2. 广东粤海饲料集团股份有限公司, 湛江 524017;
3. 广东省水产动物精准营养与高效饲料工程技术研究中心, 湛江 524088;
4. 农业农村部华南水产与畜禽饲料重点实验室, 湛江 524088
2. Guangdong Yuehai Feeds Group Co., Ltd., Zhanjiang 524017, China;
3. Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang 524088, China;
4. Key Laboratory of Aquatic, Livestock and Poultry Feed Science and Technology in South China, Ministry of Agriculture and Rural Affair, Zhanjiang 524088, China
2020年我国淡水鲈鱼的养殖产量已达到61.9万t,比2019年增长29.66%[1],其中有“淡水石斑鱼”之称的大口黑鲈(Micropterus salmoides)作为一种重要的经济鱼类[2-3],养殖产量逐年增长,但是相较于大菱鲆(Scophthalmus maxmius)[4]和乌鳢(Channa argus)[5]等其他肉食性鱼类,大口黑鲈对血糖清除相对缓慢[6],利用饲料中淀粉的能力更低[7],若摄入过量碳水化合物会导致生长迟缓和肝糖原异常积累,造成肝脏损伤[8-12]。因此,饲料中淀粉的利用效率直接影响大口黑鲈生长和养殖成本。
淀粉作为饲料中碳水化合物的主要存在形式,不但为机体提供能量,还在饲料中起着黏合和膨胀的作用,有助于改善饲料的物理品质[13]。淀粉分为直链淀粉(AM)和支链淀粉(AP),分子聚集状态与结构不同,性质和功能也不同[14-15]。AM的分支很少,可以形成具有复杂晶体结构的抗性淀粉,在动物肠道内会抵抗消化[16],而AP分支多,其分子表面积更大,分子间氢键作用力更小[17],这一特征使其更容易消化[18]。相较于AP,AM糊化温度更高,稳定性能更强,具有抗润胀性,但增稠性和黏合性较低[19]。通过控制饮食中的直链淀粉/支链淀粉(AM/AP), 可改善猪、羊和鸡等动物生长和健康状况[20-22]。AM/AP是淀粉发挥功能特性的重要因素之一,不仅对水产膨化饲料的加工产生重要的影响[23],还影响养殖动物对饲料的消化[24]、蛋白质和脂肪的代谢[25]以及能量的分配。因此,本试验通过设置饲料中不同的AM/AP,一方面评价不同AM/AP对饲料加工特性的影响,另一方面探索不同AM/AP对大口黑鲈营养物质表观消化率和肠道消化酶活性的影响,旨在明确大口黑鲈饲料中淀粉的添加形式,更有效地推动高效配合饲料替代鲜活饵料的进度,有助于实现养殖和环境的可持续性发展。
1 材料与方法 1.1 试验饲料以鱼粉、鸡肉粉和酪蛋白为主要蛋白质源,豆油和鱼油为主要脂肪源,以蜡质玉米淀粉(WCS,AM含量为0,AP含量为92.28%)和高直链玉米淀粉(HACS,AM含量为65.37%,AP含量为24.47%)来调整饲料中AM/AP,使其比值分别为0、0.22、0.56、1.18、2.62,配制5种等氮等脂等淀粉含量的试验饲料(其组成及营养水平见表 1),同时各组饲料添加0.1%三氧化二钇作为外源指示剂,用以评价大口黑鲈对营养物质的表观消化率。将原料粉碎过60目筛网,按照配方准确称取,采用逐级扩大法将原料混合均匀,在V型混合机中混合12 min,然后加入鱼油、豆油和水(25%,质量分数),用搅拌机搅拌均匀后,使用干法膨化机制作沉性膨化颗粒饲料,模板孔径为3 mm,饲料颗粒长度为3 mm,制粒完成后在25 ℃空调房中自然晾干,晾至水分含量约为10%后装入封口袋,存放于-20 ℃的冰箱中。
|
|
表 1 试验饲料组成及营养水平(饲喂基础) Table 1 Composition and nutrient levels of experimental diets (as-fed basis) |
购买遗传背景一致的大口黑鲈鱼苗(英熙鱼虾苗场,高州,广东),在桶中暂养2周,暂养期间投喂商品饲料(粗蛋白质48%,粗脂肪7%)。正式试验开始前,禁饲24 h,挑选健康、规格均匀的鱼苗600尾[初始体重(2.15±0.04) g],随机分配到20个0.4 m3的玻璃纤维钢化桶中,试验分为5组,每组4个重复,每桶30尾鱼苗。每天08:00和16:00投喂至表观饱食,投喂后用虹吸管清除桶底部的残饵。养殖试验持续56 d。养殖试验在广东湛江国联饲料有限公司室内循环水养殖系统进行,水温28~31 ℃,溶解氧含量>5 mg/L。
1.3 样品收集养殖试验到第8周结束,试验鱼禁食24 h,记录每桶鱼的数量和总重量,用于计算增重率、特定生长率和存活率;记录每桶鱼摄食饲料的重量,用于计算饲料系数。每个桶随机取3尾鱼,取肠道于冻存管中,用液氮速冻,后保存在-80 ℃冰箱中,用于测定肠道消化酶活性。样品分析前于4 ℃冰箱内解冻,在冰盘剪碎并迅速准确称取一定量组织,根据不同酶活性测定要求加入一定体积的匀浆介质,在冰浴条件下机械匀浆,4 ℃ 3 000 r/min离心10 min,取上清液测定消化酶活性。养殖试验第5周至试验结束期间收集粪便,投喂饲料3 h后用镊子收集有完整包膜的粪便,在滤纸上吸干水分放在50 mL离心管中,于-20 ℃冰箱中保存。
1.4 样品测定饲料和粪便样品中水分含量采用105 ℃常压烘箱干燥法测定(GZX-9146MBE干燥箱,上海),粗蛋白质含量采用杜马斯燃烧法测定(Primacs SN-100杜马斯定氮仪,荷兰),粗脂肪含量采用索氏抽提法测定(ANKOMXT15i自动脂肪分析仪,美国)。饲料和粪便中Y2O3含量委托上海微谱检测技术有限公司检测,使用ICP-OES检测(Perkin Elmer Optima 8000,美国)。
饲料硬度和淀粉糊化度委托中国农业科学院饲料研究所检测。饲料硬度使用Perten硬度分析仪(TVT-6700,瑞典)测定;饲料淀粉糊化度参照熊易强[26]的方法测定;饲料溶失率参照《饲料质量与安全检测技术》[27]中的方法测定;饲料沉降速度参照中国农业科学院饲料研究所杨俊成等[28]的方法测定;饲料膨化度参照Glencross等[29]的方法测定。
肠道胰蛋白酶、脂肪酶、淀粉酶活性采用试剂盒(南京建成生物工程研究所)测定。其中,胰蛋白酶活性单位定义:在pH 8.0,37 ℃条件下,每毫克蛋白中含有的胰蛋白酶每分钟使吸光度变化0.003即为1个酶活力单位。淀粉酶活性单位定义:组织中每毫克蛋白在37 ℃与底物作用30 min,水解10 mg淀粉定义为1个酶活力单位。脂肪酶活性定义:37 ℃条件下,每克组织蛋白在本反应体系中与底物反应1 min,每消耗1 μmol底物为1个酶活力单位。肠道总蛋白酶活性采用试剂盒(上海酶联生物科技公司,上海)测定。总蛋白酶活性定义:37 ℃条件下,每分钟内催化1 μmol底物转化为产物所需的酶量定为1个酶活力单位。
