瘤胃微生物对NH3-N的利用会随着饲粮中结构性碳水化合物含量的增加而降低。RAS组MCP的含量显著高于RA组,MCP是瘤胃微生物氮的主要来源,说明了补饲开食料满足了微生物对饲粮降解的蛋白质需求[32]。
反刍动物利用瘤胃微生物将饲料中的纤维素降解并转化为VFA,为机体提供70%~80%的能量需求[33]。乙酸在动物体内经过一系列变化进入三羧酸循环,也可以合成脂肪或与丁酸相互转化产生酮体,促进动物对脂肪的囤积[34]。刘敏雄[35]报道,当粗饲料水平升高时,纤维素分解菌占优势,乙酸浓度上升。本试验中RA组犊牛仅采食苜蓿干草,中性洗涤纤维和酸性洗涤纤维水平较高,导致了瘤胃乙酸浓度显著升高,这与王文奇等[36]提高饲粮中酸性洗涤纤维水平,发现乙酸浓度升高的结果一致。目前关于牦牛犊牛的培育工作正在初期探索进程中,先前研究报道采用代乳粉饲喂后,150日龄犊牛瘤胃内乙酸浓度显著升高[37],本研究结果与之不同,可能是由于海拔、饲粮结构和饲养模式的差异所导致的。
3.3 补饲开食料对牦牛犊牛瘤胃微生物区系的影响饲粮的构成影响着瘤胃微生物区系的建立,进而作用于瘤胃的形态和功能。Ben等[38]和Abecia等[39]发现,饲粮中添加单宁或甲烷抑制剂等均会改变瘤胃微生物组成,影响反刍动物的生长性能和健康发育。同时,瘤胃微生物的丰度又作用于宿主对饲粮的利用效率[40]。饲粮中高水平的非纤维性碳水化合物能降低犊牛瘤胃微生物的种类和丰富度,当饲粮中性洗涤纤维水平低于20%时,瘤胃中细菌的相对丰度有所下降[41]。本研究中,开食料中酸性洗涤纤维水平为4.10%,因此补饲开食料后犊牛瘤胃内OTU数目显著降低,这与杨硕等[42]在绒山羊上的研究结果相近。这表明尽管开食料为犊牛瘤胃微生物的定植提供充足的碳源和氮源[43],但一定程度上抑制了微生物群落的丰度。本试验中2组犊牛的优势菌门均为拟杆菌门和厚壁菌门,这与前人的研究结果[44-45]一致。Sander等[21]的研究表明,不同日龄犊牛瘤胃微生物菌群在门水平上有显著差异,本研究中犊牛采食开食料后拟杆菌门的相对丰度极显著下降,厚壁菌门的相对丰度没有显著性变化,说明开食料抑制了拟杆菌门的生长与增殖。变形菌门能够降解可溶性碳水化合物含量[46],犊牛所采食的开食料中粗蛋白质与粗脂肪水平较高,中性洗涤纤维和酸性洗涤纤维水平较低,且补饲开食料后犊牛瘤胃中变形菌门的相对丰度极显著升高,因此推测变形菌门的相对丰度随着饲粮中蛋白质水平的升高而增加,并且与饲粮中纤维水平呈负相关。
瘤胃微生物间相互作用,将纤维素等不易降解的物质转化为VFA,进一步作用于宿主的生长和维持[47]。营养水平的提高导致了微生物门水平细菌相对丰度的差异,进而影响了营养物质的利用,在属水平上,RAS组甲烷短杆菌属的相对丰度显著升高,甲烷杆菌属能够利用氢气还原甲醇,在瘤胃中,甲醇是原虫水解果胶和细菌酯酶活性的产物,这意味着补饲开食料后牦牛犊牛瘤胃发酵产生了更多的氢气,对碳水化合物的消耗增加,也为机体提供了更多的能量[48]。厚壁菌门中的瘤胃球菌属被Flint等[49]和Kabel等[50]认为在蛋白质、果胶等非纤维素植物多糖的降解等方面发挥着重要的作用,RAS组瘤胃球菌属的相对丰度显著升高进一步说明了开食料积极调控着瘤胃微生物的相对丰度。
4 结论本研究表明,哺乳期补饲开食料能够显著提高牦牛犊牛的干物质采食量和体重,改变瘤胃微生物群落的多样性和丰富度,正向调控了瘤胃形态功能发育,为青藏高原地区牦牛犊牛健康高质量培育提供了重要理论依据。
| [1] |
LIN L M, XIE F, SUN D M, et al. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model[J]. Microbiome, 2019, 7(1): 83. DOI:10.1186/s40168-019-0701-y |
| [2] |
CUI Z H, WU S R, LIU S J, et al. From maternal grazing to barn feeding during pre-weaning period: altered gastrointestinal microbiota contributes to change the development and function of the rumen and intestine of yak calves[J]. Frontiers in Microbiology, 2020, 11: 485. DOI:10.3389/fmicb.2020.00485 |
| [3] |
MISRA A K, SINGH D. Rearing of calf: a scientific approach[J]. Indian Dairyman, 2012, 64: 526-529. |
| [4] |
云强. 蛋白水平及Lys/Met对断奶犊牛生长、消化代谢及瘤胃发育的影响[D]. 硕士学位论文. 北京: 中国农业科学院, 2010. YUN Q. Effects of protein level and Lys/Met on performance, nutrient digestibility and rumen development for weaned calves[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2010. (in Chinese) |
| [5] |
高宝山. 代乳粉与开食料对犊牛生长发育影响的研究[D]. 硕士学位论文. 北京: 中国农业科学院, 2013. GAO B S. The effect of milk replacer and starter on growth and development in calves[D]. Master's Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2013. (in Chinese) |
| [6] |
KARASOV W H, MARTÍNEZ DEL RIO C, CAVIEDES-VIDAL E. Ecological physiology of diet and digestive systems[J]. Annual Review of Physiology, 2011, 73: 69-93. DOI:10.1146/annurev-physiol-012110-142152 |