1.5 计算公式

|

|
试验数据用SPSS 21.0软件进行单因素方差分析(one-way ANOVA),并用Duncan氏法进行多重比较检验,显著性水平为P < 0.05,数据以平均值±标准差表示。采用Pearson Correlation(2-tailed)方法对相关数据进行相关性分析,显著水平为P < 0.05。
2 结果 2.1 AM/AP对饲料加工特性的影响由表 2可知,D4组饲料硬度和膨化度显著低于D1、D2组(P < 0.05),与D3和D5组没有显著差异(P>0.05);D4和D5组淀粉糊化度显著低于D1、D2和D3组(P < 0.05);各组间饲料溶失率差异不显著(P>0.05);D4和D5组饲料沉降速率显著高于D1和D2组(P < 0.05)。
|
|
表 2 AM/AP对饲料加工特性的影响 Table 2 Effects of AM/AP on diet processing characteristics (n=3) |
由表 3可知,饲料AM/AP与饲料硬度、膨化度、淀粉糊化度和沉降速率呈极显著负相关(P < 0.01)。
|
|
表 3 AM/AP与饲料加工特性的相关性分析 Table 3 Correlation analysis between AM/AP and diet processing characteristics (n=3) |
由表 4可知,饲料硬度与淀粉糊化度和膨化度呈极显著负相关(P < 0.01)。
|
|
表 4 饲料硬度与淀粉糊化度和膨化度的相关性分析 Table 4 Correlation analysis between hardness and starch gelatinization degree and expansion degree of diets (n=3) |
由图 1看出,饲料膨化度在饲料AM/AP为1.81时达到最小值(y=2.418x2-8.751x+41.73,R2=0.998 2),淀粉糊化度在饲料AM/AP为1.95时达到最小值(y=7.325x2-28.5x+58.76,R2=0.930 0)。
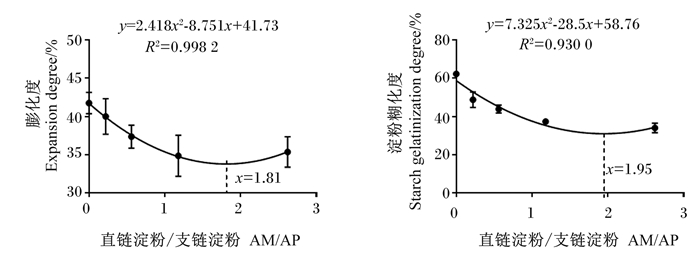
|
图 1 AM/AP和饲料膨化度和淀粉糊化度的相关性分析 Fig. 1 Relationship between AM/AP and expansion degree and starch gelatinization degree of diets |
由表 5可知,大口黑鲈的增重率、特定生长率随AM/AP的升高呈上升趋势,D4组显著高于D1和D2组(P < 0.05),与D3组和D5组没有显著差异(P>0.05)。
|
|
表 5 AM/AP对大口黑鲈生长性能的影响 Table 5 Effects of AM/AP on growth performance of largemouth bass (n=3) |
如图 2所示,经二元回归模型拟合分析后发现,特定生长率随着饲料中AM/AP的增加而上升,在AM/AP为1.83时达到最大值(y=-0.072 17x2+0.264 5x+3.303,R2=0.875 9)。饲料系数随AM/AP的升高呈上升趋势,D4组饲料系数显著高于D1组(P < 0.05),显著低于D5组(P < 0.05),和其余各组相比差异不显著(P>0.05)。
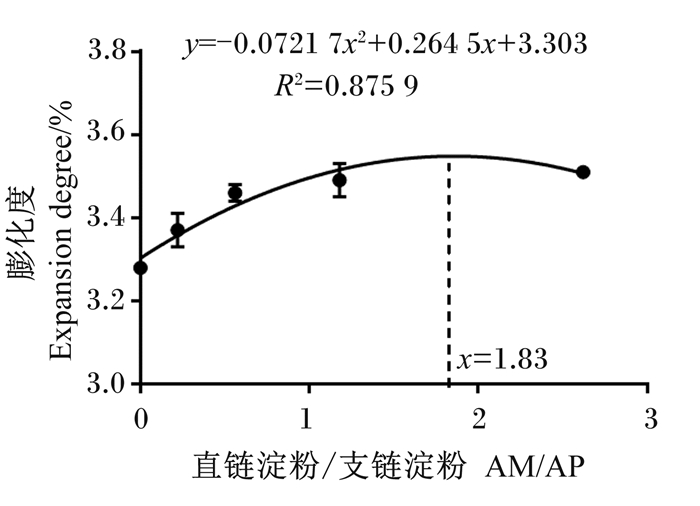
|
图 2 AM/AP对大口黑鲈特定生长率的影响 Fig. 2 Effects of AM/AP on specific growth rate of largemouth bass |
由表 6可知,D4组干物质表观消化率显著高于D1组(P < 0.05),和其余各组相比差异不显著(P>0.05);与D2、D3组相比,D4、D5组粗蛋白质表观消化率差异不显著(P>0.05),但显著高于D1组(P < 0.05)。
|
|
表 6 AM/AP对大口黑鲈营养物质表观消化率的影响 Table 6 Effects of AM/AP on apparent digestibility of nutrients of largemouth bass (n=3) |
由图 3可看出,粗蛋白质表观消化率在AM/AP为1.50时达到最大值(y=-1.879x2+5.644x+92.87,R2=0.683 7);D4组粗脂肪表观消化率与D3组差异不显著(P>0.05),但显著高于D5组(P < 0.05),显著低于D1和D2组(P < 0.05);D4和D5组显著低于其余3组(P < 0.05);淀粉表观消化率在AM/AP为2.15时达到最小值(y=7.467x2-32.03x+91.41,R2=0.906 4)。
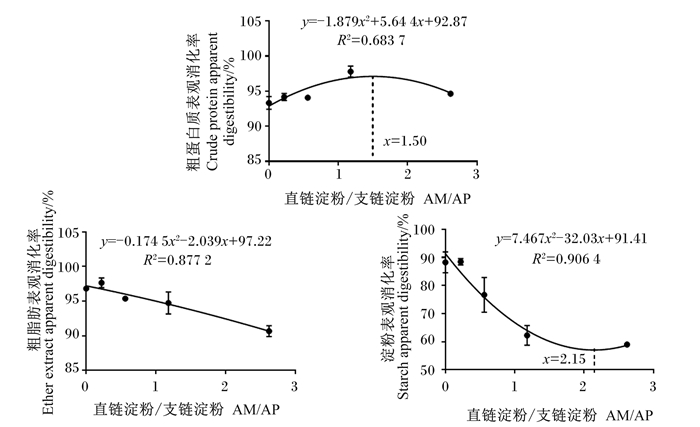
|
图 3 AM/AP对大口黑鲈粗蛋白质、粗脂肪和淀粉表观消化率的影响 Fig. 3 Effects of AM/AP on apparent digestibility of crude protein, ether extract and starch of largemouth bass |
由表 7可知,干物质表观消化率与饲料中AM/AP无显著相关(P>0.05),粗蛋白质表观消化率与饲料中AM/AP呈显著正相关(P < 0.05),粗脂肪表观消化率与饲料中AM/AP呈极显著负相关(P < 0.01),淀粉表观消化率与饲料中AM/AP呈极显著负相关(P < 0.01)。
|
|
表 7 AM/AP与大口黑鲈营养物质表观消化率的相关性分析 Table 7 Correlation analysis between AM/AP and apparent digestibility of nutrients of largemouth bass (n=3) |
由表 8可知,D4组肠道总蛋白酶和胰蛋白酶活性与D3和D5组差异不显著(P>0.05),显著高于D1组(P < 0.05);D4和D5组肠道脂肪酶活性显著低于D1和D2组(P < 0.05);与D2、D3和D5组相比,D4组肠道淀粉酶活性差异不显著(P>0.05),但显著低于D1组(P < 0.05)。
|
|
表 8 AM/AP对大口黑鲈肠道消化酶活性的影响 Table 8 Effects of AM/AP on digestive enzyme activities in intestinal tract of largemouth bass (n=3) |
由表 9可知,肠道总蛋白酶和胰蛋白酶活性与饲料中AM/AP呈极显著正相关(P < 0.01),肠道脂肪酶和淀粉酶活性与饲料中AM/AP呈极显著负相关(P < 0.01)。
|
|
表 9 AM/AP与大口黑鲈肠道消化酶活性的相关性分析 Table 9 Correlation analysis between AM/AP and digestive enzyme activities in intestinal tract of largemouth bass (n=3) |
水产动物和陆生动物相比,对饲料成形工艺要求较高。在饲料的制造加工过程中,AM/AP是对饲料质量产生影响的重要因素之一[29-30]。AP分子呈分支状,分子间氢键在膨化的高温作用下易断裂,形成不易崩塌的网状结构[31],在膨化过程中能承受较强的压力而结构不被破坏,因而容易膨化,并且膨化度较大。玉米淀粉在被挤压的过程中,AP部分会发生降解,而AM部分没有发生显著变化[32]。高直链玉米的膨化度低于普通玉米,膨化产品内部气室较小且密集[31]。AP含量较高的木薯淀粉参与形成的膨化颗粒饲料的膨化度显著高于玉米淀粉和面粉组[33-34]。袁军[35]发现,添加40%木薯淀粉组膨化度最高,而添加40%玉米淀粉组膨化度最低。本试验结果显示,饲料中AM/AP与膨化度呈极显著负相关。AP在水产膨化饲料生产过程中起到的膨胀作用强于AM,因此,饲料中较高的AP含量会提高饲料的膨化度。
淀粉糊化程度会对产品最终质量产生影响[36],糊化后的淀粉具有冷黏性,使淀粉凝胶化,增大最终黏度[37-38]。AP与淀粉溶胀现象密切相关[39],可以促进淀粉的糊化,而AM会阻碍淀粉的糊化[40-41],可能是AM分子间连接紧密不易断裂,导致淀粉糊化度降低[42],因此淀粉糊化度与饲料中AM/AP呈负相关。在本试验中,饲料硬度与膨化度和淀粉糊化度呈显著正相关,与袁军[35]关于玉米、木薯粉和面粉对膨化饲料硬度的研究结果不同,可能是饲料加工时温度影响了AM糊化温度,高直链玉米淀粉在115.3 ℃时开始糊化,完全糊化时温度为130.7 ℃[43-44],温度不足会导致AM糊化度较低,凝胶性弱,而AP较AM易糊化[45],黏结性强,因此饲料硬度较高[46]。
3.2 AM/AP对大口黑鲈生长性能和营养物质表观消化率的影响淀粉作为动物能量来源之一,可以提高动物产品生产效率,减低饲料成本。饲料中不同的AM和AP含量会影响动物对营养物质的消化利用,因此选择合适的AM/AP可以改善大口黑鲈对淀粉的利用效率。饲料中AM/AP为0.32时,暗纹东方鲀(Takifugu obscurus)可获得最佳的生长和饲料利用率[24]。饲料中AM/AP为0.24时,罗非鱼(Oreochromis nilotictus)的增重率最高,AM/AP升高则会抑制罗非鱼的生长[47]。而AM/AP为2.33时,太阳鲈(Morone chrysops ♀ ×M. saxatilis ♂)增重率高于全AP组和AM/AP为0.43组[48]。本试验研究结果显示,AM/AP的升高会促进大口黑鲈的生长,在AM/AP为2.62时,表现出最佳的生长,但饲料系数较高。这原因可能是AM相对于AP更不易消化,鱼类摄食低AM/AP的饲料后,消化后的葡萄糖快速入血,而鱼类利用淀粉的能力因鱼类种类而异[49],大口黑鲈和太阳鲈是肉食性鱼类,其调节血糖的能力弱于作为杂食性鱼类[50]的罗非鱼和暗纹东方鲀。因此,高AM/AP的饲料更有利于大口黑鲈的生长。
淀粉经膨化后,晶体结构被破坏,淀粉凝胶化,消化酶更容易接触发挥作用,提高了养殖动物对淀粉的消化利用率,降低了饲料系数[51-52]。贾艳菊等[51]发现膨化处理的饲料可以提高鳖的饲料利用率。膨化饲料相较于普通颗粒饲料可以提高异育银鲫(Carassius auratus gibelio)[53]和真鲷(Pagrus major)[54]的增重率和特定生长率,降低饲料系数。郭冉等[55]发现,淀粉糊化度为32.07%时,可以显著提高凡纳滨对虾的生长,高于该值则会抑制对虾生长。玉米和木薯等AM/AP较低的淀粉源做出的饲料其淀粉糊化度更高,显著提高了吉富罗非鱼对饲料中营养成分的吸收利用[35]。本试验研究结果显示,AM/AP较低的饲料淀粉糊化度和膨化度更高,尽管饲料系数最低,但是生长性能也降低。这原因可能是低AM/AP淀粉消化率高,但消化后的葡萄糖超出鱼体承受范围,对鱼体代谢造成障碍,表现为较慢的生长速度。
干物质表观消化率在一定程度上能反映出机体对养分的消化程度[56]。本试验结果表明,随饲料中AM/AP增加,干物质表观消化率呈先升高后降低的趋势,在D3组达到最大值。杨伟[47]在罗非鱼上的研究表明,当饲料中的AM/AP为0.24时,干物质表观消化率和粗蛋白质表观消化率最高,AM/AP增加到0.76时,粗脂肪表观消化率最高,与本试验研究结果相似,表明适当的增加饲料中AM的含量可以提高大口黑鲈对饲料中营养物质的利用能力。随饲料中AM/AP增加,粗蛋白质表观消化率和肠道中蛋白酶活性呈升高趋势,提示饲料中一定含量的AM可以影响大口黑鲈对蛋白质的消化。
3.3 AM/AP对大口黑鲈肠道消化酶活性的影响消化酶活性的强弱与营养物质结构相关,并直接影响养殖动物对营养物质的消化能力[57-58]。淀粉颗粒内AM和AP形成的晶体结构会抵抗水和酶的接触[59],影响淀粉在动物肠道内被消化的程度[60]。AM分子间氢键作用力比AP更强,难于被降解,AP越多,猪小肠淀粉的消化率越高[52],虹鳟[61]和海鲈[62]对蜡质玉米淀粉的消化率明显高于普通玉米淀粉。AM/AP为0.98时,罗非鱼淀粉表观消化率明显低于AM/AP为0.11的饲料[48],与本试验研究结果一致。相较于AP,油脂等化合物更易于和AM结合形成复合物,导致淀粉疏水性增强,膨化度降低,同时也降低了与淀粉酶的接触[63]。并且在制粒过程中,AM分子在挤压膨化作用下形成了抗性淀粉[64],而AM含量与抗性淀粉含量成正相关[21],对淀粉酶产生了一定的阻碍作用,导致饲料中的AM更不容易被消化,进而影响动物消化酶的活性以及对淀粉的消化率。本试验中大口黑鲈肠道淀粉酶的活性与饲料中AM/AP呈极显著负相关性,与淀粉表观消化率趋势相同,说明饲料中AM/AP对大口黑鲈利用碳水化合物的能力有较大的影响,与Liu等[24]在暗纹东方鲀上的研究结果一致。随饲料中AM/AP增加,脂肪消化率和肠道中脂肪酶活性呈降低趋势,这可能与AM-脂质复合物抵抗酶解,减缓脂肪的消化吸收有关。
4 结论本试验条件下,饲料中AM/AP升高会降低膨化饲料的淀粉糊化度和膨化度。适宜的AM/AP可以提高肠道蛋白酶的活性,增加大口黑鲈干物质和粗蛋白质表观消化率,促进生长。综合大口黑鲈生长和营养物质表观消化率等结果,饲料中AM/AP为1.18~2.62时效果较好。
| [1] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 中国渔业统计年鉴-2021[M]. 北京: 中国农业出版社, 2021. Fishery Administration of the Ministry of Agriculture and Rural Areas, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook-2021[M]. Beijing: Chinese Agricultural Press, 2021 (in Chinese). |