| [7] |
YEOMAN C J, WHITE B A. Gastrointestinal tract microbiota and probiotics in production animals[J]. Annual Review of Animal Biosciences, 2014, 2: 469-486. DOI:10.1146/annurev-animal-022513-114149 |
| [8] |
李洋. 亚急性瘤胃酸中毒对奶山羊瘤胃上皮挥发性脂肪酸吸收的影响及其机制研究[D]. 硕士学位论文. 呼和浩特: 内蒙古农业大学, 2019. LI Y. Effects of subacute rumen acidosis on absorption of volatile fatty acids from rumen epithelium in dairy goats and its mechanism[D]. Master's Thesis. Hohhot: Inner Mongolia Agricultural University, 2019. (in Chinese) |
| [9] |
ALIPOUR M J, JALANKA J, PESSA-MORIKAWA T, et al. The composition of the perinatal intestinal microbiota in cattle[J]. Scientific Reports, 2018, 8(1): 10437. DOI:10.1038/s41598-018-28733-y |
| [10] |
KAMADA N, CHEN G Y, INOHARA N, et al. Control of pathogens and pathobionts by the gut microbiota[J]. Nature Immunology, 2013, 14(7): 685-690. DOI:10.1038/ni.2608 |
| [11] |
CUI Z H, WU S R, LI J L, et al. Effect of alfalfa hay and starter feeding intervention on gastrointestinal microbial community, growth and immune performance of yak calves[J]. Frontiers in Microbiology, 2020, 11: 994. DOI:10.3389/fmicb.2020.00994 |
| [12] |
冯宗慈, 高民. 通过比色测定瘤胃液氨氮含量方法的改进[J]. 畜牧与饲料科学, 2010, 31(Z1): 37. FENG Z C, GAO M. The improvement of colorimetric method for determination of ammonia nitrogen in rumen fluid[J]. Animal Husbandry and Feed Science, 2010, 31(Z1): 37 (in Chinese). |
| [13] |
毕思思. 黄芪属植物对牦牛与黄牛体外发酵特征的影响[D]. 硕士学位论文. 兰州: 兰州大学, 2019. BI S S. Effects of Astragalus plants on in vitro fermentation characteristics of yak and cattle[D]. Master's Thesis. Lanzhou: Lanzhou University, 2019. (in Chinese) |
| [14] |
孙鹏飞. 简述放牧牦牛补饲精料的必要性[J]. 养殖与饲料, 2021, 20(7): 61-62. SUN P F. Brief description of the necessity of supplementary concentrate for grazing yak[J]. Animals Breeding and Feed, 2021, 20(7): 61-62 (in Chinese). DOI:10.3969/j.issn.1671-427X.2021.07.026 |
| [15] |
王书祥, 戴东文, 杨英魁, 等. 补饲精料对冷季放牧牦牛生长性能、瘤胃发酵及菌群结构的影响[J]. 动物营养学报, 2021, 33(11): 6266-6276. WANG S X, DAI D W, YANG Y K, et al. Effects of concentrate supplementation on growth performance, rumen fermentation and microbial community structure of grazing yaks in cold season[J]. Chinese Journal of Animal Nutrition, 2021, 33(11): 6266-6276 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.11.027 |
| [16] |
完玛措. 寒冷季节犊牛补饲试验研究[J]. 畜牧兽医科学(电子版), 2021(8): 4-5. WANMACUO. Experimental study on supplementary feeding of calves in cold season[J]. Graziery Veterinary Sciences (Electronic Version), 2021(8): 4-5 (in Chinese). |
| [17] |