| [2] |
刘兴旺, 朱敬强, 李国立, 等. 加州鲈营养生理研究进展[J]. 广东饲料, 2021, 30(4): 37-44. LIU X W, ZHU J Q, LI G L, et al. Research progress on nutrition and physiology of California sea bass[J]. Guangdong Feed, 2021, 30(4): 37-44 (in Chinese). DOI:10.3969/j.issn.1005-8613.2021.04.010 |
| [3] |
王秀娟, 胡嘉雯, 王悦, 等. 大口黑鲈营养需求的研究进展[J]. 饲料研究, 2019, 42(8): 112-116. WANG X J, HU J W, WANG Y, et al. Research progress of nutritional requirement for largemouth bass (Micropterus salmoides)[J]. Feed Research, 2019, 42(8): 112-116. |
| [4] |
李晓宁. 饲料糖水平对大菱鲆和牙鲆生长、生理状态参数及体组成的影响[D]. 硕士学位论文. 青岛: 中国海洋大学, 2011. LI X N. Effects of dietary carbohydrate levels on growth performance, physiological status and body composition of turbot (Scophthalmus maximus Linnaeus) and Japanese flounder (Paralichthys olivaceus)[D]. Master's Thesis. Qingdao: Ocean University of China, 2011. (in Chinese) |
| [5] |
姜大丽, 李治国, 姜永杰, 等. 乌鳢营养需求研究进展[J]. 饲料工业, 2021, 42(4): 29-35. JIANG D L, LI Z G, JIANG Y J, et al. Research advances in nutritional requirement of Channa argus[J]. Feed Industry, 2021, 42(4): 29-35 (in Chinese). |
| [6] |
SONG M Q, SHI C M, LIN S M, et al. Effect of starch sources on growth, hepatic glucose metabolism and antioxidant capacity in juvenile largemouth bass, Micropterus salmoides[J]. Aquaculture, 2018, 490: 355-361. DOI:10.1016/j.aquaculture.2018.03.002 |
| [7] |
苟仕潘, 陈乃松, 徐祥泰, 等. 饲料中可消化淀粉对大口黑鲈生长、体组成和非特异性免疫指标的影响[J]. 水产学报, 2015, 39(10): 1499-1510. GOU S P, CHEN N S, XU X T, et al. Effects of dietary digestible starch levels on growth performance, body composition, and non-specific immunological index of largemouth bass (Micropterus salmoides)[J]. Journal of Fisheries of China, 2015, 39(10): 1499-1510. |
| [8] |
刘子科, 陈乃松, 王孟乐, 等. 大口黑鲈饲料中适宜的淀粉源及添加水平[J]. 中国水产科学, 2017, 24(2): 317-331. LIU Z K, CHEN N S, WANG M L, et al. Suitable dietary starch source and supplementation level for largemouth bass (Micropterus salmoides)[J]. Journal of Fishery Sciences of China, 2017, 24(2): 317-331 (in Chinese). |
| [9] |
AMOAH A, COYLE S D, WEBSTER C D, et al. Effects of graded levels of carbohydrate on growth and survival of largemouth bass, Micropterus salmoides[J]. Journal of the World Aquaculture Society, 2008, 39(3): 397-405. DOI:10.1111/j.1749-7345.2008.00168.x |
| [10] |
邢淑娟, 孙瑞健, 马俊, 等. 饲料糖水平对大黄鱼生长和糖代谢的影响[J]. 水生生物学报, 2017, 41(2): 265-276. XING S J, SUN R J, MA J, et al. Effects of dietary carbohydrate on growth performance and glycometabolism of large yellow croaker Larimichthys crocea[J]. Acta Hydrobiologica Sinica, 2017, 41(2): 265-276 (in Chinese). |
| [11] |
郁欢欢. 高糖饲料诱导大口黑鲈代谢性肝病及胆汁酸靶向干预机制研究[D]. 博士学位论文. 北京: 中国农业科学院, 2019. YU H H. Metabolic liver disease induced by a high carbohydrate diet in largemouth bass (Micropterus salmoides), and the alleviating mechanism of bile acid supplementation[D]. Ph. D. Thesis. Beijing: Chinese Academy of Agricultural Sciences, 2019. (in Chinese) |
| [12] |
GOODWIN A E, LOCHMANN R T, TIEMAN D M, et al. Massive hepatic necrosis and nodular regeneration in largemouth bass fed diets high in available carbohydrate[J]. Journal of the World Aquaculture Society, 2002, 33(34): 466-477. |
| [13] |
SØRENSEN M, NGUYEN G, STOREBAKKEN T, et al. Starch source, screw configuration and injection of steam into the barrel affect the physical quality of extruded fish feed[J]. Aquaculture Research, 2010, 41(3): 419-432. DOI:10.1111/j.1365-2109.2009.02346.x |
| [14] |
DHITAL S, WARREN F J, BUTTERWORTH P J, et al. Mechanisms of starch digestion by α-amylase-structural basis for kinetic properties[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(5): 875-892. DOI:10.1080/10408398.2014.922043 |
| [15] |
VAN SOEST J J G, BORGER D B. Structure and properties of compression-molded thermoplastic starch materials from normal and high-amylose maize starches[J]. Journal of Applied Polymer Science, 1997, 64(4): 631-644. DOI:10.1002/(SICI)1097-4628(19970425)64:4<631::AID-APP2>3.0.CO;2-O |
| [16] |
BERRY C S. Resistant starch: formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre[J]. Journal of Cereal Science, 1986, 4(4): 301-314. DOI:10.1016/S0733-5210(86)80034-0 |
| [17] |
SINGH J, DARTOIS A, KAUR L. Starch digestibility in food matrix: a review[J]. Trends in Food Science & Technology, 2010, 21(4): 168-180. |
| [18] |
GOMINHO-ROSA M D C, RODRIGUES A P O, MATTIONI B, et al. Comparison between the omnivorous jundiá catfish (Rhamdia quelen) and Nile tilapia (Oreochromis niloticus) on the utilization of dietary starch sources: digestibility, enzyme activity and starch microstructure[J]. Aquaculture, 2015, 435: 92-99. DOI:10.1016/j.aquaculture.2014.09.035 |
| [19] |
李明. 高直链淀粉在食品和材料领域应用的研究进展[J]. 食品安全质量检测学报, 2019, 10(20): 6739-6746. LI M. Review on the application of high-amylose starch in the field of food and material[J]. Journal of Food Safety & Quality, 2019, 10(20): 6739-6746. |
| [20] |
REN W, ZHAO F F, ZHANG A Z, et al. Gastrointestinal tract development in fattening lambs fed diets with different amylose to amylopectin ratios[J]. Canadian Journal of Animal Science, 2016, 96(3): 425-433. DOI:10.1139/cjas-2015-0165 |
| [21] |
GIUBERTI G, GALLO A, MOSCHINI M, et al. New insight into the role of resistant starch in pig nutrition[J]. Animal Feed Science and Technology, 2015, 201: 1-13. DOI:10.1016/j.anifeedsci.2015.01.004 |
| [22] |
MA J, YANG T, YANG M, et al. Effects of dietary amylose/amylopectin ratio and amylase on growth performance, energy and starch digestibility, and digestive enzymes in broilers[J]. Journal of Animal Physiology and Animal Nutrition, 2020, 104(3): 928-935. DOI:10.1111/jpn.13338 |
| [23] |
韩文芳, 林亲录, 赵思明, 等. 直链淀粉和支链淀粉分子结构研究进展[J]. 食品科学, 2020, 41(13): 267-275. HAN W F, LIN Q L, ZHAO S M, et al. Recent advances in molecular structures of amylose and amylopectin[J]. Food Science, 2020, 41(13): 267-275 (in Chinese). |
| [24] |