黄文植, 张晓卫, 夏洪泽, 等. 冷季补饲矿物质盐砖对放牧牦牛犊牛体增重、瘤胃发酵和血清矿物元素含量的影响[J]. 动物营养学报, 2021, 33(11): 6300-6308. HUANG W Z, ZHANG X W, XIA H Z, et al. Effects of mineral salt brick supplement on body weight gain, rumen fermentation and serum mineral element contents of grazing yak calves in cold season[J]. Chinese Journal of Animal Nutrition, 2021, 33(11): 6300-6308 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.11.030 |
| [18] |
TERRÉ M, PEDRALS E, DALMAU A, et al. What do preweaned and weaned calves need in the diet: a high fiber content or a forage source?[J]. Journal of Dairy Science, 2013, 96(8): 5217-5225. DOI:10.3168/jds.2012-6304 |
| [19] |
崔占鸿. 牦牛犊牛培育方式对生长和消化道发育的影响[D]. 博士学位论文. 杨凌: 西北农林科技大学, 2020. CUI Z H. Effects of rearing patterns on growth and digstive tract development in yak calves[D]. Ph. D. Thesis. Yangling: Northwest A&F University, 2020. (in Chinese) |
| [20] |
BROWNLEE A. The development of rumen papillae in cattle fed on different diets[J]. British Veterinary Journal, 1956, 112(9): 369-375. DOI:10.1016/S0007-1935(17)46456-6 |
| [21] |
SANDER E G, WARNER R G, HARRISON H N, et al. The stimulatory effect of sodium butyrate and sodium propionate on the development of rumen mucosa in the young calf[J]. Journal of Dairy Science, 1959, 42(9): 1600-1605. DOI:10.3168/jds.S0022-0302(59)90772-6 |
| [22] |
ANDERSON K L, NAGARAJA T G, MORRILL J L, et al. Ruminal microbial development in conventionally or early-weaned calves[J]. Journal of Animal Science, 1987, 64(4): 1215-1226. DOI:10.2527/jas1987.6441215x |
| [23] |
SWEENEY B C, RUSHEN J, WEARY D M, et al. Duration of weaning, starter intake, and weight gain of dairy calves fed large amounts of milk[J]. Journal of Dairy Science, 2010, 93(1): 148-152. DOI:10.3168/jds.2009-2427 |
| [24] |
XIE X X, MENG Q X, LIU P, et al. Effects of a mixture of steam-flaked corn and extruded soybeans on performance, ruminal development, ruminal fermentation, and intestinal absorptive capability in veal calves[J]. Journal of Animal Science, 2013, 91(9): 4315-4321. DOI:10.2527/jas.2012-5731 |
| [25] |
LIU J H, BIAN G R, SUN D M, et al. Starter feeding supplementation alters colonic mucosal bacterial communities and modulates mucosal immune homeostasis in newborn lambs[J]. Frontiers in Microbiology, 2017, 8: 429. |
| [26] |
YANG B, LE J Q, WU P, et al. Alfalfa intervention alters rumen microbial community development in Hu lambs during early life[J]. Frontiers in Microbiology, 2018, 9: 574. DOI:10.3389/fmicb.2018.00574 |
| [27] |
GÓRKA P, KOWALSKI Z M, ZABIELSKI R, et al. Invited review: use of butyrate to promote gastrointestinal tract development in calves[J]. Journal of Dairy Science, 2018, 101(6): 4785-4800. DOI:10.3168/jds.2017-14086 |
| [28] |
张彩英, 胡国良, 曹华斌. 反刍动物瘤胃内环境的特点及调控措施[J]. 中国畜牧兽医, 2010, 37(4): 18-20. ZHANG C Y, HU G L, CAO H B. Characteristics and control measures of rumen internal environment in ruminants[J]. China Animal Husbandry & Veterinary Medicine, 2010, 37(4): 18-20 (in Chinese). |