LIU X H, YE C X, YE J D, et al. Effects of dietary amylose/amylopectin ratio on growth performance, feed utilization, digestive enzymes, and postprandial metabolic responses in juvenile obscure puffer Takifugu obscurus[J]. Fish Physiology and Biochemistry, 2014, 40(5): 1423-1436. DOI:10.1007/s10695-014-9937-4 |
| [25] |
DONA A C, PAGES G, GILBERT R G, et al. Digestion of starch: in vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release[J]. Carbohydrate Polymers, 2010, 80(3): 599-617. DOI:10.1016/j.carbpol.2010.01.002 |
| [26] |
熊易强. 饲料淀粉糊化度(熟化度) 的测定[J]. 饲料工业, 2000(3): 30-31. XIONG Y Q. Determination of starch gelatinization degree (degree of ripening)[J]. Feed Industry, 2000(3): 30-31 (in Chinese). |
| [27] |
常碧影, 张萍. 饲料质量与安全检测技术[M]. 北京: 化学工业出版社, 2008: 134-136. CHANG B Y, ZHANG P. Feed quality and safety testing techniques[M]. Beijing: Chemical Industry Press, 2008: 134-136 (in Chinese). |
| [28] |
杨俊成, 于庆龙, 秦玉昌, 等. 饲料物理性能指标的测定方法[J]. 中国饲料, 2000(17): 23-24. YANG J C, YU Q L, QIN Y C, et al. Determination method of physical properties of feed indicators[J]. China Feed, 2000(17): 23-24 (in Chinese). |
| [29] |
GLENCROSS B, BLYTH D, TABRETT S, et al. An assessment of cereal grains and other starch sources in diets for barramundi (Lates calcarifer)-implications for nutritional and functional qualities of extruded feeds[J]. Aquaculture Nutrition, 2012, 18(4): 388-399. DOI:10.1111/j.1365-2095.2011.00903.x |
| [30] |
DING X Q, YAO L, HOU Y, et al. Effects of different carbohydrate levels in puffed feed on digestive tract morphological function and liver tissue structure of snakeheads (Channa argus)[J]. Aquaculture Research, 2020, 51(2): 557-568. DOI:10.1111/are.14402 |
| [31] |
刘林三. 不同直链淀粉含量玉米的淀粉合成基因表达、淀粉结构与膨化特征分析[D]. 硕士学位论文. 杨凌: 西北农林科技大学, 2018. LIU L S. Analysis of starch synthesis gene expression, starch molecular structure and extrusion characteristics of maize with different amylose content[D]. Master's Thesis. Yangling: Northwest A & F University, 2018. (in Chinese) |
| [32] |
CHIANG B Y, JOHNSON J A. Gelatinization of starch in extruded products[J]. Cereal Chemistry, 1977, 54(3): 436-443. |
| [33] |
AH-HEN K, LEHNEBACH G, LEMUS-MONDACA R, et al. Evaluation of different starch sources in extruded feed for Atlantic salmon[J]. Aquaculture Nutrition, 2014, 20(2): 183-191. DOI:10.1111/anu.12064 |
| [34] |
KANNADHASON S, MUTHUKUMARAPPAN K. Effect of starch sources on properties of extrudates containing DDGS[J]. International Journal of Food Properties, 2010, 13(5): 1012-1034. DOI:10.1080/10942910902937416 |
| [35] |
袁军. 加工工艺及参数对饲料加工质量的影响[D]. 硕士学位论文. 大连: 大连海洋大学, 2014. YUAN J. Feed pellet qualities are affected by the processing way and the processing parameters. [D]Master's Thesis. Dalian: Dalian Ocean University, 2014. (in Chinese) |
| [36] |
DINTZIS F R, BAGLEY E B. Effects of thermomechanical processing on viscosity behavior of corn starches[J]. Journal of Rheology, 1995, 39(6): 1483-1495. DOI:10.1122/1.550719 |
| [37] |