| [29] |
FIRKINS J L, YU Z, MORRISON M. Ruminal nitrogen metabolism: perspectives for integration of microbiology and nutrition for dairy[J]. Journal of Dairy Science, 2007, 90(Suppl.1): E1-E16. |
| [30] |
MICHALSKI J P, KOWALCZYK J, CZAUDERNA M, et al. Incorporation of endogenous urea nitrogen into the amino acids of bacterial protein in the rumen of goats fed diets with various protein levels[J]. Journal of Animal and Feed Sciences, 2013, 22(4): 311-315. DOI:10.22358/jafs/65918/2013 |
| [31] |
AGLE M, HRISTOV A N, ZAMAN S, et al. Effect of dietary concentrate on rumen fermentation, digestibility, and nitrogen losses in dairy cows[J]. Journal of Dairy Science, 2010, 93(9): 4211-4222. DOI:10.3168/jds.2009-2977 |
| [32] |
ØRSKOV E R, MCDONALD I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage[J]. The Journal of Agricultural Science, 1979, 92(2): 499-503. DOI:10.1017/S0021859600063048 |
| [33] |
VAN HOUTERT M F J. The production and metabolism of volatile fatty acids by ruminants fed roughages: a review[J]. Animal Feed Science and Technology, 1993, 43(3/4): 189-225. |
| [34] |
SATTER L D, SUTTIE J W, RAUMGARDT B R. Dietary induced changes in volatile fatty acid formation from α-cellulose-C14 and hemicellulose-C14[J]. Journal of Dairy Science, 1964, 47(12): 1365-1370. DOI:10.3168/jds.S0022-0302(64)88919-0 |
| [35] |
刘敏雄. 反刍动物消化生理学[M]. 北京: 北京农业大学出版社, 1991. LIU M X. Digestive physiology of ruminants[M]. Beijing: Beijing Agricultural University Press, 1991 (in Chinese). |
| [36] |
王文奇, 罗永明, 刘艳丰, 等. 不同棉秆水平全混合日粮对绵羊生长性能和瘤胃发酵参数的影响[J]. 新疆农业科学, 2015, 52(11): 2111-2116. WANG W Q, LUO Y M, LIU Y F, et al. Effects of diets with different cotton stalk levels of total mixed ration on growth performance and rumen fermentation parameters in sheep[J]. Xinjiang Agricultural Sciences, 2015, 52(11): 2111-2116 (in Chinese). |
| [37] |
马涛, 崔凯, 张成福, 等. 不同培育模式对牦牛犊牛生长性能、瘤胃发酵参数和血清生化指标的影响[J]. 动物营养学报, 2021, 33(4): 2055-2062. MA T, CUI K, ZHANG C F, et al. Effects of different feeding modes on growth performance, rumen fermentation parameters and serum biochemical indices of yak calves[J]. Chinese Journal of Animal Nutrition, 2021, 33(4): 2055-2062 (in Chinese). DOI:10.3969/j.issn.1006-267x.2021.04.025 |
| [38] |
BEN SALEM H, NEFZAOUI A, MAKKAR H P S, et al. Effect of early experience and adaptation period on voluntary intake, digestion, and growth in Barbarine lambs given tannin-containing (Acacia cyanophylla Lindl.foliage) or tannin-free (oaten hay) diets[J]. Animal Feed Science and Technology, 2005, 122(1/2): 59-77. |
| [39] |
ABECIA L, WADDAMS K E, MARTÍNEZ-FERNANDEZ G, et al. An antimethanogenic nutritional intervention in early life of ruminants modifies ruminal colonization by archaea[J]. Archaea, 2014, 2014: 841463. |