VAN DEN EINDE R M, VAN DER GOOT A J, BOOM R M. Understanding molecular weight reduction of starch during heating-shearing processes[J]. Journal of Food Science, 2003, 68(8): 2396-2404. DOI:10.1111/j.1365-2621.2003.tb07036.x |
| [38] |
HOOVER R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review[J]. Carbohydrate Polymers, 2001, 45(3): 253-267. DOI:10.1016/S0144-8617(00)00260-5 |
| [39] |
MAHANTA C L, BHATTACHARYA K R. Relationship of starch changes to puffing expansion of parboiled rice[J]. Journal of Food Science and Technology, 2010, 47(2): 182-187. DOI:10.1007/s13197-010-0038-9 |
| [40] |
TESTER R F, MORRISON W R. Swelling and gelatinization of cereal starches.Ⅰ.Effects of amylopectin, amylose, and lipids[J]. Cereal Chemistry, 1990, 67(6): 551-557. |
| [41] |
LII C Y, SHAO Y Y, TSENG K H. Gelation mechanism and rheological properties of rice starch[J]. Cereal Chemistry, 1995, 72(4): 393-400. |
| [42] |
ROMANO N, KUMAR V. Starch gelatinization on the physical characteristics of aquafeeds and subsequent implications to the productivity in farmed aquatic animals[J]. Reviews in Aquaculture, 2019, 11(4): 1271-1284. DOI:10.1111/raq.12291 |
| [43] |
陈旭. 高直链玉米淀粉的形态和糊化行为研究[D]. 硕士学位论文. 合肥: 安徽农业大学, 2018. CHEN X. Morphologies and gelatinization behaviours of high-amylose maize starches during heat treatment[D]. Master's Thesis. Hefei: Anhui Agricultural University, 2018. (in Chinese) |
| [44] |
章显光, 黄永楷. 稻米直链淀粉含量、糊化温度和胶稠度的初步研究[J]. 湖北农学院学报, 1992(1): 10-15. ZHANG X G, HUANG Y K. Preliminary study of amylose content, gelatinization temperature and gel consistency in rice[J]. Journal of Hubei Agricultural College, 1992(1): 10-15 (in Chinese). |
| [45] |
周慧颖, 彭小松, 欧阳林娟, 等. 支链淀粉结构对稻米淀粉糊化特性的影响[J]. 中国粮油学报, 2018, 33(8): 25-30, 36. ZHOU H Y, PENG X S, OUYANG L J, et al. Effects of amylopectin structure on gelatinization characteristics of rice starch[J]. Journal of the Chinese Cereals and Oils Association, 2018, 33(8): 25-30, 36 (in Chinese). |
| [46] |
孙永泰. 如何调控颗粒饲料的颗粒硬度[J]. 江西饲料, 2011(5): 31-32. SUN Y T. How to control pellet hardness of pellet feed[J]. Jiangxi Feed, 2011(5): 31-32 (in Chinese). |
| [47] |
杨伟. 饲料中直链/支链淀粉比对罗非鱼生长、饲料利用及肠道健康的影响[D]. 硕士学位论文. 厦门: 集美大学, 2012. YANG W. Effects of dietary amylose/amylopectin ratios on the growth, feed utilization and intestinal health of tilapia, Oreochromis nilotictus[D]. Master's Thesis. Xiamen: Jimei University, 2012. (in Chinese) |
| [48] |
RAWLES S, LOCHMANN R. Effects of amylopectin/amylose starch ratio on growth, body composition and glycemic response of sunshine bass Morone chrysops×M. saxatilis[J]. Journal of the World Aquaculture Society, 2003, 34(3): 278-288. DOI:10.1111/j.1749-7345.2003.tb00066.x |
| [49] |
KAMALAM B S, MEDALE F, PANSERAT S. Utilisation of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies[J]. Aquaculture, 2017, 467: 3-27. |
| [50] |
NRC. Nutrient requirements of fish and shrimp[M]. Washington D.C.: National Academies Press, 2011: 135-162.