| [40] |
CARBERRY C A, WATERS S M, KENNY D A, et al. Rumen methanogenic genotypes differ in abundance according to host residual feed intake phenotype and diet type[J]. Applied and Environmental Microbiology, 2014, 80(2): 586-594. DOI:10.1128/AEM.03131-13 |
| [41] |
马满鹏, 王炳, 屠焰, 等. 日粮纤维水平和来源影响犊牛生长和胃肠道发育的研究[J]. 家畜生态学报, 2019, 40(5): 7-12. MA M P, WANG B, TU Y, et al. Effects of dietary fiber levels and sources on calf growth and gastrointestinal development[J]. Journal of Domestic Animal Ecology, 2019, 40(5): 7-12 (in Chinese). DOI:10.3969/j.issn.1673-1182.2019.05.002 |
| [42] |
杨硕, 卢洋洋, 韦玥瑞, 等. 补饲精料对大青山绒山羊瘤胃细菌及产甲烷菌多样性的影响[J]. 畜牧与兽医, 2020, 52(8): 50-55. YANG S, LU Y Y, WEI Y R, et al. Effect of supplementary feeding on the diversity of rumen bacteria and methanogens in grazing Daqingshan cashmere goats[J]. Animal Husbandry & Veterinary Medicine, 2020, 52(8): 50-55 (in Chinese). |
| [43] |
DORRESTEIN P C, MAZMANIAN S K, KNIGHT R. Finding the missing links among metabolites, microbes, and the host[J]. Immunity, 2014, 40(6): 824-832. DOI:10.1016/j.immuni.2014.05.015 |
| [44] |
DE OLIVEIRA M N V, JEWELL K A, FREITAS F S, et al. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer[J]. Veterinary Microbiology, 2013, 164(3/4): 307-314. |
| [45] |
吴琼, 王思珍, 张适, 等. 基于16S rRNA高通量测序技术分析中国西门塔尔牛瘤胃微生物多样性和功能预测的研究[J]. 中国畜牧兽医, 2019, 46(5): 1370-1378. WU Q, WANG S Z, ZHANG S, et al. Analysis of rumen microbial diversity and functional prediction of Chinese Simmental cattle based on 16S rRNA high-throughput sequencing technology[J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(5): 1370-1378 (in Chinese). |
| [46] |
PITTA D W, PINCHAK W E, INDUGU N, et al. Metagenomic analysis of the rumen microbiome of steers with wheat-induced frothy bloat[J]. Frontiers in Microbiology, 2016, 7: 689. |
| [47] |
MORGAVI D P, KELLY W J, JANSSEN P H, et al. Rumen microbial (meta) genomics and its application to ruminant production[J]. Animal, 2013, 7(Suppl.1): 184-201. |
| [48] |
HOOK S E, WRIGHT A D G, MCBRIDE B W. Methanogens: methane producers of the rumen and mitigation strategies[J]. Archaea, 2010, 2010: 945785. |
| [49] |
FLINT H J, BAYER E A, RINCON M T, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis[J]. Nature Reviews Microbiology, 2008, 6(2): 121-131. DOI:10.1038/nrmicro1817 |
| [50] |
KABEL M A, YEOMAN C J, HAN Y J, et al. Biochemical characterization and relative expression levels of multiple carbohydrate esterases of the xylanolytic rumen bacterium Prevotella ruminicola 23 grown on an ester-enriched substrate[J]. Applied and Environmental Microbiology, 2011, 77(16): 5671-5681. DOI:10.1128/AEM.05321-11 |