|
| [51] |
贾艳菊, 陈颖, 杨振才. 饲料膨化处理对中华鳖氮和能量收支的影响[J]. 四川动物, 2008, 27(5): 949-951. JIA Y J, CHEN Y, YANG Z C. Effects of feed extrusion on nitrogen and energy budgets of Chinese soft-shelled turtle (Pelodiscus sinensis Wiegmann)[J]. Sichuan Journal of Zoology, 2008, 27(5): 949-951 (in Chinese). |
| [52] |
黄伟, 杨秀娟, 曹志勇, 等. 日粮淀粉直支比对断奶仔猪养分消化率和生长性能的影响[J]. 中国饲料, 2017(14): 20-23. HUANG W, YANG X J, CAO Z Y, et al. Effects of the ratio of amylase and amylopectin on nutrient digestibility and growth performance in piglets[J]. China Feed, 2017(14): 20-23 (in Chinese). |
| [53] |
GAO S Y, JUN J Y, LIU H K, et al. Effects of pelleted and extruded feed of different ingredients particle sizes on feed quality and growth performance of gibel carp (Carassius gibelio var. CAS V)[J]. Aquaculture, 2019, 511: 734236. |
| [54] |
JEONG K S, TAKEUCHI T, WATANABE T. Improvement of nutritional quality of carbohydrate ingredients by extrusion process in diets of Red Sea bream[J]. Nippon Suisan Gakkaishi, 1991, 57(8): 1543-1549. |
| [55] |
郭冉, 刘永坚, 田丽霞, 等. 不同淀粉糊化度对凡纳滨对虾生长和体营养成分的影响[J]. 大连水产学院学报, 2010, 25(5): 402-406. DUO R, LIU Y J, TIAN L X, et al. The effects of pre-gelatinization of cornstarch on the growth performance and body composition in Pacific white leg shrimp Litopenaeus vannamei[J]. Journal of Dalian Ocean University, 2010, 25(5): 402-406. |
| [56] |
卢德勋. 系统动物营养学导论[M]. 北京: 中国农业出版社, 2004: 401-402. LU D X. An introduction to systems-nutrition of animals[M]. Beijing: China Agriculture Press, 2004: 401-402 (in Chinese). |
| [57] |
PÉREZ-JIMÉNEZ A, CARDENETE G, MORALES A E, et al. Digestive enzymatic profile of Dentex dentex and response to different dietary formulations[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2009, 154(1): 157-164. |
| [58] |
孟祥科, 孙阳, 屈菲, 等. 植酸酶对红鳍东方鲀幼鱼生长、消化酶及消化率的影响[J]. 大连海洋大学学报, 2013, 28(4): 323-328. MENG X K, SUN Y, QU F, et al. Effects of phytase on growth, digestive enzyme activity and digestibility in juvenile redfin puffer Takifugu rubripes[J]. Journal of Dalian Fisheries University, 2013, 28(4): 323-328 (in Chinese). |
| [59] |
CUI R, OATES C G. The effect of retrogradation on enzyme susceptibility of sago starch[J]. Carbohydrate Polymers, 1997, 32(1): 65-72. |
| [60] |
ZHANG G Y, Ao Z H, HAMAKER B R. Slow digestion property of native cereal starches.[J]. Biomacromolecules, 2006, 7(11): 3252-3258. |
| [61] |
BERGOT F. Digestibility of native starches of various botanical origins by rainbow trout (Oncorhynchus mykiss)[J]. Colloques de l'INRA(France), 1993, 286(6383): 2067-2067. |
| [62] |
ENES P, PANSERAT S, KAUSHIK S, et al. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles[J]. Comparative Biochemistry and Physiology.Part A: Molecular & Integrative Physiology, 2006, 143(1): 89-96. |
| [63] |
PÉREZ S, BALDWIN P M, GALLANT D J. Chapter 5-structural features of starch granules Ⅰ[M]//BEMILLER J, WHISTLER R. Starch: chemistry and technology. 3rd ed. London: Academic Press, 2009: 149-192.
|
| [64] |
HSIEN-CHIH H W, SARKO A. The double-helical molecular structure of crystalline α-amylose[J]. Carbohydrate Research, 1978, 61(1): 27-40. |




